Translate this page into:
Isolation and characterization of encapsulated plant growth-promoting Enterobacter sp. SA10 for enhancing chili growth
⁎Corresponding author at: Faculty of Plantation and Agrotechnology, Universiti Teknologi Mara (UiTM) Malacca, Jasin Campus, 77300 Merlimau, Melaka, Malaysia. Nurmaizatul@uitm.edu.my (N.M.I. Othman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction
Plant growth-promoting rhizobacteria (PGPR) can increase plant growth and encapsulation of PGPR with biochar ensures the viability and survival of PGPR. However, this approach is still underexplored.
Objectives
The objectives of this study: 1) to isolate and select a potential PGPR from rice rhizosphere based on plant growth-promoting characterization, 2) encapsulate the selected PGPR strain SA-10 using biochar and sodium alginate, and 3) assess the effect of encapsulated and non-encapsulated SA-10 on chili plant growth compared to a non-inoculated SA-10 and control.
Methodology
SA-10 was isolated from rice rhizosphere and characterized for plant growth promoting traits including, the nitrogen fixation, phosphate solubilization, production of siderophore, production of indole acetic acid and production of cellulose degradation enzyme. SA-10 was encapsulated in alginate beads along with biochar. A pot experiment conducted using encapsulated SA-10, non-encapsulated SA-10, and a control group to assess the effects of encapsulated SA-10 application on chili plants using complete randomized design (CRD) experimental design with four replications.
Results
About 28 bacteria isolates were isolated from the rhizosphere and endospheric of rice crops to determine their effects on the growth of chili plants. The isolates were characterized with zinc, phosphate, and potassium solubilization, siderophores production, and cellulose degradation. The findings showed isolate SA-10 solubilized zinc at a rate of 41.6 %, phosphate at 50 %, and potassium at 76.9 %. It also fixed nitrogen, produced siderophores and degraded cellulose. The isolate also produced the highest IAA at 4.007 mg/L. Isolate SA-10 was then encapsulated in a mixture of biochar and sodium alginate. The results showed that plants treated with encapsulated SA-10 had significantly higher leaf area (3.68 cm2), leaf diameter (6.55 cm), and dry weight (0.39 g) compared to those treated with non-encapsulated and the control treatment. Through 16S rRNA gene sequencing, it was identified that SA-10 belongs to the Enterobacter genus, closely resembling Enterobacter chuandaensis.
Conclusion
The findings suggest that Enterobacter sp. SA-10 enhances plant growth, and the use of encapsulated SA-10 with biochar contributes to the growth of chili plants. The encapsulated Enterobacter sp. SA-10 has a promising potential in enhancing crop yield production and promoting sustainable agriculture.
Keywords
Biofertilizer
Encapsulation
Bacteria
Capsicum annuum L
Biochar
1 Introduction
Chili (Capsicum annum L.) is the universal spice widely cultivated crop of the Solanaceae family. There are several types of plant chili cultivated in the world and which are the most distributed in Asia. The genus Capsicum encompasses five well-known species, namely Capsicum. annuum, Capsicum frutescens, Capsicum pubescens, Capsicum baccatum, Capsicum chinense. Capsicum annuum L. and Capsicum frutescens L. are the most widely cultivated chili peppers in Malaysia (Hassan et al., 2019). In chili plantation, chemical fertilizers are widely used by Malaysian farmers to obtain a high chili yield to meet the demand. Sofyan et al. (2019) highlighted the long-term effects of inorganic fertilizer usage, including soil acidification, decreased soil fertility, and pollution of air and water. The negative consequences of inorganic fertilizer usage have prompted the exploration of alternative fertilization methods to mitigate these issues. Plant growth-promoting rhizobacteria (PGPR) has been found widely explored to enhance sustainability plantation as nitrogen fixer, phosphate solubilizer, zinc solubilizer, potassium solubilizer, growth hormone producer and siderophore producer (Othman et al., 2022). In a recent study, Kashyap et al. (2022) highlighted the positive effects of Pseudomonas fluorescens PDS1 and Bacillus subtilis KA9 in terms of chili plant growth and its biocontrol efficiency against Ralstonia solanacearum. Other than that, PGPR also have mechanisms to help host plant through solubilization or release of nutrient elements from sparingly available sources while not causing any harm to their plant host (Boleta et al., 2020). Studies have shown that Azotobacter sp., Bacillus megaterium, Bacillus coagulants, Flavobacterium sp. and Pseudomonas atmuta relatively effective to provide an increased on the growth and seed germination of chili plants from the variety PM999 (V1) and Kiyo F1 (V2), suggesting that they have the potential to enhance crops growth and yield (Syamsuddin et al., 2022; Hu et al., 2023). This finding opens possibilities for targeted application in the future.
Other than chili plant, inoculation of Azospirilum brasilense on wheat plants caused the top part of the plants to have more micronutrients (Boleta et al., 2019; Iqbal et al., 2023a). This inoculation has the potential to reduce reliance on chemical fertilizer while effectively enhancing plant growth and nutrition. Commensalism occurs when bacteria colonize varied parts of plant foliage without inflicting harm or providing considerable benefits to the host plant (Iqbal et al., 2023a; Iqbal et al., 2023b). The presence of unfavorable environmental conditions and abiotic stresses presents a major challenge to the adoption of sustainable agricultural methods for chili (Capsicum annuum L.) (Achari and Kowshik, 2018). Abiotic stresses, such as drought, salinity, and heat, negatively impact crop yields but PGPR can lessen the bad effects of stress as occurred in the Brassicaceae family (Casagrande et al., 2022; Jalal et al., 2021; Jalal et al., 2023). Plant growth-promoting rhizobacteria can help plants grow in several mechanisms, such as breaking down nutrients, making phytohormones, and fixing nitrogen and it also can integrate with zinc nanomaterial (He et al., 2023).
Plant growth-promoting rhizobacteria have many beneficial properties, however, effectively protecting these microorganisms can be challenging due to environmental stress (Adedeji et al., 2020). Encapsulation of PGPR with sodium alginate has emerged as a guaranteed approach to enhance the survival and activity of beneficial bacteria in plants. This method causes the progressive release of encapsulated bacteria into the soil, enhancing the lifespan of bacterial isolates and thus, boosting their potency. (Lee at al. 2021). Martínez-Cano et al. (2022) reviewed alginate-encapsulated plant growth-promoting bacteria for diverse agricultural crops. Microbial encapsulation may safeguard beneficial microbes and improve plant root colonization. Encapsulation might help to protect the heavy metal effects on microbial communities in soil which contribute to optimum condition in nutrient cycling and fertility level in soil (Iqbal et al., 2023b; Iqbal et al., 2024). To develop the encapsulation formulation, other organic mixtures might help to boost plant growth like biochar (Xu et al., 2022). A study showed that biochar treatment on chili production in Bandung, Indonesia (Rematwa et al., 2022). These studies demonstrate the efficacy of using biochar and microbial inoculants for sustainable crop production. However, sodium alginate-biochar encapsulation remains underexplored as biofertilizer production including Malaysia.
Given the advantages of PGPR and encapsulation technique as potential biofertilizer, this study proposed three hypotheses. The hypotheses are as follows: 1) PGPR can be isolated and selected based on its plant growth-promoting traits; 2) encapsulating SA-10 with biochar and sodium alginate protects the PGPR; and 3) determining whether encapsulated SA-10 can be used as a biofertilizer for chili cultivation. Thus, the objectives of this study are to: 1) isolate, identify and select a potential PGPR from rice rhizosphere based on plant growth-promoting characterization, 2) encapsulate the selected PGPR using biochar and sodium alginate, and 3) assess the effect of encapsulated and non-encapsulated SA-10 on chili plant growth compared to a non-inoculated SA-10 and control.
2 Materials and methods
2.1 Location of study
Rice soil samples were obtained to isolate microorganisms from the non-rhizosphere, rhizosphere, and endospheric environments. The samples were collected from a rice irrigation region and planted using the MR 269 rice variety. The site is in Sungai Udang, North Malacca, Southern Malaysia (coordinates: 2°15ʹ45″N, 102°9ʹ9″E). A soil auger was utilized to collect soil samples from a depth of 15 cm. The samples were obtained using an auger during the rice vegetative period (about 40 days after seeding). It was assumed that the bacteria were active during this period. The samples were chilled to 4 °C to reduce microbial activity.
2.2 Preparation of bacteria isolation from rice cultivation
Root samples and soil were collected from rice plants growing in the field. The sample was grouped into three categories: non-rhizosphere, rhizosphere, and endophytes. Bacteria was isolated from the non-rhizosphere; 10 g of sample soil was weighed, and 90 mL of sterile water was added into the glass bottle. The solution was shaken until homogenized. Then, 0.1 mL of the solution is directly poured and spread using sterilized glass rod onto the nutrient agar (NA) plate. The plate is placed upside down and incubated at 28 °C for 48 h. Bacteria isolate from the rhizosphere, the root part sample was cut and gently washed. Then, one g of root sample was weighed, and 9 mL of sterile water. The solution was shaken until homogenized. The solution is poured and spread on the nutrient agar exactly like the soil sample in the previous step. The isolation of endophytic bacteria method was adopted from Sondang et al. (2019). The root sample was cut and sterilized with ethanol and sodium hypochlorite. One gram of root was scraped with distilled water into test test. A small amount of the solution was spread onto NA and incubated to observe colony formation.
2.3 Characterization of plant growth-promoting bacteria
Six isolates with different colors and morphology were selected for the following test: Nitrogen fixation qualitative test (Dobereiner and Day, 1976), phosphate solubilizing test (National Botanical Reserch Insitute Phosphate (NBIRP), potassium solubilizing test (Aleksandrov et al., 1967), zinc solubilization ability test (Saravanan et al., 2003). qualitative cellulose degrading enzyme production test (Padaria et al., 2014), qualitative siderophore production test (Schwyn and Neilands, 1987) and indole-acetic acid (IAA production test) (Gordon and Weber et al. 1951) and Qualitative Chrome Azurol-S test for siderophore production.
2.4 Encapsulation of plant growth-promoting bacteria
Encapsulation of an inoculant was modified from the method by Bashan (1986). About one loopful of bacteria isolates SA-10 were transferred into a liquid nutrient broth medium and were grown for 24 h at 30 °C. Fifty mililitres of bacterial culture was aseptically mixed with 2 % sodium alginate powder and stirred gently for one hour. Then, the mixtures of bacteria, sodium alginate and biochar were drawn using a syringe into a solution of 0.1 M Calcium dichloride. Beads immediately formed in the CaCl2 solution and beads were washed twice with sterilized tap water and then were dried for one to two hours.
2.5 Non-encapsulated plant growth-promoting bacteria preparation
One loopful of bacteria Isolates SA-10 were transferred into a nutrient broth media. It was incubated for 24 h at 30 °C in incubator. After 24 h, the liquid was centrifuged at 7000 rpm for 10 mins and washed the liquid with phosphate buffered saline (PBS) pH 7.4 three times where the cell pelleted became small. PBS pH 7.4 is prepared following ingredients, 8 g NaCl, 200 mg KCl, 1.44 g Na2HPO4, 245 mg KH2PO4 to the 800 mL of distilled water. The solution was adjusted to pH 7.4 before applying to the plant.
2.6 Seed, soil, and plant preparation
In this study, chili (Capsicum annum L.) was used as the model plant organism. Chili seeds were imbibed for 24 h in tap water at room temperature. Then, the seed germinated in a tissue towel. The seed that grows was transferred into the seedling tray for 25 days. After 25 days, the plant was transferred to a polybag with the treatment. The soil was collected from the farm site at UiTM Jasin and mixed well at the ratio of 4:1 of topsoil and sand. This soil was sieved to remove gravel and other material. The media was mixed with the following composition for three kg for topsoil, and one kg sand was put into the pot 15′ × 16′. The experiment was designed as a completely randomized design (CRD) with three treatments and four replications. A total number of 12 pots were used for the experiment. The chili crop was grown as a control, non-encapsulated SA-10, and encapsulated SA-10. For non-encapsulated (liquid bio-fertilizer). Growth parameters such as plant height (shoot length), leaves number per plant, leaves width (leaf area), diameter of leaves, fresh and dry weight were measured.
2.7 16S rRNA gene sequencing molecular identification of soil microbial isolates
Isolate SA-10 was selected and incubated in sterile nutrient broth for 24 h at 37 °C. The DNA genome was extracted using HiYieldTM Genomic DNA Mini Kit (Bacteria)(Real Biotech, Taiwan). 16S rDNA was polymerase chain reaction (PCR) amplified using thermal cycler (peqSTAR, Germany) and universal bacterial primers 27F Forward (5′ AGA GTT TGA TCC TGG CTC AG 3′) and 1492R Reverse (5′ TAC GGT TAC CTT GTT ACG ACT T 3′). The total volume of 50 uL per reaction PCR was prepared: deionised water (31.5 μL); 25 mM magnesium chloride (Lucigen, USA) (3 μL); 10 mM dNTP (Lucigen, USA)(4.0 μL); 10x reaction buffer (5.0 μL), 100 uM forward universal primers (0.5 μL); 100 μM reverse universal primers (0.5 μL); 5 U/mL EconoTaq Polymerase (Lucigen, USA) (0.5 μL) and genomic DNA as template (50 to 200 ng)(5 μL). The PCR thermal cycle setting are: (a) preheating at 94 °C for 2 min; (b) denaturation (35 cycles at 94 °C for 30 s); (c) annealing (52 °C for 30 s); (d) extension (72 °C for 1 min); (d) final elongation (72 °C for 10 min). Purification of PCR product was done using HiYieldTM Gel/PCR DNA Mini Kit (Real Biotech, Taiwan). Sequence was obtained by Apical Scientific Sdn. Bhd.
The forward and reverse sequences, multiple alignment and phylogenetic tree were constructed using MEGA Software Version 11.0.8 (Tamura et al., 2021). The 16S rRNA sequence of SA 10 and related species were used to generate phylogenetic locality of the strain by comparing SA 10 sequence with published sequences in NCBI Genbank using Basic Local Alignment Search Tool (BLAST) (https://blast.ncbi.nlm.nih.gov/) and EzBiocloud (EzBioCloud.net). The phylogenetic tree was constructed with the following setting: (a) analysis by statistical neighbour-joining method, (b) maximum composite likelihood model; (c) 1,000 replicates bootstraps.
2.8 Statistical analysis
The experiment was conducted using a fully randomized design with four replications. Data analysis was carried out using one-way ANOVA with the SPSS statistical program. The Tukey's test was utilized to compare the treatment means at 5 % significance level.
3 Results
3.1 Bacteria isolation from the rice cultivation soil
In this study, about 28 bacteria isolates with different color and morphology were successfully isolated from the rice cultivation soil and plant, providing a foundation for comprehensive screening. Non-rhizosphere, rhizosphere, and endophytes bacteria were observed to fully grown on agar plate only after two days of incubation. However, non-rhizosphere and rhizosphere bacteria showed a high bacteria population with the amount exceeding 300 colony forming unit (CFU) after two days of incubation and only four endophytes CFU per plate after two-day incubation was observed (Supplementary Fig. 1).
3.2 Characterization of PGPR and encapsulation using biochar and sodium alginate
For the zinc solubilization test, isolate SA-8 showed the highest zinc oxide solubility index at 41.66 %. Isolate SA-8, SA-10, and SA-17 showed the highest phosphate solubilization ability at 50 % (Table 1). The highest potassium solubilization activity (90.90 %) was SA-9 (Table 1). All the isolated bacteria showed positive results for nitrogen fixation and siderophores production (Supplementary Fig. 2). In the cellulose degradation, only five isolated bacteria showed positive results, but SA-8 showed negative results. The highest production of indole-acetic acid (IAA) was 4.00 (mg/L) by isolate SA-10 (Table 1). Thus, SA-10 was chosen and successfully encapsulated using biochar and sodium alginate with 2–3 mm size beads as a potential biofertilizer in the plant growth performance test.
Isolates
Solubilization index ZnO (%)
Phosphate solubilisation ability (%)
Potassium solubilisation ability (%)
Nitrogen fixation
Siderophores production
Cellulose degradation
IAA production (mg/L)
SA-8
41.66.a
50.00a
81.81b
+
+
–
2.559b
SA-9
19.04.b
31.57c
90.90a
+
+
+
2.099c
SA-10
13.04.c
50.00a
76.92c
+
+
+
4.007a
SA-14
20.00.d
40.00c
66.66d
+
+
+
1.901c
SA-17
31.25.e
50.00a
90.00a
+
+
+
2.822b
SA-20
21.24.f
47.61b
80.00b
+
+
+
2.23b
3.3 Plant growth performance of chili plant
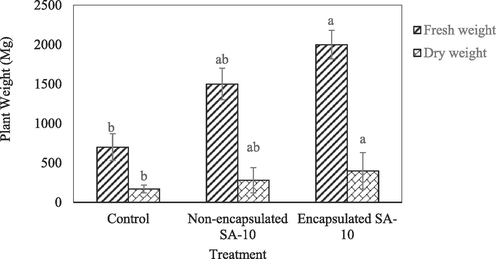
The treatment with encapsulated bio-fertilizer showed highest of plant height, where the mean value was 18.25 cm per plant. The non-encapsulated bio-fertilizer the mean values was 14.5 cm per plant (Fig. 1). Control treatment showed lower plant height at 7.37 cm. A comparison of the treatment means showed that the encapsulated PGPR was significantly different from the non-encapsulated PGPR and control treatments in terms of plant height (Fig. 1). For number of leaves per plant, there was no significant difference among all the treatments at p < 0.05 (Fig. 1). The chili plant treated with encapsulated biofertilizer showed a significantly high measurement of leaf area (3.68 cm2) compared to non-encapsulated biofertilizer treatment (3.10 cm2) and control (2.13 cm2) at p < 0.05 (Fig. 1). The chili plants treated with encapsulated PGPR showed highest leaf diameter (6.55 cm) compared to non-encapsulated biofertilizer treated plant at 5.10 cm and control treatment (3.5 cm) at p < 0.05 (Fig. 1). However, the fresh weight of all the treatments showed there were no significant difference at p < 0.05 for encapsulated biofertilizer plant, non-encapsulated and control (Fig. 2). For dry weight, encapsulated biofertilizer showed significantly highest reading at 0.39 g compared to non-encapsulated biofertilizer treatment (0.28 g) and control (0.18 g) at p < 0.05 (Fig. 2).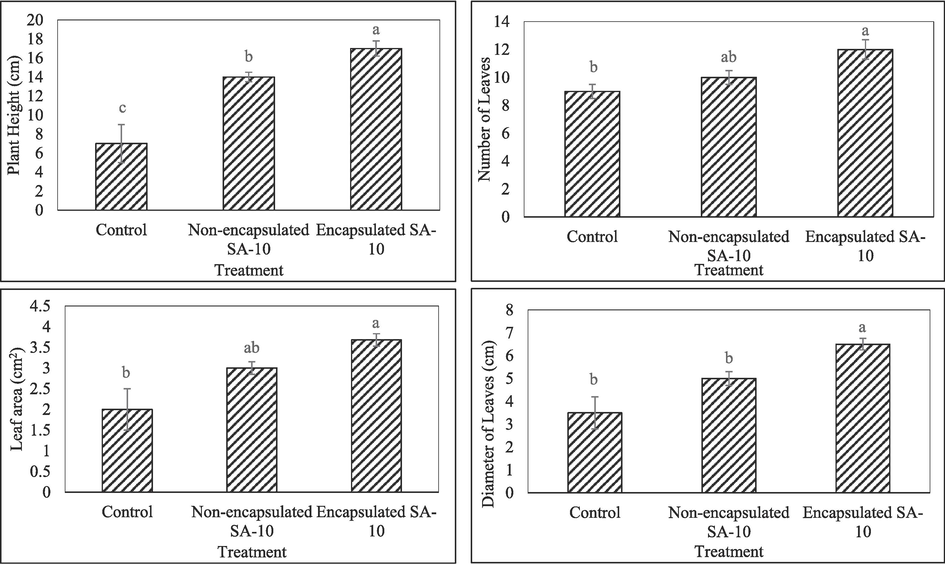
Mean value for different treatments was observed on plant height, leaf number per plant, leaf area, diameter of leaf at p < 0.05.
Mean value for different treatments was observed on plant height, leaf number per plant, leaf area, diameter of leaf at p < 0.05.
3.4 Molecular identification based on 16S rRNA gene sequence
Based on a similarity comparison using BLAST and EzBiocloud, SA 10 was closely related to Enterobacter ludwigii, Leclercia adecarboxylata, Enterobacter bugandensi, Enterobacter chuandaensis, Enterobacter kobei, and Enterobacter sichuanensis (Fig. 2). All the species showed pairwise sequence similarities up to 99.0 % (Supplementary Table 1).
4 Discussion
The study successfully isolated 28 bacterial isolates from rice soil samples. Previous studies have explored bacterial isolates associated with rice plants relevant to these findings. Othman et al. (2022) explored that isolated Malaysian rice root rhizosphere and endophytic bacteria, demonstrating their potential biocontrol activity and successful isolation from plant. Similarly, Sharma and Mallubhotla, (2022) found endophytes showed lower carbohydrate use and stress tolerance compared to rhizosphere and non-rhizosphere population. These isolation studies are consistent with previous findings that explore rice-associated bacteria communities. The current findings that endophytes grew slower than rhizosphere bacteria. This support differences in niche populations which were influenced by carbohydrate utilization. The finding of niche-specific growth rates suggests microbial adaptations deserve deeper study to elucidate colonization mechanisms due to changes in microbial community structure (Nie et al., 2023). Another study also highlights the potential for discovering suitable PGPR among candidates within endophytic habitats. In contrast, a study of sugarcane found a significant number of nitrogen-fixing bacteria in sugarcane roots and leaves, which were influenced by soil salinity levels (Pirhadi, 2018). Approximately 55 saline soil endophytic strains were found in saline soils which are higher than the rhizosphere. While the study focused only on nitrogen fixer, Bacillus sp., the current studdy focus on culturable bacteria population in rice samples. Thus, differences in population might be due to differences in the environment where the bacteria might obtain their needs and also their culturability.
In this study, encapsulated PGPR SA-10, was identified as a potential biofertilizer candidate due to its consistently high activity throughout PGPR characterization test. The hypothesis that SA-10 isolate can promote plant growth via multiple mechanisms and that encapsulation safeguards its viability are supported by the results. The study's findings on SA-10 bacteria's multifunctional plant growth-promoting features match previous research on PGPR and crop nutrition and yields. Similarly, Bhatt and Maheshwari (2020) identified Bacillus megaterium from cow manure and showed that they promote plant development, including Capsicum annuum L. Indole-3-acetic acid produced by SA-10 bacteria may promote plant development, via specific enzymatic pathways. Plant growth-promoting rhizobacteria use auxin production as one of their main direct ways to benefit plants (Wagi and Ahmed, 2019). This shows that SA-10 bacteria's IAA synthesis directly promotes plant development. Similarly, Srithaworn et al. (2023) examined the effects of Pseudomonas protegens RY2 and Bacillus megaterium on green soybean growth that showed similar increase due to plant growth promoting traits by both bacteria. While, Zahra et al. (2023) found rhizosphere Bacillus sp. predominates in the wheat (Triticum aestivum L.) compared to endospheric like current study, the findings still demonstrate the diverse roles of PGPR in promoting plant growth under stress condition. Through these findings, PGPR show high ability to promote plant growth through nutrient acquisition mechanism such as synthesizing IAA, improving stress tolerance, and acquiring nutrients.
Encapsulated SA-10 with biochar showed encouraging outcomes in enhancing plant growth parameters such as leaf diameter, leaf area, and dry weight which supports the hypothesis that encapsulated SA-10 can be used as a biofertilizer for chili cultivation. Queroz-Sarmiento et al. (2019) encapsulated PGPR which led to a significant 35 % increase in chili seedling growth compared to the control group. Coatings like sodium alginate, can promote bacterial survival and better plant growth in arid soil (Riseh et al., 2021). Zego et al. (2019), found that encapsulated Azospirillium brasilience with alginate-humic acid-maintained shelf-life viability, supporting seedlings growth. In contrast, Beula et al. (2019) found humic acid helps improve encapsulation. stressed on soil and climate can influence plant phenotype. In the current study, biochar helped successfully increase chili cultivation. Similarly, a finding showed that biochar protects encapsulated bacteria by providing habitat and protecting them from stress, maintaining viability which can help for plant growth (Lee et al., 2021). Encapsulation methods like sodium alginate and biochar mixture are highly effective in enhancing the effects of PGPR on plant growth. However, it should be noted that further validation is required in real-life, changeable field situations. More validation and research are required under varied agro-climate (Xue et al., 2023; Xiao et al., 2019).
The findings from current study showed SA-10 was molecularly identified as Enterobacter chuandesis. Similarly, Enterobacter sp. has also been studied as a biofertilizer in black pepper growth and root disease suppression (Nguyen et al., 2020). Similarly, Lucero et al. (2020) examined the effects of fermentation, endophytic bacteria, and bioactive compounds on plant growth and nutrient concentrations on soybeans, maize and peanut using phosphate-solubilizing Enterobacter sp. Endophytic Enterobacter ludwigi EB4B has also been researched for biological control and illness suppression in tomato growth (Bendaha and Belaouni, 2020). The discovery of SA-10 as Enterobacter sp. links this study to a broader range of research that highlights the ability of this genus to enhance plant growth. Research has shown that Enterobacter sp. has the potential to enhance crop yields by promoting nutrient cycling, suppressing diseases, and producing phytohormones in various crop systems (Fig. 3).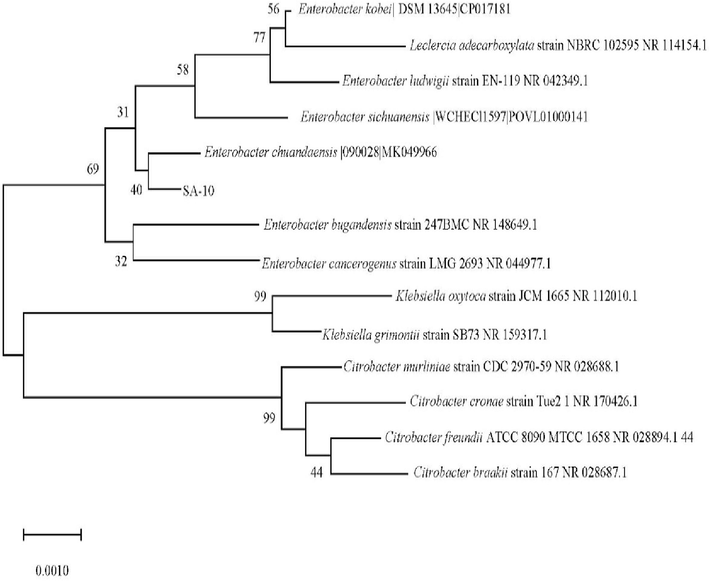
Neighbor-joining phylogenetic tree of SA 10 based on 16S rRNA sequences analysis.
5 Conclusion
The results of this study showed 28 bacteria isolates were successfully isolated from rice soi. Bacterial strain SA-10 has several characteristics that promote plant growth in a laboratory environment. SA-10 was able to dissolve zinc at a rate of 41.6 %, phosphate at a rate of 50 %, and potassium at a rate of 76.9 %. Additionally, SA-10 fixed nitrogen positively, produced siderophore, degraded cellulose, and created growth hormone and IAA at a rate of 4.007 mg/L. The 16 s rRNA molecular analysis identified the bacterial strain as Enterobacter chuandaensis, which has significant potential for enhancing plant growth. The use of encapsulated SA-10 resulted in better plant growth in various ways. Plants treated with encapsulated SA-10 had significantly higher leaf area (3.68 cm2), diameter (6.55 cm), and dry weight (0.39 g). Encapsulated PGPR SA-10 biofertilizer can enhance soil health in chili farming. It reduces the need for overusing chemical fertilizers, helps in policy making for sustainable agriculture approach and has the potential to be a global climate-resilient strategy. However, more research is needed to improve formulations, assess field performance, and commercialize innovations.
Disclosure of funding
This work has been supported by Ministry of Higher Education (MOHE), Malaysia for sponsoring this research under Fundamental Research Grant Scheme (FRGS) (Ref: FRGS/1/2023/STG02/UITM/02/4).
Acknowledgments
Authors acknowledge the Ministry of Higher Education (MOHE), Malaysia for funding under Fundamental Research Grant Scheme (FRGS) (Ref: FRGS/1/2023/STG02/UITM/02/4) and Faculty of Plantation and Agrotechnology, UiTM Malacca, Jasin, Melaka, Malaysia.
References
- Recent developments on nanotechnology in agriculture: plant mineral nutrition, health, and interactions with soil microflora. J. Agric. Food Chem.. 2018;66(33):8647-8661.
- [CrossRef] [Google Scholar]
- Sustainable agriculture in Africa: plant growth-promoting rhizobacteria (PGPR) to the rescue. Sci Afr. 2020;9:e00492.
- [Google Scholar]
- Liberation of phosphoric acid from apatite by silicate bacteria. Mikrobiol. Z.. 1967;29(2):111-114.
- [Google Scholar]
- Alginate beads as synthetic inoculant carriers for the slow release of bacteria that affect plant growth. Appl. Environ. Microbiol.. 1986;51(5):1089-1098.
- [Google Scholar]
- Effect of the endophytic plant growth promoting Enterobacter ludwigii eb4b on tomato growth. Hell. Plant Prot. J.. 2020;13(2):54-65.
- [CrossRef] [Google Scholar]
- Encapsulation of Pseudomonas fluorescens for a slow release biofertilizer, presented at conference: international conference on medical and industrial biotechnology (ICAMIB), Chennai, India, 2019. Chennai: CSIR Publishing; 2019.
- Zinc solubilizing bacteria Bacillus megaterium with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. Biotech. 2020;10(2):3-15.
- [CrossRef] [Google Scholar]
- Inoculation with growth-promoting bacteria Azospirillum brasilense and its effects on productivity and nutritional accumulation of wheat cultivars. Front. Sustain. Food Syst.. 2020;4:607262
- [CrossRef] [Google Scholar]
- Production and nutritive value of Tifton 85 bermudagrass pastures overseeded with annual ryegrass and inoculated with diazotrophic bacteria. J. Agric. Sci.. 2022;160(1–2):117-126.
- [CrossRef] [Google Scholar]
- Associative sysbiosis and dinitrogen fixing site, presented at proceedings of the international symposium on nitrogen fixation, Pullman, United State of America, 1976. Pullman, WA: Washington State University Press; 1976.
- Carotenoids of Capsicum fruits: pigment profile and health-promoting functional attributes. Antioxidants. 2019;8(10):469.
- [CrossRef] [Google Scholar]
- Precise analysis of potassium isotopic composition in plant materials by multi-collector inductively coupled plasma mass spectrometry. Spectrochim. Acta B At. Spectrosc.. 2023;209:106781
- [CrossRef] [Google Scholar]
- Parallel genetic adaptation of Bacillus subtilis to different plant species. Microb. Genomics. 2023;9(7):1-17.
- [CrossRef] [Google Scholar]
- Influence of soil microplastic contamination and cadmium toxicity on the growth, physiology, and root growth traits of Triticum aestivum L. South Afr J Bot.. 2023;160:369-375.
- [CrossRef] [Google Scholar]
- Advancing environmental sustainability through microbial reprogramming in growth improvement, stress alleviation, and phytoremediation. Plant Stress.. 2023;10:100283
- [CrossRef] [Google Scholar]
- Microplastics meet invasive plants: unravelling the ecological hazards to agroecosystems. Sci. Total Environ.. 2024;906:167756
- [CrossRef] [Google Scholar]
- Diazotrophic bacteria is an alternative strategy for increasing grain biofortification, yield and zinc use efficiency of maize. Plants. 2021;11(9):1125.
- [CrossRef] [Google Scholar]
- Nanozinc and plant growth-promoting bacteria improve biochemical and metabolic attributes of maize in tropical Cerrado. Front. Plant Sci.. 2023;13:1046642.
- [CrossRef] [Google Scholar]
- Unravelling microbial volatile elicitors using a transparent methodology for induction of systemic resistance and regulation of antioxidant genes at expression levels in chili against bacterial wilt disease. Antioxidants. 2022;11(2):404.
- [CrossRef] [Google Scholar]
- From lab to farm: elucidating the beneficial roles of photosynthetic bacteria in sustainable agriculture. Microorganism. 2021;9(12):2453.
- [CrossRef] [Google Scholar]
- Motility and biofilm production involved in the interaction of phosphate solubilizing endophytic strains with peanut, maize and soybean plants. Rhizosphere. 2020;15:100228
- [CrossRef] [Google Scholar]
- Review and perspectives of the use of alginate as a polymer matrix for microorganisms applied in agro-industry. Molecules. 2022;27(13):4248.
- [CrossRef] [Google Scholar]
- Combined application of rhizosphere bacteria with endophytic bacteria suppresses root diseases and increases productivity of black pepper (Piper nigrum L.) Agriculture. 2020;11(1):15.
- [CrossRef] [Google Scholar]
- Coupling effects of nitrate reduction and sulfur oxidation in a subtropical marine mangrove ecosystem with Spartina alterniflora invasion. Sci. Total Environ.. 2023;862:160930
- [CrossRef] [Google Scholar]
- Isolation, characterization, and identification of zinc-solubilizing bacteria (ZSB) from wetland rice fields in peninsular Malaysia. Agriculture. 2022;12(11):1823.
- [CrossRef] [Google Scholar]
- Molecular characterization of cellulose degrading Bacillus pumilus from the soil of tea garden, Darjeeling Hills. J. Environ. Biol.. 2014;35(3):555-561.
- [Google Scholar]
- Pirhadi, M., 2018. Impact of soil salinity on diversity and community of sugarcane endophytic plant growth promoting bacteria (Saccharum officinarum L. var. cp48). Appl Ecol Environ Res. 16(1), 725-739. doi: 10.15666/aeer/1601_725739.
- Biofertilizers of rhizobacteria in the growth of Poblano chili seedlings. Rev. Mex. Cienc. Agric.. 2019;10(8):1733-1745.
- [Google Scholar]
- Application of biochar and poschar from several types of animal manure on the growth and yield of red chili plants (Capsicum annuum l.) Agriwar Journal. 2022;2(1):1-6.
- [CrossRef] [Google Scholar]
- Reducing drought stress in plants by encapsulating plant growth-promoting bacteria with polysaccharides. Int. J. Mol. Sci.. 2021;22(23):12979.
- [CrossRef] [Google Scholar]
- Assessing in-vitro solubilisation potential of different zinc solubilising bacteria isolates. Braz. J. Microb.. 2003;34:121-125.
- [Google Scholar]
- Universal chemical assay for the detection and determination of siderophores. Anal. Biochem.. 1987;160(1):47-56.
- [CrossRef] [Google Scholar]
- Diversity, antimicrobial activity, and antibiotic susceptibility pattern of endophytic bacteria sourced from Cordia dichotoma L. Fron. Microb.. 2022;13:1-17.
- [CrossRef] [Google Scholar]
- The effect of organic and inorganic fertilizer applications on N, P-uptake, K-uptake, and yield of sweet corn (Zea mays saxxharata Sturt), presented at IOP conference series: earth and environmental science, Bandung West Java, Indonesia, 2019. Bandung, IND: IOP publishing; 2019.
- Identification of endophytic and rhizosphere bacteria in Maize, presented at IOP conference series: earth and environmental science, Bandung West Java, Indonesia, 2019. Bandung, IND: IOP publishing; 2019.
- Zinc solubilizing bacteria and their potential as bioinoculant for growth promotion of green soybean (Glycine max L Merr.) Peerj. 2023;11:e15128.
- [Google Scholar]
- Seed treatment using rhizobacteria as plant growth promotion of two chili variety (Capsicum annuum L.) IOP Conf. Se.: Earth Enviro. Sci.. 2022;951:012060
- [CrossRef] [Google Scholar]
- Bacillus spp.: potent microfactories of bacterial indole acetic acid (IAA) PeerJ. 2019;7:e7258.
- [Google Scholar]
- Variation in rhizosphere microbiota correlates with edaphic factor in an abandoned antimony tailing dump. Environ. Pollut.. 2019;253:141-151.
- [CrossRef] [Google Scholar]
- Biochar combined with nitrogen alters rhizosphere soil nutrients and microbial communities and promotes growth of moso bamboo seedlings. Forests. 2022;13(7):1043.
- [CrossRef] [Google Scholar]
- Spring photosynthetic phenology of Chinese vegetation in response to climate change and its impact on net primary productivity. Agric. Met.. 2023;342:109734
- [CrossRef] [Google Scholar]
- Dominance of Bacillus species in the wheat (Triticum aestivum l.) rhizosphere and their plant growth promoting potential under salt stress conditions. PeerJ. 2023;11:e14621.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103197.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







