Translate this page into:
Understanding the cross-talk of major abiotic-stress-responsive genes in rice: A computational biology approach
⁎Corresponding author at: Department of Genetics and Plant Breeding, Institute of Agriculture, Visva-Bharati University, Sriniketan, West Bengal 731236, India. sandip.debnath@visva-bharati.ac.in (Sandip Debnath)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
This study aims to identify key abiotic-stress-responsive genes in Oryza sativa and investigate their expression profiles, protein–protein interactions, and co-expression patterns to better understand the molecular mechanisms underlying stress response in rice. The ultimate goal is to employ these findings in the development of gene-based molecular markers to breed rice cultivars with enhanced resistance to challenging environmental conditions. A total of 14 abiotic-stress-responsive genes in O. sativa were identified through literature mining. These genes were analyzed for their chromosomal distribution, transcript length, CDS length, and translated amino acid length. Pairwise similarity matrix, phylogenetic analysis, and protein–protein interaction networks were employed to understand the evolutionary relationships and functional interactions among these genes. Expression profiles of six key genes (AOX1a, AOX1b, ALDH2a, ALDH2b, OsNAC6, OsDHN1) were investigated using the electronic fluorescent pictogram (eFP) program. KEGG pathway analysis and co-expression studies were also conducted to further understand the roles of these genes in abiotic stress response pathways. The 14 abiotic-stress-responsive genes were distributed across different chromosomes of O. sativa, suggesting the presence of interconnected cascades regulating abiotic-stress-response. Phylogenetic analysis revealed four clusters of genes, indicating their potential shared ancestry. Protein-protein interaction analysis identified three prominent clusters of interactions, with the strongest interactions occurring among Aldh2a, Aldh2b, OS07T0188800-01, and OsJ_04113 in one cluster, and between AOX1a and AOX1b in another cluster. Expression profiles of the six key genes varied across different stages of the rice life cycle. KEGG pathway analysis showed that ALDH2a and ALDH2b participated in almost all pathways except propanoate metabolism.
The study demonstrated that the six key genes play significant regulatory roles in abiotic stress responses in O. sativa. The expression profiles, phylogenetic analysis, protein–protein interactions, and gene co-expression studies revealed interconnected cascades and cross-talk in response mechanisms of these genes. These findings can be employed to develop gene-based molecular markers for breeding rice cultivars with enhanced resistance to abiotic stresses, and contribute to the successful application of computational biology in plant breeding towards sustainable development goal.
Keywords
Oryza sativa
Abiotic stress
Gene expression
Phylogenetic analysis
Protein-protein interaction
Transcription Factor
- MSA
-
Multiple Sequence Alignment
- DNA
-
Deoxyribonucleic Acid
- RNA
-
Ribonucleic Acid
- JTT
-
Jones-Taylor-Thornton
- ML
-
Maximum Likelihood
- eFP
-
Electronic Fluorescence Pictogram
- KEGG
-
Kyoto Encyclopedia of Genes and Genomes
- BIC
-
Bayesian Information Criterion
- CDS
-
Coding Sequenc
- UTR
-
Untranslated Region
- GCOS
-
GeneChip Operating Software
- SAM
-
Shoot Apical Meristem
- AOX
-
Alternative Oxidase
- ALDH
-
Aldehyde Dehydrogenase
- ORF
-
Open Reading Frames
- OsNAC
-
Oryza sativa NAC-domain transcription factor
- OsDHN
-
Oryza sativa Dehydrin
- OsDREB
-
Oryza sativa DRE-binding protein
- OsERF
-
Oryza sativa Ethylene-Responsive Factor
- OsSTLK
-
Oryza sativa Serine/Threonine Protein Kinase
- OsPP2C
-
Oryza sativa Protein Phosphatase 2C
- OsSADR
-
Oryza sativa Salt and Drought Resistance protein
- OsNIN
-
Oryza sativa NIN-like protein
- OsNCA
-
Oryza sativa Non-Coding Acidic protein
- SDG
-
Sustainable Development Goal
Abbreviations
1 Introduction
Plants inhabit dynamic environments, subject to continuous fluctuations that can be detrimental to their growth and development (Zhu et al., 2016). Environmental stressors can be broadly classified into biotic and abiotic stress (Debnath et al., 2013). Biotic stress refers to adverse effects resulting from herbivore attacks and pathogen infections, while abiotic stress pertains to unfavourable environmental conditions such as cold, heat, drought, and excessive salt and calcium concentrations. Plants possess inherent physiological processes to cope with these stresses, relying on diverse genetic expressions regulated by multiple transcription factors (Atkinson et al., 2012). The ability to withstand stress varies among plant species, with different genes being responsible for stress tolerance against specific stressors (Seth et al., 2020). Rabadanova et al., 2018 emphasize the importance of enhancing plant stress tolerance for increased productivity and environmental sustainability, as crops lacking stress tolerance require excessive inputs like fertilizers, pesticides, and irrigation. Understanding plant stress response necessitates examining stress signals, which facilitate communication between various molecular responses related to stress resistance. This study will specifically focus on the abiotic stress response of rice (Oryza sativa).
Plants employ various strategies (Fig. 1) to tolerate or avoid abiotic stresses, including reduced photosynthesis, stomatal closure, increased reactive oxygen species (ROS) scavenging, stunted leaf growth, and elongated roots (Cohen and Leach, 2019). Whereas, biotic stress factors, such as pathogens, can also induce stomatal closure and decrease photosynthesis. Defensive mechanisms against biotic stress include secretion of phytotoxins (e.g., ROS, phytoalexins, and secondary metabolites) and localized cell death. Phytohormones, such as salicylic acid (SA), jasmonic acid (JA), and ethylene, are crucial for plant immunity against pathogenic factors (Lata et al., 2011). Transcription factor families involved in abiotic stress response include abscisic acid (ABA)-dependent and ABA-independent transcription factors. Kim et al., 2020 identified stress-responsive genes in O. sativa using expressed sequence tags (ESTs) generated from drought-stressed seedlings, revealing distinct gene families responsible for abiotic stress response.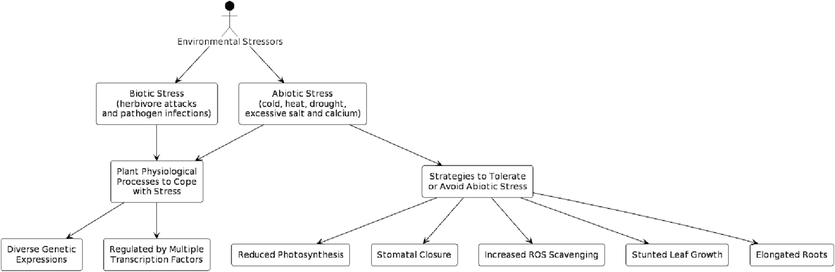
Strategies employed by plants against environmental stressors (Source: Author’s creation with PlantText UML Editor).
According to Zhao et al. (2010), dehydration-responsive element-binding (DREB) genes confer resistance to drought, low temperature, and high salinity stress in rice. Most DREB genes are thought to regulate downstream stress-responsive genes by binding directly to drought-responsive elements (DRE) and cis-elements (GCC box) (Fig. 2). Studies by Zhang et al. (2013) and Ranawake et al., 2012 investigated the expression patterns of DREB genes and identified numerous stress-responsive genes in rice, respectively. Rabbani et al., 2003 used a cDNA microarray technique to profile rice gene expression under various environmental stresses, such as drought, cold, high salinity, and abscisic acid. Sevanthi et al. (2021) identified six highly heat-sensitive genes in rice, while Riccio-Rengifo et al. (2021) categorized stress-induced proteins into functional and regulatory proteins.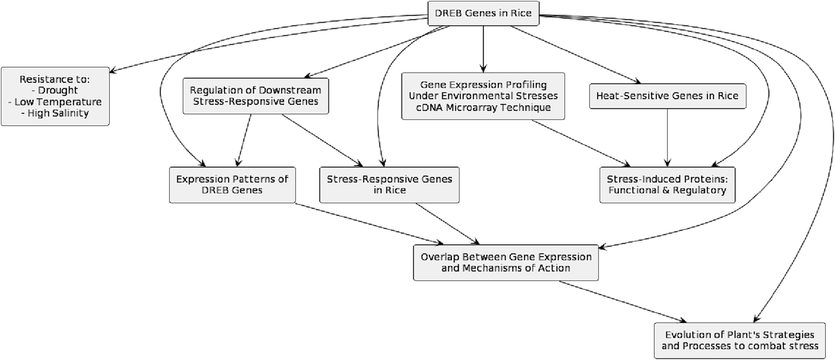
Functions of DREB gene in rice combating different abiotic stresses (Source: Author’s creation with PlantText UML Editor).
Mondini et al. (2015) suggested significant overlap between gene expression and mechanisms of action in response to abiotic and external stress stimuli. Consequently, abiotic stress response genes play a critical role in stress regulation and assisting rice plants in coping with adverse conditions induced by abiotic stress. Throughout their evolution, plants have developed various strategies and processes to deal with stressors such as dryness, heat, cold, and excessive salinity (Debnath et al., 2022).
Plant stress responses are crucial not only for their economic value but also for maintaining environmental homeostasis. If rice plants lose their stress resistance capacity, they will require more water and fertilizers, which is neither cost-effective nor environmentally sustainable, given the immense agricultural significance of rice (Seth et al., 2020). Shi and Chan (2014) proposed enhancing plant stress responses through modulation of gene expression and post-translational modification. Gaining a comprehensive understanding of the genes responsible for abiotic stress response in rice plants and their expression patterns in response to abiotic stress can contribute valuable insights for modulating genetic expression. Additionally, studying the roles of different abiotic stress-responsive genes can help in understanding their importance in developing stress-tolerant rice varieties.
Despite the wealth of information on regulating various stress-responsive genes in rice, there remains a significant gap in the literature regarding a comprehensive study that investigates the expressions of abiotic stress-responsive genes and their interplay within different stress response pathways in rice plants. Numerous primary articles found in electronic databases offer insight into the expression patterns of the aforementioned genes through systematic reviews. However, considering the lack of a comprehensive study on this topic, it is essential to formulate a hypothesis for a comprehensive analysis of the expression patterns of abiotic stress-responsive genes in O. sativa. This will enable researchers to identify key factors and mechanisms that can be harnessed to improve stress tolerance in rice plants, ultimately enhancing productivity and minimizing negative environmental impacts to achieve sustainable development goal.
2 Materials and methods
2.1 Identification of genes
Data mining is essential for the effective screening and analysis of the retrieved information. In this study, a systematic approach to literature mining was employed to identify abiotic stress-sensitive genes in O. sativa by searching public databases. Various electronic databases, such as PubMed, CINAHL, EBSCO (HOST), Web of Science and Google Scholar, were used as sources for collecting relevant literature. However, this research focused on publications available in CINAHL, PubMed, and Google Scholar databases due to their public accessibility. To perform a comprehensive search, several keywords and Boolean operators were used, including “rice plant,” “abiotic stress,” “stress-responsive genes,” and “abiotic stress-responsive genes.” A two-step screening process was applied to ensure the relevance of the literature retrieved in relation to the research question (Supplementary file 1). Initially, title and abstract screening were used to collect publications from online databases. Subsequently, a full-text assessment was conducted to evaluate the relevance of the selected articles.When mining the literature for genes of interest, it is crucial to establish and apply appropriate inclusion and exclusion criteria. Using an evidence-based screening strategy alone may not be sufficient to maintain the relevance of the search results if no control is exercised during the literature mining process. Inclusion of extraneous publications in the search results may occur without proper guidance. To ensure the validity of the gathered literature for this study, the authors applied specific inclusion and exclusion criteria (Supplementary file 2). By adhering to these criteria, the research team was able to focus on pertinent publications, thereby enhancing the reliability and accuracy of the data analysis.
2.2 Exploration of identified genes
To systematically explore the identified genes, the Oryzabase (https://shigen.nig.ac.jp/rice/oryzabase/) was utilized. Oryzabase is a comprehensive rice research database established in 2000 by a group of rice researchers in Japan. The initial aim of the database was to consolidate information ranging from traditional rice genetics to modern genomics. The database is supported by the National BioResource Project (NBRP).The Michigan State University (MSU) IDs of these genes were extracted from Oryzabase, which facilitated locating the genes in The Rice Annotation Project Database (RAP-DB) (https://rapdb.dna.affrc.go.jp/). This allowed for the extraction of specific chromosomal locations of the genes. The genomic sequence data of the genes in FASTA format (.fasta /.fa) were obtained using the G-Browse tool of RAP-DB. To identify the ORF of the genes and reveal the protein-coding sequences (CDS), the NCBI ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) was employed. By analyzing the data acquired from RAP-DB and NCBI ORF Finder, the lengths of the 5′ untranslated region (UTR), CDS, and 3′ UTR for the genes were determined. Finally, a Basic Local Alignment Search Tool (BLAST) analysis was conducted for all the gene sequences using the NCBI BLASTX (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to obtain the translated protein sequences of the genes. This methodology allowed for a detailed characterization of the identified abiotic stress-sensitive genes in O. sativa.
2.3 Multiple sequence alignment and phylogenetic analyses
To investigate the evolutionary relationships and identify similar patterns across genes, Multiple Sequence Alignment (MSA) is employed. The translated protein sequences of the genes were subjected to Multiple Sequence Alignment using the Clustal Omega 1.2.2 program (Sievers et al., 2014). Pairwise sequence comparisons were carried out by calculating the percentage similarity matrix using the BLOSUM62 matrix in the Geneious Prime 2022.2.2 software. Phylogenetic analysis was conducted by constructing a Maximum Likelihood (ML) phylogenetic tree based on the Jones-Taylor-Thornton (JTT) substitution model (Jones et al., 1992). To assess the reliability and accuracy of the generated phylogenetic tree branches, one thousand bootstrap replications were performed during tree construction using the MEGA11 software (Tamura et al., 2021).This methodological approach provides a comprehensive understanding of the evolutionary relationships among the identified abiotic stress-sensitive genes in O. sativa, facilitating further analysis of their biological functions and potential roles in stress tolerance.
2.4 Protein-Protein interaction and gene co-occurrence study
The investigation of interactions among proteins and small molecules is vital for a deeper comprehension of molecular and cellular functions, including metabolism, signalling, and drug treatments, as they may apply. Gaining insight into the interactions between proteins and other biomolecules is imperative for a thorough understanding of molecular and cellular processes such as metabolic pathways and signalling cascades.
STRING (https://string-db.org/) is an online resource that provides information on functional associations between various biomolecules for different species by integrating known and predicted protein–protein interactions (Szklarczyk et al., 2021). The interactions among the translated proteins from the identified genes were examined using STRING. To enhance the understanding of how these genes interact across different taxa beyond Oryza, gene co-occurrence and co-expression were also investigated within the STRING platform which provided greater insights into their interactions across various taxa.
2.5 In silico expression study
The electronic fluorescence pictogram (eFP) program is a widely recognized tool for visualizing transcriptome data and has been employed in various model organisms. To understand the expression levels of the six identified genes under stress conditions, their expression profiles were analyzed using the Rice eFP Browser (https://bar.utoronto.ca/transcriptomics/efp_rice/cgi-bin/efpWeb.cgi?dataSource = rice_leaf_gradient). Developed at the University of Toronto, this tool facilitates the visual analysis of gene expression by providing illustrative graphics for different tissue types, with distinct colors representing the expression levels of a specific gene in each tissue (Jung et al., 2011).Bioinformatic analysis revealed that six out of the fourteen genes, namely AOX1a, AOX1b, ALDH2a, ALDH2b, OsNAC6 and OsDHN1, exhibited the potential to be expressed under all abiotic stresses investigated. Consequently, these six genes were further screened using the Rice eFP Browser to evaluate their expression potential through in silico analysisfor exploring a more exhaustive understanding of the stress-responsive expression patterns of the identified genes in O. sativa.
These methodological approaches followed in this study (Fig. 3) collectively allows for a comprehensive examination of the characterization, evolutionary relationship, functional associations, potential roles and expression patterns of the identified abiotic stress-sensitive genes in O. sativa.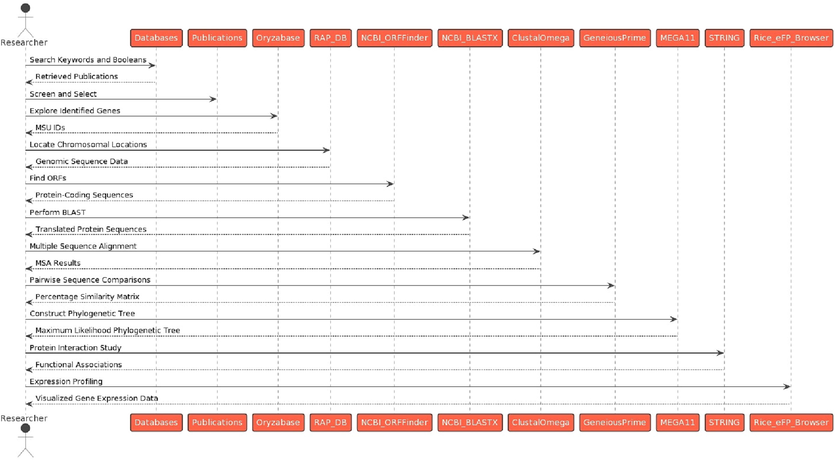
Methodological approaches followed in this study to identify abiotic stress-responsive genes in O. sativa. (Source: Author’s creation with PlantText UML Editor).
3 Results
3.1 Identification of genes
A total of 14 abiotic stress-responsive genes of rice have been identified through a comprehensive literature mining process. These genes were classified into three significant abiotic stresses that rice plants experience throughout their lifetime, i.e., drought, salt, and low temperature. Only three identified genes are expressed under individual stresses: OsDREB1G under cold stress response (Moon et al., 2019), OsERF28 under drought stress response (Mawlong et al., 2014) and OsSTLK under salt stress response (Lin et al., 2020). The other 11 genes showed significant overlapping in their mechanisms of action, as reported by Mondini et al. (2015). OsPP2C1 and OsSADR1 are expressed under salt and drought stress (Jiang et al., 2011; Park et al., 2018), OsNIN6 and OsNCA1A are expressed in response to salt and low temperatures (Liu et al., 2019; Yao et al., 2009), and OsDREB1B is expressed under both drought and cold stress (Figueiredo et al., 2012). Remarkably, six genes (AOX1a, AOX1b, ALDH2a, ALDH2b, OsNAC6 and OsDHN1) are found to be expressed under all three categories of abiotic stress, i.e., drought, salt, and low temperature (Feng et al., 2009; Tsuji et al., 2003; Nakashima et al., 2007; Kumar et al., 2014) (Supplementary file 3).
3.2 Exploration of identified genes
The specific chromosomal locations of the identified genes were determined, revealing that the genes are distributed across multiple chromosomes of O. sativa. The top three genes are located on chromosome 2, followed by two genes on chromosomes 1, 4, 9, and 11 each, and only one gene is located on chromosomes 5, 6, and 8 each. The transcript length, 5′ UTR length, CDS length, 3′ UTR length, and translated protein length for each gene are listed in Table 1.
Sl. No.
Gene Name
MSU ID
Chromosome Number
Exhaustive Position
Transcript length
5′ UTR length
CDS length
3′ UTR length
Protein length
1
OsPP2C1
LOC_Os09g15670.1
9
chr09:9567471.0.9568877
1407 bp
80 bp
1077 bp
250 bp
358 aa
2
OsDREB1G
LOC_Os02g45450.1
2
chr02:27652935.0.27654206
1272 bp
45 bp
675 bp
552 bp
224 aa
3
OsERF28
LOC_Os08g43210.1
8
chr08:27320678.0.27325475
981 bp
981 bp
326 aa
4
OsSTLK
LOC_Os05g24010.1
5
chr05:13834116.0.13839997
3312 bp
194 bp
2832 bp
286 bp
943 aa
5
OsSADR1
LOC_Os11g07450.1
11
chr11:3749274.0.3755192
1680 bp
60 bp
1437 bp
183 bp
478 aa
6
OsDREB1B
LOC_Os09g35010.1
9
chr09:20395279.0.20396175
897 bp
15 bp
657 bp
225 bp
218 aa
7
NCA1A
LOC_Os01g01420.1
1
chr01:209771.0.214173
1642 bp
165 bp
1092 bp
385 bp
363 aa
8
OsNIN6
LOC_Os11g07440.1
11
chr11:3739630.0.3743522
2320 bp
325 bp
1647 bp
348 bp
548 aa
9
AOX1a
LOC_Os04g51150.1
4
chr04:30287197.0.30289860
2712 bp
413 bp
999 bp
107 bp
332 aa
10
AOX1b
LOC_Os04g51160.1
4
chr04:30291463.0.30293040
1580 bp
207 bp
1008 bp
146 bp
335 aa
11
ALDH2a
LOC_Os02g49720.1
2
chr02:30392547.0.30396729
4110 bp
964 bp
1752 bp
107 bp
553 aa
12
ALDH2b
LOC_Os06g15990.1
6
chr06:9091026.0.9096474
5664 bp
226 bp
1650 bp
479 bp
549 aa
13
OsNAC6
LOC_Os01g66120.1
1
chr01:38398996.0.38401481
3017 bp
984 bp
744 bp
161 bp
303 aa
14
OsDHN1
LOC_Os02g44870.1
2
chr02:27165514.0.27166898
1465 bp
379 bp
873 bp
119 bp
300 aa
3.3 Multiple sequence alignment and phylogenetic analyses
The multiple sequence alignment (MSA) of the translated proteins indicates an average protein length of 416 aa and a pairwise identity of 9.2% (Supplementary file 4). The MSA revealed that the proteins have an average molecular weight of 45.353 kDa and an average isoelectric point of 6.88. The percentage similarity matrix (Fig. 4) was constructed using the BLOSUM62 matrix to assess the amino acid substitution rates in clusters of the relevant protein translated by these genes. The matrix primarily highlights the evolutionary relationships among the genes. In terms of evolutionary divergence, genes sharing higher percentages of similarities are more closely related, as confirmed by the subsequent phylogenetic analysis.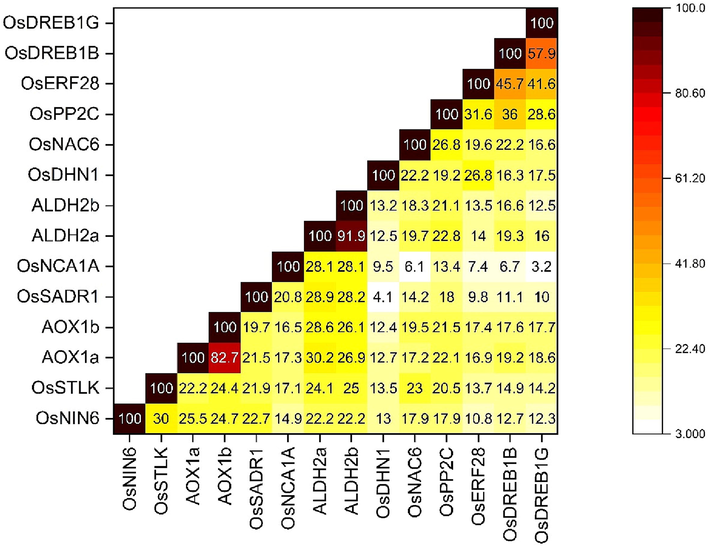
Percentage similarity matrix of 14 identified abiotic-stress responsive genes in O. sativa. The colour scheme indicates the heatmap of similarities among the genes (Source: Author’s creation with OriginPro 2022 v.9.9.0.225).
The phylogenetic analysis, based on the Maximum Likelihood method, resulted in four clusters of genes (Fig. 5). The resulting dataset had 948 positions from the 14 amino acid sequence analyses. In agreement with the similarity matrix constructed, ALDH2a, ALDH2b, AOX1a and AOX1b formed cluster 1 in the phylogenetic tree, as they showed the highest values of percentage similarities among them, ranging from 82.7 to 91.9%. Cluster 2 is formed by OsERF28, OsDREB1B and OsDREB1G, corresponding to percentage similarities ranging from 41.6 to 57.9%. OsNAC6 and OsDHN1 formed cluster 3, showing a 22.2% similarity. Lastly, cluster 4 is formed by OsSTLK, OsNCA1A and OsNIN6, expressing percentage similarities ranging from 14.9 to 30%. OsSADR1 and OsPP2Care not clustered in the phylogenetic tree, although they show a percentage similarity of 18% per the matrix constructed.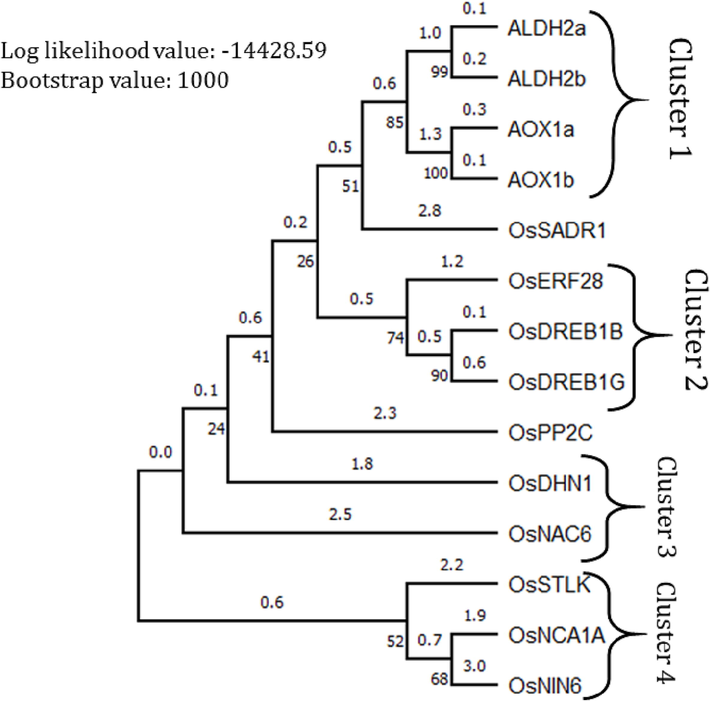
Phylogenetic tree of 14 identified abiotic-stress responsive genes in Oryza sativa made in MEGA11 (20). The Maximum Likelihood (ML) phylogenetic tree is computed with the highest log likelihood value of −14428.59. Above the branches is the branch length and below is the proportion of replicate trees in which the related taxa grouped together in the bootstrap test with 1000 repetitions.
3.4 Protein-protein interaction and gene co-occurrence study
The phylogenetic analysis suggests that these genes share homology, potentially evolving from a single ancestral gene. The protein–protein interaction (PPI) network, based on high confidence values as defined by the STRING software, revealed 19 nodes in the network of proteins located in functional subsystems, with an average node degree of 1.68, an average local clustering coefficient of 0.458, and a PPI enrichment p-value of 0.000639 (Fig. 6). In addition to the 14 proteins in the search query (Supplementary file 5), five more proteins (OsJ_04113, OsJ_24269, OS03T0297600-01, OS07T0188800-01, and OsJ_06966) were predicted as functional partners by the network. The STRING software recorded 17 interactions among the query proteins (Supplementary file 6). The k-means clustering identified 3 clusters of interactions at the protein level with a high level of interaction confidence. Cluster 1 groups 6 genes (AOX1a, AOX1b, DHN1, DREB1G, OS11T0175500-02, OsJ_33156), Cluster 2 groups 7 genes (DREB1H, NAC6, OS01T0104100-01, OS03T0297600-01, OS05T0305900-01, OS08T0545500-00, OsJ_027745), and Cluster 3 groups 6 genes (Aldh2a, Aldh2b, OS07T0188800-01, OsJ_04113, OsJ_06966, OsJ_24269). The KEGG pathway analysis identified that the query proteins are involved in 14 potential KEGG pathways (Table 2). The co-occurrence patterns of the 14 identified abiotic-stress responsive genes in rice and 5 predicted functional protein partners are also examined across various taxa (Supplementary file 7). These genes were further subjected to a co-expression study in STRING, which provided an initial understanding of the pattern of expression of these genes under various abiotic stresses (Supplementary file 8).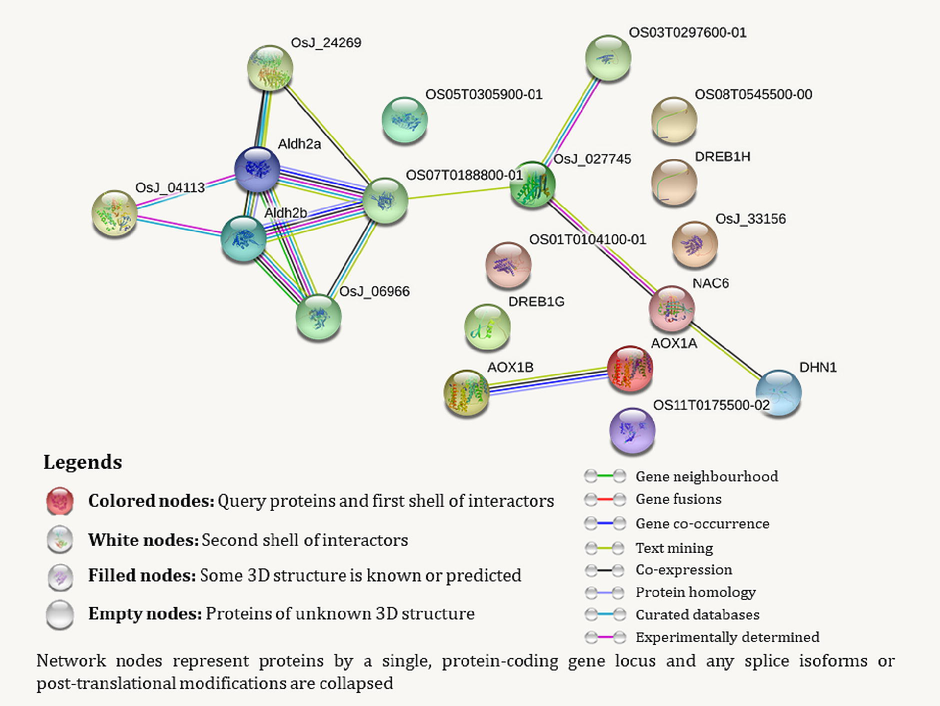
Protein-protein interaction among 14 identified abiotic-stress responsive genes in O. Sativa along with 5 predicted functional protein partners made in STRING.
KEGG Pathways
Observed gene count
Background gene count
Strength
False discovery rate
Matching proteins in network
beta-Alanine metabolism
4
55
2.14
3.97E-06
Aldh2a, Aldh2b, OS07T0188800-01, OsJ_24269
Fatty acid degradation
3
60
1.97
0.00026
OsJ_04113, Aldh2a, Aldh2b
Valine, leucine and isoleucine degradation
3
65
1.94
0.00026
Aldh2a, Aldh2b, OS07T0188800-01
Pantothenate and CoA biosynthesis
3
52
2.03
0.00026
Aldh2a, Aldh2b, OsJ_24269
Limonene and pinene degradation
2
9
2.62
0.00046
Aldh2a, Aldh2b
Pyruvate metabolism
3
110
1.71
0.00075
OsJ_06966, Aldh2a, Aldh2b
Histidine metabolism
2
24
2.19
0.0019
Aldh2a, Aldh2b
Glycolysis / Gluconeogenesis
3
179
1.5
0.0023
OsJ_06966, Aldh2a, Aldh2b
Propanoate metabolism
2
42
1.95
0.0043
OsJ_06966, OS07T0188800-01
Ascorbate and aldarate metabolism
2
65
1.76
0.009
Aldh2a, Aldh2b
Arginine and proline metabolism
2
80
1.67
0.0122
Aldh2a, Aldh2b
Lysine degradation
2
88
1.63
0.0135
Aldh2a, Aldh2b
Tryptophan metabolism
2
104
1.56
0.0172
Aldh2a, Aldh2b
Glycerolipid metabolism
2
110
1.53
0.0178
Aldh2a, Aldh2b
3.5 In silico expression study
The expression potential of each of the six genes was examined individually using an electronic fluorescent pictogram (eFP) to better understand how these genes may respond under stressful conditions (Fig. 7). The expression potential of each gene was calculated through the GCOS expression signal (Supplementary file 9). ALDH2a showed the highest and lowest expression potential during inflorescence P3 and lowest in young leaves ALDH2b showed the highest expression potential in young leaves and the lowest in SAM Meanwhile, AOX1ashowed the highest expression potential in seed S1 and the lowest in SAM.AOX1b showed the highest expression potential in seed S1 and the lowest in inflorescence P2. OsDHN1 showed the highest expression potential in young inflorescence and the lowest in seed S5. OsNAC6 showed the highest expression potential in the mature leaves and the lowest in SAM.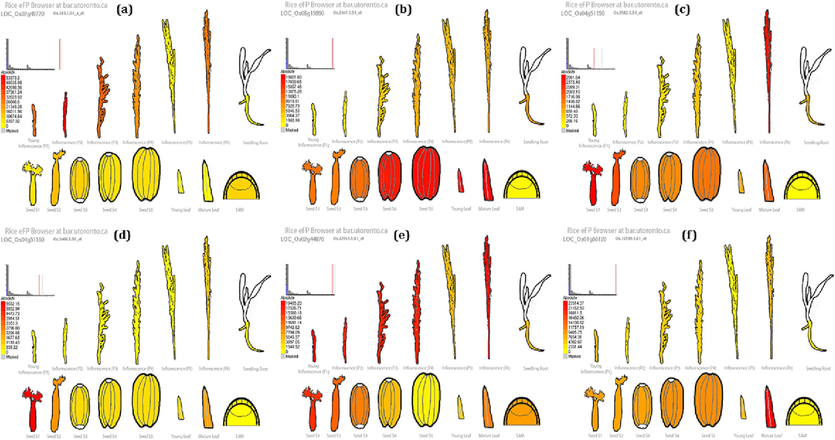
Expression profiling of 6 identified abiotic-stress responsive genes in Oryza sativa, analysis done in Rice eFP browser: (a) ALDH2a, (b) ALDH2b, (c) AOX1a, (d) AOX1b, (e) OsDHN1, (f) NAC6.
In summary, the comprehensive analysis of 14 abiotic stress-responsive genes in rice, including their expression patterns, evolutionary relationships, protein–protein interactions, and co-expression, provides valuable insights into their potential roles in abiotic stress response. Further experimental validation and functional characterization of these genes will contribute to a better understanding of their roles in stress tolerance and may help develop strategies for improving rice crop resilience.
4 Discussion
The 14 abiotic-stress-responsive genes in O. sativa identified for this study are distributed across different chromosomes, suggesting that multiple interconnected cascades regulate the abiotic-stress-response. The presence of more than one gene on a single chromosome further indicates the prominent cross-talk in the response pathways due to the prevalence of abiotic stress (Zarattini et al., 2021). The transcript length, CDS length, and translated amino acid length (Supplementary file 10) of these genes varied significantly, as demonstrated by the analysis carried out in this study. This variation implies individual gene occupancy in the abiotic stress response pathways.
A pairwise similarity matrix is a fundamental but effective tool for understanding the co-expression of various genes. The matrix revealed a wide range of variation in percentage similarity among the genes of interest, ranging from 3.2 to 91.9%. The broad range of similarity percentages is well supported by the phylogenetic analysis conducted in this study.
The base tree(s) for the heuristic search were automatically generated during the development of the phylogenetic tree using the Neighbor-Join and BioNJ algorithms and then determining the topology with the highest log likelihood value. The branch lengths of the phylogenetic trees were inferred by computing the number of substitutions per site, providing abundant information on the evolutionary development of the genes involved in the analysis. The phylogenetic tree was constructed using the Maximum Likelihood (ML) approach, which employs various substitution models to account for multiple changes at the exact sequence location along the evolutionary timeline. The Jones-Taylor-Thornton (JTT) substitution model (Jones et al., 1992) was considered while constructing the phylogenetic tree, as this model best fits the phylogeny by corresponding to the lowest Bayesian Information Criterion (BIC) score calculated. Bootstrapping was performed using 1000 replicates (Felsenstein, 1985; Hillis and Bull, 1993). The clusters in the phylogenetic tree indicate the similar potential ancestry of genes within the same cluster. Although each cluster is somehow interrelated according to the phylogenetic tree, genes within the same cluster showed significant percentage similarity in the pairwise similarity matrix, justifying the phylogenetic tree.
The protein–protein interaction analysis revealed three prominent clusters of interactions. The most robust interactions were found among Aldh2a, Aldh2b, OS07T0188800-01 and OsJ_04113 in one cluster and between AOX1a and AOX1b in another cluster. Notably, the KEGG pathway analysis identified two genes, ALDH2a and ALDH2b, to participate in every pathway except propanoate metabolism. The profiles of potential expression levels of the analyzed six genes indicated that resistance or tolerance mechanisms under various abiotic stress stimuli gradually develop throughout the plant's lifetime. Each gene demonstrated peaks of expression at different stages of plant growth. These findings underscore the significant roles of these genes in the abiotic-stress response in O. sativa and suggest intense cross-talk of response mechanisms.
From an agricultural perspective, understanding the abiotic stress-responsive genes in O. sativa is crucial for improving crop resilience and maintaining yield stability under adverse environmental conditions (Paul et al., 2023). Rice is a staple food crop for more than half of the world's population, and its production must keep pace with the growing demand. However, the increasing frequency and severity of abiotic stress factors, such as drought, salinity, and cold, due to climate change pose significant challenges to rice production worldwide. The findings of this study on the 14 abiotic-stress responsive genes, their interactions, and their potential expression levels under various stress conditions provide valuable insights for developing rice varieties with enhanced tolerance to multiple abiotic stresses (Fig. 8). Identifying and characterizing these genes and their roles in abiotic stress response pathways can help plant breeders and biotechnologists to design targeted breeding strategies, including marker-assisted selection and genetic engineering approaches, for developing stress-tolerant rice cultivars (Sun et al., 2022). The resulting improved rice varieties would enhance agricultural productivity and ensure food security in the face of climate change.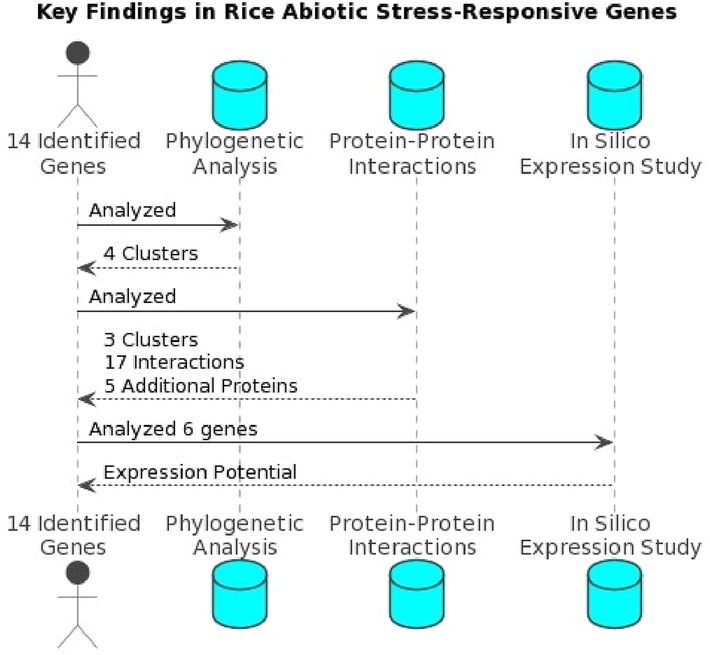
Key findings of the study conducted on the genes playing significant regulatory roles in abiotic stress responses in O. Sativa (Source: Author’s creation with PlantText UML Editor).
Furthermore, understanding the cross-talk of response mechanisms among these abiotic stress-responsive genes can contribute to the development of rice cultivars with broad-spectrum stress tolerance (Husaini, 2022). This can be achieved by manipulating multiple genes or regulatory elements involved in the cross-talk, thereby enabling the plant to withstand multiple stress factors simultaneously. Future research should focus on validating the roles of these identified genes through functional genomics approaches, such as gene knockout or over-expression studies, in rice plants grown under controlled stress conditions. Moreover, the study of gene regulatory networks and the identification of transcription factors involved in the regulation of these stress-responsive genes will provide a comprehensive understanding of the molecular mechanisms underlying abiotic stress responses in rice. Therefore, the insights gained from this study on the abiotic stress-responsive genes in O. sativa have significant implications for agriculture, especially in the context of climate change. By harnessing the potential of these genes and understanding their interactions and cross-talk, researchers and plant breeders can develop new rice varieties with enhanced tolerance to multiple abiotic stresses, thus ensuring sustainable rice production and global food security.
5 Conclusion
The findings of the present study suggest that six key genes (AOX1a, AOX1b, ALDH2a, ALDH2b, OsNAC6, OsDHN1) play significant regulatory roles in abiotic stress responses in O. sativa. The expression profiles of these genes were found to vary across different stages of the rice life cycle, such as young and mature leaves, young inflorescence, and seed development stages. These results indicate that these genes participate in interconnected cascades throughout the plant's life cycle, providing protection against abiotic stresses. The cross-talk between response mechanisms of these abiotic-stress responsive genes was also corroborated by phylogenetic analysis, protein–protein interaction, and gene co-expression studies. Therefore, using these genes as targets for developing gene-based molecular markers could enable the breeding of rice cultivars with enhanced resistance to challenging environmental conditions. Furthermore, these findings have the potential to contribute to the successful application of computational biology in plant breeding by reducing the expense, complexity, and time required for traditional biological studies.
CRediT authorship contribution statement
Sandip Debnath: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Shaik Aisha: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing - original draft, Writing - review & editing. Ayushman Malakar: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing - original draft, Writing - review & editing. Kahkashan Perveen: Manuscript preparation, reviewing, and editing. Alanoud Alfagham: Manuscript preparation, reviewing, and editing. Mehrun NishaKhanam: Manuscript preparation, reviewing, and editing. Biswajit Pramanik: Manuscript preparation, reviewing, and editing. Ahmed Mohammed: Manuscript preparation, reviewing, and editing.
Acknowledgement
The author, SA, would like to sincerely thank Visva-Bharati University for all the support and resources offered to conduct research during the master's program.
The authors would also like to acknowledge the support provided by Researchers Supporting Project Number RSP2023R358, King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot.. 2012;63(10):3523-3543.
- [CrossRef] [Google Scholar]
- Abiotic and biotic stresses induce a core transcriptome response in rice. Sci. Rep.. 2019;9(1):6273.
- [CrossRef] [Google Scholar]
- Pathotype characterization of Xanthomonasoryzaepv. oryzae isolates of West Bengal and evaluation of resistance genes of bacterial blight of rice (Oryza sativa L.) Journal of Crop and Weed.. 2013;9(1):198-200.
- [Google Scholar]
- Structural and Functional Characterization at the Molecular Level of the MATE Gene Family in Wheat in silico. Contrast Media Mol. Imaging. 2022;2022
- [CrossRef] [Google Scholar]
- Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783-791.
- [Google Scholar]
- Enhanced expression of alternative oxidase genes is involved in the tolerance of rice (Oryzasativa L.) seedlings to drought stress. ZeitschriftfürNaturforschung C. 2009;64(9–10):704-710.
- [Google Scholar]
- Seven zinc-finger transcription factors are novel regulators of the stress responsive gene OsDREB1B. J. Exp. Bot.. 2012;63(10):3643-3656.
- [Google Scholar]
- An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol.. 1993;42(2):182-192.
- [Google Scholar]
- High-value pleiotropic genes for developing multiple stress-tolerant biofortified crops for 21st-century challenges. Heredity. 2022;128(6):460-472.
- [CrossRef] [Google Scholar]
- Isolation of a novel PP2C gene from rice and its response to abiotic stresses. Afr. J. Biotechnol.. 2011;10(37):7143-7154.
- [Google Scholar]
- The rapid generation of mutation data matrices from protein sequences. Bioinformatics. 1992;8(3):275-282.
- [Google Scholar]
- Web tools for rice transcriptome analyses. Journal of Plant Biology. 2011;54:65-80.
- [Google Scholar]
- Root response to drought stress in rice (Oryza sativa L.) Int. J. Mol. Sci.. 2020;21(4):1513.
- [CrossRef] [Google Scholar]
- Over-expression of dehydrin gene, OsDhn1, improves drought and salt stress tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.) Journal of Plant Biology. 2014;57:383-393.
- [Google Scholar]
- Role of plant transcription factors in abiotic stress tolerance. Abiotic Stress Response in Plants, INTECH Open Access Publishers. 2011;10:269-296.
- [Google Scholar]
- A leucine-rich repeat receptor-like kinase, OsSTLK, modulates salt tolerance in rice. Plant Sci.. 2020;296:110465
- [Google Scholar]
- Two NCA1 isoforms interact with catalase in a mutually exclusive manner to redundantly regulate its activity in rice. BMC Plant Biol.. 2019;19:1-10.
- [Google Scholar]
- Isolation and characterization of an AP2/ERF-type drought stress inducible transcription factor encoding gene from rice. J. Plant Biochem. Biotechnol.. 2014;23:42-51.
- [Google Scholar]
- Allelic variants in durum wheat (Triticum turgidum L. var. durum) DREB genes conferring tolerance to abiotic stresses. Mol. Genet. Genomics. 2015;290:531-544.
- [CrossRef] [Google Scholar]
- Ectopic expression of OsDREB1G, a member of the OsDREB1 subfamily, confers cold stress tolerance in rice. Front. Plant Sci.. 2019;10:297.
- [Google Scholar]
- Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J.. 2007;51(4):617-630.
- [Google Scholar]
- A negative regulator in response to salinity in rice: Oryza sativa salt-, ABA-and drought-induced RING finger protein 1 (OsSADR1) Plant Cell Physiol.. 2018;59(3):575-589.
- [Google Scholar]
- Identification of major and stable QTLs conferring drought tolerance in rice RIL populations. Current Research in Biotechnology 2023100125
- [CrossRef] [Google Scholar]
- Cellular and molecular mechanisms controlling autophagy: A perspective to improve plant stress resistance and crop productivity. Sel'skokhozyaistvennayaBiologiya. 2018;53(5):881-896.
- [CrossRef] [Google Scholar]
- Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol.. 2003;133(4):1755-1767.
- [CrossRef] [Google Scholar]
- Expression profiles of stress responsive genes in rice (Oryza sativa L.) under abiotic stresses. Biotechnol. Biotechnol. Equip.. 2012;26(2):2838-2843.
- [CrossRef] [Google Scholar]
- Identifying stress responsive genes using overlapping communities in co-expression networks. BMC Bioinf.. 2021;22:1-17.
- [CrossRef] [Google Scholar]
- In silico analysis of functional linkage among arsenic induced MATE genes in rice. Biotechnol. Rep,. 2020;26:e00390.
- [Google Scholar]
- Integration of dual stress transcriptomes and major QTLs from a pair of genotypes contrasting for drought and chronic nitrogen starvation identifies key stress responsive genes in rice. Rice. 2021;14(1):1-28.
- [CrossRef] [Google Scholar]
- Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Integr. Plant Biol.. 2014;56(2):114-121.
- [CrossRef] [Google Scholar]
- Clustal Omega, accurate alignment of very large numbers of sequences. Multiple sequence alignment methods 2014:105-116.
- [Google Scholar]
- MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol.. 2021;38(7):3022-3027.
- [CrossRef] [Google Scholar]
- Organ-specific expressions and chromosomal locations of two mitochondrial aldehyde dehydrogenase genes from rice (Oryzasativa L.), ALDH2a and ALDH2b. Gene. 2003;305(2):195-204.
- [Google Scholar]
- Analysis of the rice SHORT-ROOT5 gene revealed functional diversification of plant neutral/alkaline invertase family. Plant Sci.. 2009;176(5):627-634.
- [Google Scholar]
- Every cloud has a silver lining: how abiotic stresses affect gene expression in plant-pathogen interactions. J. Exp. Bot.. 2021;72(4):1020-1033.
- [CrossRef] [Google Scholar]
- OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean. PLoS One. 2013;8(12):e83011.
- [CrossRef] [Google Scholar]
- ARAG1, an ABA-responsive DREB gene, plays a role in seed germination and drought tolerance of rice. Ann. Bot.. 2010;105(3):401-409.
- [CrossRef] [Google Scholar]
- Abiotic stress signaling and responses in plants. Cell. 2016;167(2):313-324.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102786.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







