Translate this page into:
The potential mosquitocidal activity of cry4A toxic region crystal protein gene from local isolates of Bacillus thuringiensis against Aedes aegypti
⁎Corresponding author. rehman.mmg@pu.edu.pk (Abdul Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study was conducted to isolate Bacillus thuringiensis (B.t.) strains from different ecological regions of Pakistan and determined the mosquitocidal activity of cry4A crystal protein gene against the dipteran insects. Out of 25 B.t. isolates, 5 isolates were selected on the basis of toxicity and 4 were identified as Bacillus thuringiensis GCU-DAB-TK-04 (MN922746), GCU-DAB-TK-06 (MT032397), GCU-DAB-TK-09 (MN922747), and GCU-DAB-TK-13 (MT032398). GCU-DAB-TK-04 was found to be the most toxic strain against the 3rd instar larvae of Aedes aegypti. The presence of Cry 4A protein was confirmed by the amplification of cry4A gene (accession number MT001910) from GCU-DAB-TK-04. The highest spores toxicity was shown by GCU-DAB-TK-04 (LC50 = 104 μg/ml) while LC50 values of other strains were GCU-DAB-TK-13 (LC50 = 602 ± 0.43 μg/ml) GCU-DAB-TK-06 (LC50 = 812 ± 0.63 μg/ml), GCU-DAB-TK-12 (LC50 = 1230 ± 1.14 μg/ml), and GCU-DAB-TK-09 (LC50 = 7585 ± 1.17 μg/ml). Similarly, the highest toxicity of total cell protein was also shown by GCU-DAB-TK-04 (LC50=676 ± 0.53 μg/ml while LC50 values of other strains were GCU-DAB-TK-13 (LC50 = 724 ± 1.12 μg/ml), GCU-DAB-TK-06 (LC50 = 741 ± 0.64 μg/ml), GCU-DAB-TK-12 (LC50 = 912 ± 0.65 μg/ml), and GCU-DAB-TK-09 (LC50 = 1621 ± 1.13 μg/ml). The order of toxicity of B.t. strains was GCU-DAB-TK-04 > GCU-DAB-TK-13 > GCU-DAB-TK-06 > GCU-DAB-TK-12 > GCU-DAB-TK-09. Protein analysis showed that 130 kDa (probably Cry4A, B), 70 kDa (Cry4C, D) and 20 kDa (Cyt enhancer protein of Cry4) proteins were present in all B.t. strains. These B.t. strains have found great potential to grow into bio-insecticidal formulation for the eco-friendly control of mosquitoes.
Keywords
B.t. strains
Cry4A gene
Cry 4A protein
Biotoxicity
Aedes aegypti
1 Introduction
The insecticides used in the earliest 19th century include organic and inorganic compounds, organochlorides, carbamates, pyrethroids, formamidines, etc. (Glazer and Nikaido, 1995). These chemicals have certain characteristics which make them useful for a broad range of organisms, such as residual action and toxicity (Khan et al., 2021). However, they cause resistance to insects, are harmful to humans and kill beneficial insects when used improperly (Cetinkaya, 2002; Kegley and Wise, 1994).
Like all organisms, insects are susceptible to infection by pathogenic microorganisms. Biological pesticides are, therefore, becoming key components of integrated pest management strategies. The tremendous success in microbial pesticides has come from the uses of Bacillus thuringiensis (Zakeel et al., 2009; Nair et al., 2020).
B. thuringiensis (B.t) is a gram-positive, aerobic and spore-forming bacterium that produces parasporal crystals encoded by plasmid-based cry and cyt genes (Das et al., 2015). These proteins, known as insecticidal crystal proteins (ICPs), are specifically toxic to insect larvae and are used against the insects of Lepidoptera, Diptera, Coleoptera, certain nematodes, protozoan pathogens and cancer (Frutos et al., 1999; Aboul-Soud et al., 2019; Bedini et al., 2020; Dhamana et al., 2020a,b).
Larvicidal activity of B. thuringiensis is based on crystals, produced during sporulation. These parasporal inclusions comprise a high amount of glycoproteins known as endo-toxins that exhibit highly specific insecticidal activity. B. thuringiensis subspecies aizawai, sotto, kurstaki (Btk), entomocides, and berliner are active against the insects of order Lepiodoptera. B. thuringiensis subspecies sandiego, and tenebrionis are effective against the insects of the order Coleoptera. B. thuringiensis subspecies israelensis (Bti), kyushuensis, and gallariae are highly toxic to the insects of order Diptera (Donovan et al., 1988; Chilcott and Wigley, 1993; Bedini et al., 2020).
The objective of the present study was to characterize B. thuringiensis strains, isolated from different ecological areas of Pakistan, through biochemically and 16S rRNA sequencing. The strains were also screened for the presence of cry4A gene and their toxicity against Aedes aegypti larvae was also evaluated.
2 Material and methods
2.1 Sample collection and isolation of B.t. isolates
Soil samples were collected from different areas of Lahore, Gujranwala, Kashmir, Faisalabad, Sialkot, and Kasur. Soil samples were taken 10 cm below the surface using sterile spatula in sterile plastic bags and were stored at 4 °C until processed. For the isolation of local strains of B. thuringiensis, samples were processed according to Martin and Travers (1989) by sodium acetate selection method. Briefly, 0.5 g soil of each sample was mixed in medium containing 0.25 M sodium acetate (2.05 g/100 ml of autoclaved distilled water, pH 6.8) and then incubated for microbial growth, filtered with the help of syringe filter (0.45 µm) and were heat shocked at 80 °C for 10–15 min to remove all the vegetative and non-spore forming cells. The processed samples were then diluted to 1:2, 1:3, and 1:4 and so on. The suspension was spread on LB agar medium, incubated at 37 °C for 24 h, and checked for microbial growth on the next day.
2.2 Bacterial identification
Gram staining, endospore staining, crystal staining and motility test were performed according to procedures described in Cheesebrough (1993) and Bukhari and Shakoori (2010). Various biochemical tests including catalase test, blood agar test, lecithinase activity, hydrolysis of casein, starch hydrolysis test, and indole test were also performed (James and Natalie, 2014). Isolation of genomic DNA from B. thuringiensis isolates was carried out according to Martin and Travers (1989). Universal primers were used for the conserved region of the 16S rRNA gene.
F 5′ TGAAAACTGAACGAAACAAAC 3′; R 5′ CTCTCAAAACTGAACAAAACGAAA 3′.
The PCR was performed according to the procedure described in Saiki et al. (1988) using Humanizing Genomics macrogen reagents. The full-length gene was amplified for 30 cycles by programming the Thermocycler (Progene, Techne) with initial denaturation at 94 °C (5 min), denaturation at 94 °C (2 min), annealing at 52 °C (1:30 min), elongation at 72 °C (2 min) with final elongation at 72 °C (7 min). The PCR products were loaded on agarose gel (1%) and electrophoresis was performed at 90 V for 45 min. Amplified PCR products were visualized on ultra-violet (UV) trans-illuminator.
2.3 PCR based detection of cry4A gene
For confirmation of cry4A gene, a 459 bp fragment of cry4A gene was amplified from three local isolates of B. thuringiensis using specific primers. The sequence of the primers is as follows:
F 5′ TCAAAGATCATTTCAAAATTACATG 3′; R 5′ CGGCTTGATCTATGTCATAATCTGT 3′.
PCR was performed with crude DNA as described by Carozzi et al. (1991). The cry4A gene was amplified for 30 cycles, with initial denaturation at 94 °C for 5 min, denaturation at 94 °C for 2 min, annealing at 56 °C for 30 sec, elongation at 72 °C for 2 min with a final extension at 72 °C for 7 min. PCR products were loaded on 1% agarose gel and electrophoresis was performed at 90 V for 45 min. Amplified PCR products were visualized on UV trans-illuminator.
PCR products were sent to Macrogen Korea for sequencing. The genes were sequenced to find out the evolutionary relationship between sequences and to confirm the identification of local isolates of B. thuringiensis to subspecies level. Sequences of cry4A gene aligned and blasted using the Nucleotide BLAST program NCBI. The gene sequences of isolated B.t. strains were submitted in the NCBI DNA databases.
2.4 Biotoxicity assays of B.t. isolates
2.4.1 Preparation of bacterial spore diet and cell protein
Biotoxicity assays against 3rd instar larvae of A. aegypti were performed by using local isolates of B. thuringiensis. Both spores as well as total cell protein were used for bioassays. The preparation of bacterial spores was done according to the procedure described in Makino et al. (1994). Total cell protein was also extracted from bacterial cultures and toxin concentration was determined by the method of Bradford (1976).
2.4.2 Procedure adopted for bioassays
For biotoxicity assays, 3rd instar larvae of A. aegypti were used. Various doses of B.t. spores i.e. 0, 100, 200, 300, 400, 500, 600, 700, 800, 900, and 1000 μg/ml were prepared for positive control HD 500 (Kindly provided by Bacillus Genetic Stock Center, Columbus, Ohio State, USA) and for each bacterial strain. Likewise, B.t. total cell protein doses [activated i.e. trypsin treated (Bukhari and Shakoori, 2010)] were prepared in 10 ml of distilled water ranging from 25 to 250 μg/ml. The wide-mouthed plastic cups were used for the preparation of doses, in each cup ten (10) third instar larvae were added and finally covered with a fine net. The room temperature was kept at 25 °C. The larval mortality was recorded in each cup after 24 to 48 h. The larvae brought down at the bottom of the cup and unable to swim to the water surface were contemplated as dead (Fig. 1). Then larval mortality against each concentration was calculated and mean, standard deviation, and standard error of the mean were calculated according to Shakol and Rohlf (1981). Toxicity was assessed with Log-Probit analysis (Finney, 1971).
Bio-toxicity assays (set up) with B.t. spores and total cell protein under specified conditions.
2.5 Extraction of total cell protein
The total proteins from local B.t. strains were isolated according to the procedure described in Bukhari and Shakoori (2010). Briefly, the isolated single B.t. colony after 24 h incubated culture on LB agar plate was streaked on T3 plates and plates were kept for 72 h at 30 °C. The culture was washed away by adding autoclaved distilled water (3 ml) from the plate. After this, culture was centrifuged at 4500 xg at 4 °C for 15 min, and the pellet was suspended in autoclaved distilled water. The suspension was centrifuged and two washings were performed with cold autoclaved distilled water. Then pellet was again suspended in alkaline buffer (Na2CO3 0.265g, DTT 0.08g, autoclaved distilled water 50 ml, pH 10.5–11), incubated at 37 °C for 3 h, spun at 4500 xg at 4 °C for 20 min. The concentration of protein in the supernatant was determined by Lowry method (Lowry et al., 1951). Finally proteins were resolved by SDS-PAGE on 12% acrylamide gel, stained with Coomassie Brilliant Blue and photographed after destaining (Laemmli, 1970).
3 Results
3.1 Isolation of B.t. isolates
For B.t. screening, 25 samples from different ecological habitats of Pakistan under sterile conditions were collected. Both shaken flask technique and sodium acetate selection method were used for B.t. isolation. The distinct 25 colonies appeared on LB agar plates with B.t. like morphology, entire margin, off white color, and with dry and rich growth and were processed for further work.
3.2 Characterization of B.t. isolates
Gram staining results showed that the isolated bacterial strains were rod shape, and gram positive bacilli (Fig. 2). Most of the strains were spore formers and the position of endospores was sub-terminal and paracentral. Most of the bacterial strains formed crystals after 24 h of incubation (Fig. S1a) and were active motile (Fig. S1b). It was found that out of 25 isolates, 21 were catalase positive (Fig. S1c), 14 showed hemolysis by forming zones around the bacterial culture (Fig. S2a), and 20 produced white precipitation around the colony (Fig. S2b). It was also found that out of 25 isolates, 19 formed clear zones around the bacterial colony (Fig. S2c), 20 produced amylase to hydrolyze the starch resulting in the formation of a clear zone around the bacterial culture (Fig. S2d), and 18 were indole positive (Fig. S2e).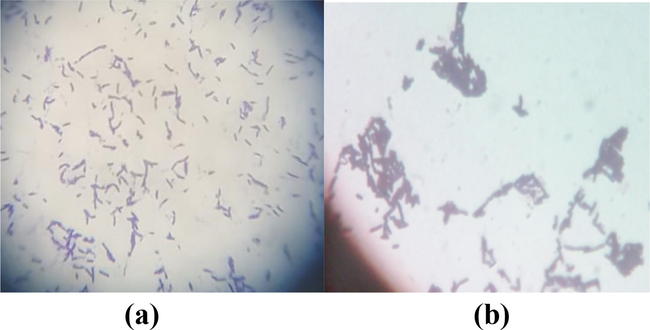
Gram staining of bacterial strains (a) GCU-DAB-TK-04, (b) HD500.
The nucleotide sequence of the full-length 16S rRNA gene was done for the identification of B. thuringiensis isolates up to species and subspecies level. The sequence alignment of four B.t. strains showed maximum homology with the sequences of already reported strains (Fig. S3) and their sequences were deposited to GenBank database under accession numbers of MN922746 (GCU-DAB-TK-04), MT032397 (GCU-DAB-TK-06), MN922747 (GCU-DAB-TK-09), and MT032398 (GCU-DAB-TK-13).
3.3 Presence of cry4A gene in B.t. strains
A fragment of a 459 bp base pair of cry4A gene was amplified through PCR technique (Fig. 3a), sequenced, and the gene sequences were then submitted to GenBank under accession number of MT001910. A phylogenetic relationship of cry4A gene sequence was established with the sequences of already reported cry4A gene (Fig. 3b). The cry4A gene sequences were translated into amino acids and a 3D model of Cry4A protein computed from SWISS-MODEL Expasy Tools was made. The α-helical structure is involved in pore forming units and responsible toxicity while the β-pleated sheets are involved in the receptor region (Fig. 3c).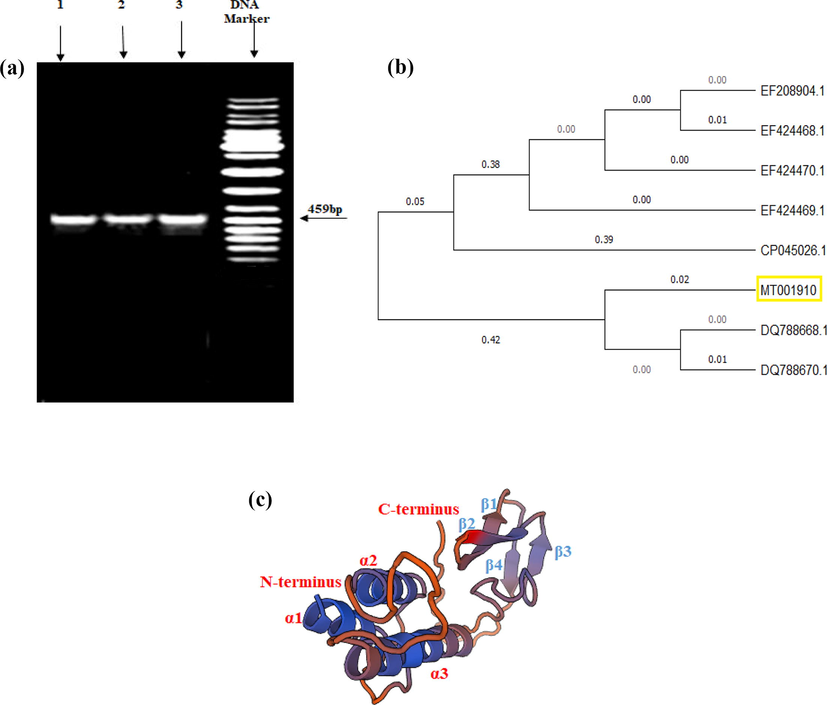
(a) Agarose gel showing PCR products of cry4A gene by using B. thuringiensis; Lane 1, 2, and 3 represent (GCU-DAB-TK-06), (GCU-DAB-TK-04), and (GCU-DAB-TK-13), respectively, (b) Phylogenetic relationship of shorter fragment of cry4A gene from the most toxic B.t. strain GCU-DAB-TK-04 with already reported genes, (c) The predicted 3D model of Cry4A protein computed from SWISS-MODEL Expasy Tools.
3.4 Bioassay
3.4.1 With bacterial spore diet
Among the most toxic B.t. isolates, GCU-DAB-TK-04 (LC50 = 104 µg/ml) was isolated from moist and sticky soil from field area, Kasur, GCU- DAB-TK-13 (LC50 = 602 µg/ml) was isolated from dry and sandy soil field area Cantt road, Lahore, GCU-DAB-TK-06 (LC50 = 812 µg/ml) was isolated from moist soil field farm Johar Town, Lahore, GCU-DAB-TK-12 (LC50 = 1230 µg/ml) was isolated from Canal area, Gujranwala, and GCU-DAB-TK-09 (LC50 = 7585 µg/ml) was found to be least toxic and isolated from Nursery farm Canal view society, Lahore (Table 1).
Strain ID
Area of collection
Soil texture
LC50 (Spores) (µg/ml)
LC50 (Total cell protein) (µg/ml)
GCU-DAB-TK-04
Field area, Kasur
Moist and sticky
104 ± 0.53
676 ± 0.53
GCU-DAB-TK-06
Field farm JT, Lahore
Moist soil
812 ± 0.63
741 ± 0.64
GCU-DAB-TK-09
Nursery farm, Lahore
Moist soil
7585 ± 1.17
1621 ± 1.13
GCU-DAB-TK-12
Canal area, Gujranwala
Moist soil
1230 ± 1.14
912 ± 0.65
GCU-DAB-TK-13
Field area, Lahore
Dry and sandy
602 ± 0.43
724 ± 1.12
HD500
Reference strain
588 ± 1.15
575 ± 0.18
3.4.2 With total cell protein
In bioassays with total cell protein again the highest toxicity was shown by GCU-DAB-TK-04 i.e. LC50 = 676 µg/ml (Table 1) while the LC50 values of other strains were 724 µg/ml (GCU-DAB-TK-13), 741 µg/ml (GCU-DAB-TK-06), 912 µg/ml (GCU-DAB-TK-12) and 1621 µg/ml (GCU-DAB-TK-09). The order of toxicity of B.t. strains was GCU-DAB-TK-04 > GCU-DAB-TK-13 > GCU-DAB-TK-06 > GCU-DAB-TK-12 > GCU-DAB-TK-09.
3.5 Protein analysis of B.t. strains
Overall the proteins extracted from the B.t. isolates were resolved on 12% SDS-PAGE. A great number of protein bands are found in all B.t. strains and 130, 70, and 40 kDa were the most prominent (Fig. 4). After sporulation the prominent bands presumably belong to Cry proteins. SDS-PAGE analysis also showed several low molecular weight proteins, including 20 kDa proteins, were present in all B.t. strains (Fig. 4).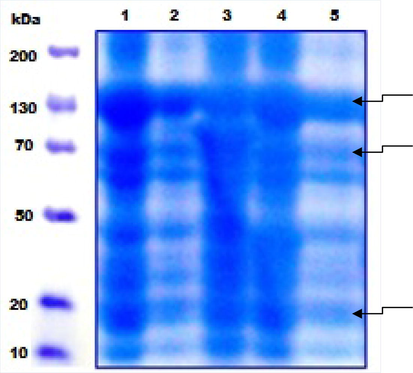
SDS-Polyacrylamide gel electrophoretic pattern of total cell proteins of sporulated B.t. strains. The lanes 1–5 indicate GCU-DAB-TK 04, 06, 13, 12, and 09, respectively. The gel was 12% and stained with Coomassie Brilliant Blue.
4 Discussion
This current study was designed to screen different ecological areas of Pakistan for cry4A positive B. thuringiensis isolates which could be later helpful to form bio-pesticides against mosquitoes. B. thuringiensis is able to synthesize some targeted specific insecticidal proteins in huge quantities. During sporulation these proteins have various forms of parasporal crystalline inclusions. The spores add an important contribution to the toxicity of B. thuringiensis δ-endotoxins.
B. thuringiensis specificity is its toxicity against different insect orders and it is totally non-toxic to mammals including human beings. So, it is eco-friendly. Frederiksen et al. (2006) reported that the Cry protein’s specificity is due to presence of specific receptors in insect midgut. Dried spores and toxin crystal products of B. thuringiensis have commercial importance and are used worldwide (Alberola et al., 1999; Roh et al., 2007). Lobo et al. (2018) isolated 300 local isolates of B.t. from 45 separated soil samples and PCR technique was used to detect toxic genes in these bacterial isolates. Most of the strains were found positive for cry4A, cry11Aa, and cyt1 genes. The bioassays were performed to assess the pathogenicity against the A. aegypti (3rd instar larvae) and 12 strains were found to present larvicidal activity.
The insecticidal crystal protein genes are normally found to be associated with plasmids having large molecular size (Gonzales and Carlton, 1980). The cry genes are mainly divided into four classes: cry I, cry II, cry III, and cry IV are specific for insects belonging to various orders (Schnepf et al., 1998). Each of the B. thuringiensis strains contains one or more types of crystal toxin genes, and therefore, more than one crystal protein can be synthesized by a single strain of the organism (Thomas and Ellar, 2001).
Cry proteins are globular proteins encoded by cry genes within a range of 50–140 kDa. The cry4 gene is specifically known to be active against mosquitoes and black flies worldwide. The plasmid encodes cry4A, cry4B, cry4C, and cry4D genes of dipteran specific proteins having 134, 128, 78, and 72 kDa molecular masses, respectively. All proteins (Cry4 toxins) are synthesized at different phases of sporulation and are accumulated in ovoid inclusion (Bukhari and Shakoori, 2010; Faiz and Bukhari, 2018). The inclusion proteins are made up of one or may be several insecticidal proteins also called δ-endotoxins. These δ-endotoxins are grouped into two main classes, namely crystal (cry) and cytolytic (cyt) toxins, on the basis of their amino acid sequence (Hofte and Whiteley, 1989; Bukhari and Shakoori, 2010; Zhang et al., 2016). Rodríguez-González et al. (2020) reported that larvae and adults of Acanthoscelides obtectus can be controlled by the application of Cry proteins. Khan et al. (2022) studied the function of various target genes of Paederus fuscipes, a medically and agriculturally important insect, through RT-qPCR for correct reference genes.
Bravo et al. (2007) determined the tertiary structure of Cry4Aa and Cry4Ba toxin protein through X-ray crystallography and described that the mosquitocidal active Cry proteins including Cry4A, Cry4B, and Cry11Aa share similar structures with Cry1Aa. Abdullah et al. (2006) reported that bacterial crystalline inclusions, composed of Cry4A protein, are toxic to different insects of medical importance belonging to the genera Culex, Aedes, and Anopheles. Generally, Cry4A and Cry4B proteins are most toxic against insects of genus Aedes (Table 1) while Cry4C (Cry 10) and Cry4D (Cry 11) are most potent against insects of Anopheles genus. Our results are in good agreement with Abdullah et al. (2006). Goje et al. (2020) reported that domain I is responsible for the specificity of Cry2A proteins against insects and 4 amino acids in the N-terminal region the Cry2A protein confer activity against A. aegypti when mutated Cry2Ab. This motif containing the region is usually removed during proteolysis of the protein.
5 Conclusion
In conclusion, the B. thuringiensis based bio-pesticides production depends on the high quality and formulations processes. The formulations used in this study are safe and easy to use having long shelf time and are very effective against the insects of order Diptera. The amplification of cry4A gene indicates the presence of Cry 4A protein responsible for toxicity against dipteran insects. From B.t. isolates, GCU-DAB-TK-04 was found the most toxic B.t. strain with LC50 = 104 µg/ml against 3rd instar larvae and isolated from moist and sticky soil from the field area, Kasur, Pakistan. Protein analysis showed that 130 kDa (probably Cry4A, B), 70 kDa (Cry4C, D) and 20 kDa (Cyt enhancer protein of Cry4) proteins were present in all B.t. strains.
Acknowledgements
None.
Conflict of interest
The authors have declared that no competing interests exist.
Funding
No funding was received for this work from any organization.
References
- Identification of a Bacillus thuringiensis Cry11Ba toxin-binding aminopeptidase from the mosquito, Anopheles quadrimaculatus. BMC Biochem.. 2006;7:16.
- [Google Scholar]
- Specific cytotoxic effects of parasporal crystal proteins isolated from native Saudi Arabian Bacillus thuringiensis strains against cervical cancer cells. Molecules. 2019;24(3):506.
- [Google Scholar]
- Insecticidal activity of strains of Bacillus thuringiensis on larvae and adults of Bactrocera oleae Gmelin (Dipt. Tephritidae) J. Inverterb. Pathol.. 1999;74:633-636.
- [Google Scholar]
- Insecticidal potential of Brevibacillus laterosporus against dipteran pest species in a wide ecological range. J Inverterb. Pathol.. 2020;177:107493.
- [Google Scholar]
- A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon.. 2007;49:423-435.
- [Google Scholar]
- Isolation and molecular characterization of cry4 harbouring Bacillus thuringiensis isolates from Pakistan and mosquitocidal activity of their spores and total proteins. Pakistan J. Zool.. 2010;42(1):1-15.
- [Google Scholar]
- Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl. Environ. Microbiol.. 1991;57:3057-3061.
- [Google Scholar]
- Isolation of Bacillus thuringiensis and Investigation of its Crystal Protein Genes. MSc. Thesis. Turkey: Izmir Institute of Technology; 2002. p. :55.
- Medical Laboratory Manual for Tropical Countries. Cambridge: ELBS, University Press; 1993.
- Isolation and toxicity of Bacillus thuringiensis from soil and insects habitats in New Zealand. J. Inverteb. Pathol.. 1993;61:244-247.
- [Google Scholar]
- Morphological and biochemical, characterization of four new Bacillus thuringiensis strains. Intl. J. Curr. Res.. 2015;7:17143.
- [Google Scholar]
- Insecticidal activity of bacteria from larvae breeding site with natural larvae mortality: Screening of separated supernatant and pellet fractions. Pathogens. 2020;9(6):486.
- [Google Scholar]
- Biological control of Aedes albopictus: Obtained from the new bacterial candidates with insecticidal activity. Insects. 2020;11(7):403.
- [Google Scholar]
- Molecular characterization of a gene encoding a 72-kilodalton mosquito-toxic crystal protein from Bacillus thuringiensis subsp. israelensis. J. Bacteriol.. 1988;170:4732-4738.
- [Google Scholar]
- Mosquitocidal activity of cyt positive Bacillus thuringiensis isolated from soil samples. Pakistan. J. Zool.. 2018;50(3):843-849.
- [Google Scholar]
- Probit Analysis (3rd Edition). Cambridge: Cambridge University Press; 1971.
- Occurrence of natural Bacillus thuringiensis contaminants and residues of Bacillus thuringiensis-based insecticides on fresh fruits and vegetables. Appl. Environ. Microbiol.. 2006;72(5):3435-3440.
- [Google Scholar]
- Managing insect resistance to plants producing Bacillus thuringiensis toxins. Crit. Rev. Biotechnol.. 1999;19:227-276.
- [Google Scholar]
- Goje, L.J., Elmi, E.D., Bracuti, A., Courty, T., Rao, T., Ay Alzahrani, F., Crickmore, N., 2020. Identification of Aedes aegypti specificity motifs in the N-terminus of the Bacillus thuringiensis Cry2Aa pesticidal protein. J. Inverteb. Pathol. 174, 107423.
- Patterns of plasmid DNA in crystalliferous strains of B thuringiensis. Plasmid. 1980;3:92-98.
- [Google Scholar]
- Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev.. 1989;53:242-255.
- [Google Scholar]
- Microbiology. A Laboratory Manual. Pearson Education; 2014.
- Pesticides in Fruit and Vegetables. Sausalito, CA: University Science Books; 1994.
- Screening and validation of reference genes using in RT-qPCR for gene expression studies in Paederus fuscipes, a medically and agriculturally important insect. J. King Saud Uni. Sci.. 2022;34(1):101654.
- [Google Scholar]
- Residual toxicity and sublethal effects of fenvalerate on the development and physiology of Spodoptera exigua reared on different hosts. J. King Saud Uni. Sci.. 2021;33(7):101593.
- [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685.
- [Google Scholar]
- Isolation and molecular characterization of Bacillus thuringiensis found in soils of the Cerrado region of Brazil, and their toxicity to Aedes aegypti larvae. Rev. Bras. Entomol.. 2018;62:5-12.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- A spore-lytic enzyme released from Bacillus cereus spore during germination. Microbiology. 1994;140:1403-1410.
- [Google Scholar]
- Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl. Environ. Microbiol.. 1989;55:2437-2442.
- [Google Scholar]
- Bacillus thuringiensis strains isolated from Qatari soil, synthesizing δ-endotoxins highly active against the disease vector insect Aedes aegypti Bora Bora. Heliyon. 2020;6(10):e05003.
- [Google Scholar]
- Toxicity of five Cry proteins against the insect pest Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae) J. Inverebr. Pathol.. 2020;169:107295.
- [Google Scholar]
- Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487-491.
- [Google Scholar]
- Bacillus thuringiensis and its insecticidal proteins. Microbiol. Mol. Biol. Rev.. 1998;62:774-806.
- [Google Scholar]
- Biometry. The Principles of Statistics in Biological Research (2nd ed. W.H.). San Francisco. CA: Freeman and Company; 1981.
- Mechanism of action of Bacillus thuringiensis var. israelensis insecticidal ẟ-endotoxin. FEBS Lett.. 2001;154:362-368.
- [Google Scholar]
- Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol.. 2007;17(4):547-559.
- [Google Scholar]
- Molecular characterization of Bacillus thuringiensis strains isolated from a selected site in Nechchiyagama, Anuradhapura in Sri-Lanka. Trop. Agric. Res. Ext.. 2009;12(1):31-34.
- [Google Scholar]
- Effects and mechanisms of Bacillus thuringiensis crystal toxins for mosquito larvae. Insect. Sci.. 2016;24(5):1744-1747.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102191.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







