Translate this page into:
The influence of zinc supplementation on IGF-1 levels in humans: A systematic review and meta-analysis
⁎Corresponding author. lym19225@sina.com (Yimin Liang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The effect of supplementation with zinc on levels of IGF-1 remains relatively unexplored, and many of previous studies have reported equivocal findings. Thus, the aim of this study was to elucidate the influence of zinc on IGF-1. A complete systematic search was executed in Scopus, Web of Science, Embase, and PubMed/MEDLINE, by reviewers, from database inception until June 2019. Weighted mean difference (WMD) with the 95% CI was used for assessing the effects of zinc on IGF-1. We evaluated between study heterogeneity using the I-squared and the Q-test statistic. Ten studies reported changes in plasma levels of IGF-1. Combined results ascertained an increase in IGF-1 levels following zinc administration (WMD: 8.620 ng/ml, 95% CI: 1.126, 16.113, I2 = 97.3%). Subgroup analyses demonstrated that zinc intake dosage ≤10 mg/day (WMD: 9.50 ng/ml, 95% CI: 1.47, 17.53) and intervention length ˃8 weeks (WMD: 10.08 ng/ml, 95% CI: 0.67, 19.48) significantly greater increased IGF-1 levels. The present study demonstrated that zinc supplementation can elicit significant increases in IGF-1 in humans. In addition, greater increments were observed when zinc intake dosage was ≤10 mg/day and intervention duration ˃8 weeks.
Keywords
Zinc
IGF-1
Humans
Meta-analysis
1 Introduction
Zinc is a ubiquitous, divalent, metal cation that is an essential component in 10% of human proteins and is a key micronutrient in cell signaling. Zinc is found in high concentrations in the β-cells of the human pancreas, where it plays significant role in insulin and glucagon secretion (Zhao et al., 2019). The effects of zinc on human health are, however, pleiotropic, where it is involved in the activation of certain enzymes, immune response, cell growth and proliferation, and, as a co-factor, in conferring protection against oxidative stress and inflammation (Prasad and Bao, 2019). Disturbances of zinc homeostasis have been shown to be involved in various non-communicable diseases, such as type 2 diabetes, growth retardation, age-mediated macular deterioration, alcohol-related liver disorder or sickle cell anemia (Fernandez-Cao et al., 2019; Prasad and Bao, 2019). Zinc is an important factor in growth and development in humans, mainly due to its crosstalk at a cellular level with insulin-like growth factor-binding protein 3 (IGFBP-3), growth hormone (GH), and insulin-like growth factor 1 (IGF-1). Thus, it is unsurprising that zinc deficiency can result in growth retardation and impaired bone metabolism (Adriani and Wirjatmadi, 2014; Alves et al., 2012).
Insulin-like growth factor 1 (IGF-1) is a growth factor synthesized in the liver, and elicits a myriad of effects on health due to its participation in the GH-IGF-1 axis, where it is involved in tissue homeostasis, has anti-apoptotic, mitogenic, anti-inflammatory, antioxidant and metabolic actions, contributes to skeletal muscle plasticity, maintenance of muscle strength and muscle mass, neural and cardiovascular protection, development of the skeleton, possesses insulin-like effects, and is a key factor in brain, eye and lung development during fetal development (Blanco-Alvarez et al., 2015; Hellstrom et al., 2016; Maggio et al., 2013; Vitale et al., 2019). As an anabolic hormone, IGF-1 plays important roles in both growth and development, and its levels vary depending on age, with peaks generally observed in the postnatal period and at puberty (Cho et al., 2019; Rahmani et al., 2019). Via a negative feedback loop, IGF-1 levels influence the release of GH from the hypophysis (Himoto and Masaki, 2018), where some of the actions of GH include, stimulation of glucose and amino acid, cell cycle regulation, are IGF-1 dependent (Alvarez-Nava and Lanes, 2017; MacDonald, 2000).
IGF-1 levels are not constant throughout the life course but decrease with age as a reflection of the actions of GH. Following puberty, during the third decade of life, a rapid decrease in IGF-1 levels is registered. Whilst between the third and the eighth decade of life, IGF-1 levels decrease gradually, but appear unrelated to functional decline (Janssen, 2018; Newman et al., 2016; Wennberg et al., 2018).
The impact of zinc on plasma levels of IGF-1 remains relatively unexplored and many of the previous studies have shown conflicting results (Berger et al., 2015; Blostein-Fujii et al., 1997; Clark et al., 1999; Ninh et al., 1996; Rodondi et al., 2009). Thus, the aim of this study was to elucidate the influence of zinc intervention on IGF-1 levels in humans.
2 Methods
2.1 Study design and search strategy
This present study was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (Moher et al., 2015). An electronic search was performed in Web of Science, Scopus, PubMed/MEDLINE, and EMBASE by the authors, from database inception until June 2019. We provided search strategy keywords in Supplementary Table 1.
2.2 Selection criteria
We followed the participant, intervention, comparison, outcome, time, and study design (PICOTS) items to define publication inclusion/exclusion criteria.
Two independent investigators screened the title and abstract of all articles to ascertain eligible studies and in the next step, reviewed the full-manuscripts of the selected studies inclusion criteria were: 1. Controlled Trial studies in human adults (either parallel or crossover designs); 2. Studies measured circulating IGF-1 at baseline and end of treatment, or reported IGF-1 change for intervention/control groups 2. Compared oral zinc administration with the control group. Moreover, studies not providing concentrations of IGF-1 pre and post treatment, animal studies, studies without a comparative group, review articles, abstracts from conferences, and commentaries were excluded.
2.3 Data extraction
Two reviewers performed the data extraction and a chief researcher resolved any disputes with discussion. Study authors, country, year of publication, sample size of studies, participants’ gender, duration of intervention, mean age, study design (parallel/cross-over), zinc dosage, and averages and associated standard deviations (SD) of IGF-1 circulation at beginning, end intervention or/and alterations between beginning and end intervention.
2.4 Quality assessment
The quality assessment of RCT’s were surveyed by means the Cochrane Collaboration’s tool (Higgins et al., 2011), which composed of the following tenets: 1) allocation concealment, 2) random sequence generation, 3) incomplete outcome data, 4) blinding of participants, 5) personnel, blinding of outcome assessment, 6) selective reporting, 7) and other possible sources of biases.
2.5 Statistical analysis
Weighted mean differences (WMD) in addition to 95% CI were used to assess effect of zinc supplementation on IGF-1 levels. If the mean change SD was not identifiable in the included trials, we utilized the next formula to calculate it: SDalteration = square root [(SD baseline*SDbaseline + SDfinal*SDfinal) − (2 × R × SDbaseline × SDfinal)] (Borenstein et al., 2009). Pooled WMD from qualified trials was calculated using the derSimonian and Laird random-effects approach. We evaluated the heterogeneity between studies using the Q-test and the I-squared statistic with significant levels set at a p-value <0.10. Subgroup analyses were used to identify the sources of heterogeneity among the included studies. Publication bias was discovered through funnel plot examination, in addition to Egger’s method and Begg’s method, respectively. The possible impact of zinc dosage and treatment duration was assessed by means fractional polynomial approaches within non-linear dose-response analyses, in addition to conducting a meta-regression. All statistical analyses were implemented utilizing Stata program (Stata Corp. College Station, Texas, USA) and an a priori p-value ≤ 0.05 was used to demarcate statistical significance.
3 Results
The initial search yielded 606 studies from PubMed/Med-line, Scopus, Web of Science, and Embase. After duplicates were removed, 317 studies remained for further assessment. Following screening against inclusion criteria, 286 publications were excluded and 31 trials were eligible for full-text extraction. Subsequently, 21 studies were ruled out for the following reasons: 1) no data of interest were evident 2) and non-randomized controlled trial (RCT) design, Lastly, ten publications were inserted in the quantitative meta-analysis (Adriani and Wirjatmadi, 2014; Berger et al., 2015; Blostein-Fujii et al., 1997; Clark et al., 1999; Diaz-Gomez et al., 2003; Hershkovitz et al., 1999; Ninh et al., 1996; Nishiyama et al., 1999; Porto et al., 2000; Rodondi et al., 2009).
3.1 Study characteristics
Features of all included trials are summarized in Table 1. There were 247 and 218 sample size in zinc and control groups, respectively. Dose of zinc intervention ranged between 0.37 and 34 mg/day. Two arms were employed in the USA (Berger et al., 2015; Blostein-Fujii et al., 1997), one in Indonesia (Adriani and Wirjatmadi, 2014), one in the UK (Clark et al., 1999), one in Vietnam (Ninh et al., 1996), one in Switzerland (Rodondi et al., 2009), one in Spain (Diaz-Gomez et al., 2003), one in Brazil (Porto et al., 2000), one in Japan (Nishiyama et al., 1999) and one in Israel (Hershkovitz et al., 1999). Included studies were published between 1996 and 2015. The mean follow up of treatment was 12 weeks. All publication were clinical trials. Most publications were implemented on both genders (Adriani and Wirjatmadi, 2014; Diaz-Gomez et al., 2003; Hershkovitz et al., 1999; Ninh et al., 1996; Porto et al., 2000; Rodondi et al., 2009) and four conducted on women (Berger et al., 2015; Blostein-Fujii et al., 1997; Clark et al., 1999; Nishiyama et al., 1999). Table 2 detailed the summary results of the quality assessment of meta analyses. Three studies were of fair quality (Blostein-Fujii et al., 1997; Clark et al., 1999; Rodondi et al., 2009), 6 were of good quality (Adriani and Wirjatmadi, 2014; Berger et al., 2015; Diaz-Gomez et al., 2003; Hershkovitz et al., 1999; Ninh et al., 1996; Porto et al., 2000), and one was of poor quality (Nishiyama et al., 1999).
Author
Country (year)
Participants
Duration (week)
Gender
Sample Size case/placebo
Dose
Berger et al.
USA (2015)
Premenarcheal Girls
4
female
75/75
9 mg.day
Adriani et al.
Indonesia (2014)
stunted children
24
both
12/12
0.37 mg.day
Rodondi et al.
Switzerland (2009)
FRAIL ELDERLY
4
both
25/22
30 mg.day
Az-Gomez et al.
Spain (2003)
Preterm Infants
24
both
18/16
10 mg.day
Porto1 et al.
Brazil (2000)
Children with Short Stature
24
both
18/18
5 mg.kg.day
Nishiyama et al.
Japan (1999)
Pregnant Women
8
female
17/10
34 mg.day
Hershkovitz et al.
Israel (1999)
Infants with Nonorganic Failure to Thrive
12
both
14/11
2 mg.kg.day
Blostein et al.
USA (1997)
women with noninsulin-dependent diabetes mellitus
3
female
20/20
30 mg.day
Clark et al.
UK (1999)
pubertal girls
6
female
24/19
15 mg.day
Ninh et al.
Vietnam (1996)
children
20
both
24/18
10 mg.day
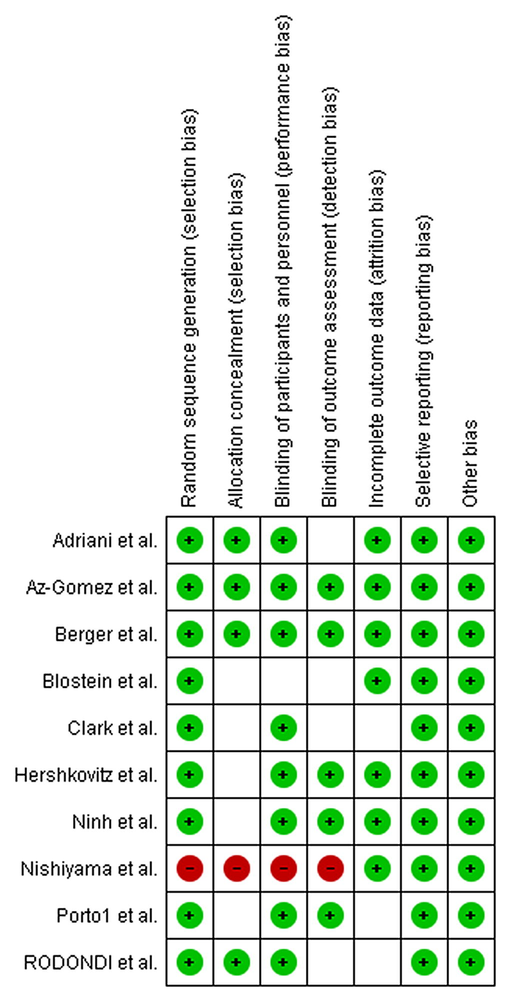
3.2 Meta-analysis results
Ten studies providing a total of 465 (case = 247, control = 218) individuals published alterations in IGF-1 plasma levels as an outcome measure. The random-effects model asserted an significant elevate in IGF-1 after zinc administration (WMD: 8.620 ng/ml, 95% CI: 1.126–16.113, p = 0.024; Fig. 1). Nevertheless, a significant high of heterogeneity was discovered in the meta-analysis (p = 0.000, I2 = 97.3%).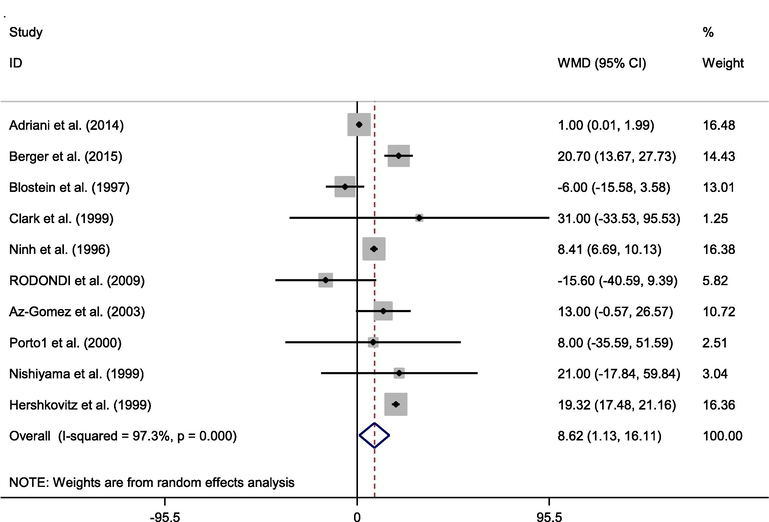
Forest plot of randomized controlled trials investigating the effects of zinc supplementation on IGF-1 levels.
3.3 Subgroup analysis
We stratified trials across zinc intake dosage and intervention duration (week) (Table 3). The analyses demonstrated that zinc intake dosage ≤10 mg/day (WMD: 9.50 ng/ml, 95% CI: 1.47–17.53, I2 = 98.5%) increased IGF-1 significantly more than zinc intake dosage >10 mg/day (WMD: 2.45 ng/ml, 95% CI: −18.56–23.47, I2 = 17.5%). Furthermore, follow up duration >8 weeks (WMD: 10.08 ng/ml, 95% CI: 0.67–19.48, I2 = 98%) improved IGF-1 significantly more than ≤8 weeks (WMD: 6.27 ng/ml, 95% CI: 12.67–25.21, I2 = 83%). Additionally, among preterm infants, zinc administration resulted in a greater increase in IGF-1 (WMD: 19.20 ng/ml, 95% CI: 4.99–6.54, I2 = 0.0%) than children (WMD: 9.26 ng/ml, 95% CI: 2.24–16.27, I2 = 94%); however, it did not influence IGF-1 levels in adult subjects (WMD: −5.48 ng/ml, 95% CI: −17.43–6.46, I2 = 17%).
Group
No of comparisons
WMD (95% CI)
P value
P-heterogeneity
I2 (%)
Zinc supplementation dosage (mg/day)
≤10
6
9.50 (1.47–17.53)
0.020
0.000
98.5
˃10
4
2.45 (−18.56 to 23.47)
0.819
0.304
17.5
Intervention duration (week)
≤8
5
6.27 (−12.67 to 25.21)
0.517
0.000
83.6
˃8
5
10.08 (0.67–19.48)
0.036
0.000
98.7
Type of population
Preterm Infants
2
19.20 (17.37–21.03)
0.000
0.366
0.0
Children
5
9.26 (2.24–16.27)
0.010
0.000
94.9
adult
3
−5.48 (−17.43 to 6.46)
0.368
0.298
17.4
3.4 Dose-response analysis and meta regression
Dose-response analysis and meta regression of the follow-up duration and zinc intake dosage with alterations in plasma IGF-1 did not reveal a significant association (Fig. 2).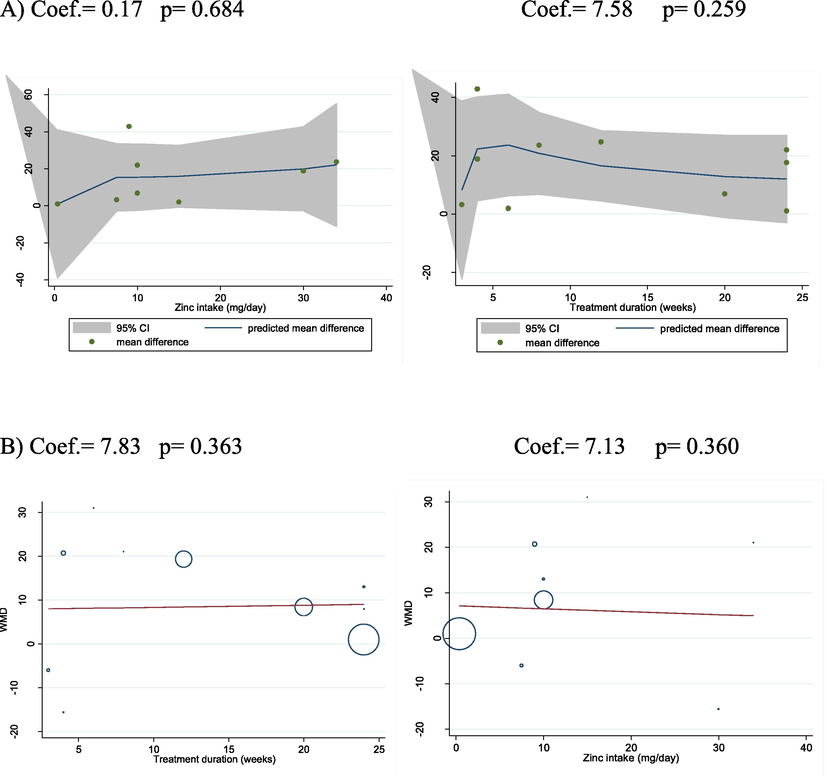
A) Dose-response analysis – zinc intake dosage (mg/day) and intervention duration (week) with IGF changes. Weighted mean difference, WMD, B) Meta regression analysis (Zinc intake dosage (mg/day) and intervention duration (week) with IGF changes).
3.5 Sensitivity analysis and publication bias
The Begg’s and Egger’s tests, did not highlight any publication bias in the meta-analysis (p = 0.721 and p = 0.531, respectively). Visual inspection of funnel plot also demonstrated no evidence of the presence of publication bias (Fig. 3). To explore the influence of any individual study on the pooled effect, we iteratively omitted each study and assessed the impact. Accordingly, we found no significant effects of any one trial on the overall effect.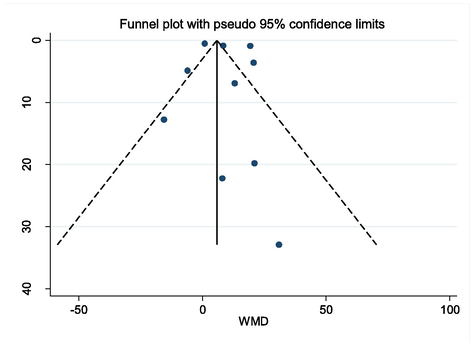
Funnel plot of the weighted mean difference (WMD) versus the s.e. of the weighted mean difference (WMD).
4 Discussion
Our meta-analysis of 10 clinical trials, which included 465 subjects, revealed that zinc supplementation yields a significant rise in IGF-1 levels in humans. Our findings from subgroup analyses suggested that the effect of zinc supplementation on levels of IGF-1 in humans depends, not only on the dosage, but also on the duration of the intervention and on the age of the participants.
Our results show that daily zinc intake ≤10 mg increased IGF-1 significantly more versus a daily intake of zinc >10 mg/day. Thus, we can infer that the dose of daily zinc is an important factor in increasing IGF-1 levels and that an excessive daily intake of zinc does not yield any considerable benefits. However, some studies have argued that zinc supplementation has no effect on IGF-1 levels. Our results contradict the findings of a prior study by Barffour et al. who concluded that the administration of zinc (in 7 mg tablets or micronutrient powder consisting of 10 mg zinc + 6 mg iron +13 other micronutrients) in 419 Laotian children did not increase IGF-1 levels (Barffour et al., 2019). Normal daily intake of zinc in humans is estimated at 14–30 mg/day, but values between 2.8 and 40 mg/day can, reportedly, yield physiological zinc homeostasis, whilst excess zinc is eliminated mainly via the gastrointestinal tract (Roohani et al., 2013).
Further, we showed that zinc supplementation >8 weeks increased IGF-1 significantly more versus zinc supplementation ≤8 weeks. Our findings might be related to the prior serum zinc concentrations of the subjects who were given zinc supplements, and that a supplementation of >8 weeks is required to replenish zinc deposits in zinc deficient patients. Zinc supplementation increases IGF-1 levels in both zinc-deficient and normal, non-zinc-deficient, subjects (Rocha et al., 2015). However, previous reports have asserted that zinc supplementation is more effective in patients who have a zinc deficiency and non-normal serum zinc levels. Park et al. studied the effect of zinc supplementation on IGF-1 levels in children diagnosed with failure-to-thrive; however, study cessation, they found no significant modifications in serum IGF-1 levels in these participants, attributing this result to the fact that the study group had normal zinc and IGF-1 values prior to the zinc intervention (Park et al., 2017).
The most prominent finding of the present study was that the influence of zinc supplementation on IGF-1 levels depends on the age category in which the intervention was delivered. Among preterm infants, zinc administration resulted in a greater increase in IGF-1 levels versus children, whereas it did not influence IGF-1 levels in adults. Firstly, these results confirm the hypothesis of Akram et al., that zinc is an essential factor in preventing premature birth (Akram et al., 2011). Zinc is an essential trace element for human health, earning its colloquial title of ‘the metal of life’, due to its participation in cell growth, immunity, tissue repair, synthesis of proteins and of the DNA, thyroid gland and optimal bone functioning (Kaur et al., 2014; Maggio et al., 2013). Along with proteins, phosphorus, magnesium, sodium and potassium, zinc is a type 2 nutrient whose deficiency results in the inhibition of linear growth (Millward, 2017). Zinc is required as early as fetal-placental development, where subjects who are small for their gestational age have lower body mass index, hemoglobin, iron, zinc and placental protein levels of IGF-1 as compared to infants measured as large for gestational age group (Akram et al., 2011). Thus, the authors suggested that zinc supplementation in pregnancy might decrease preterm birth risk, and elicit a positive impact on the outcome of the pregnancy and on the infant’s birthweight (Akram et al., 2011). Interestingly, in murine models with zinc deficiency, IGF-1 levels can be corrected by stimulating caloric intake or by external administration, but these measures do not correct the growth retardation (MacDonald, 2000).
Our results reinforce the concept that zinc supplementation provides benefits in children with growth disturbances related to zinc deficiency. Hamza et al. demonstrated that zinc supplementation leads to an increase in IGF-1 levels in Egyptian children who had a serum zinc deficit and short stature. In Hamza et al, the authors administered zinc supplements for a period of 3 months in 50 zinc-deficient pre-pubertal children and observed an elevate in serum zinc in 100%, IGF-1 concentrations in 40% and IGFBP-3 levels in 40% of the study group, respectively, as well as an elevation of the height standard deviation score. However, despite the zinc supplementation, normal ranges of serum zinc, IGF-1 and IGFBP-3 were only noted in 64%, 40% and 22% of the children, respectively. Thus, an intervention >3 months might have been required in these children to normalize the concentrations of serum zinc, IGF-1 and IGFBP-3 (Hamza et al., 2012). Imamoglu et al. also confirmed the stimulatory effect of zinc treatment on IGF-1 levels, where 22 pre-pubertal children who received zinc supplements for 6 weeks registered an elevate in IGF-1 and IGFBP-3 concentrations as compared to baseline. On the other hand, children who had normal zinc levels and continued zinc supplementation for 6–12 months did not register higher standard deviation scores for weight or height, implying that zinc supplementation provides greater benefits mainly in zinc-deficient subjects (Imamoglu et al., 2005).
We also demonstrated that zinc supplementation at an older age did not increase IGF-1 levels. Maintaining high or low levels of IGF-1 in adults and the elderly remains a controversial topic, lacking consensus. Zinc is a micronutrient of paramount importance to the bioactivity of IGF-1, nevertheless, it should be taken into account that an increased bioactivity of IGF-1 has been linked to several types of malignancies, such as breast, prostate or colorectal cancer (Rahmani et al., 2019). On the other hand, there is evidence suggesting that higher IGF-1 are cardio- and neuro-protective and thus beneficial (Janssen, 2018). Contrastingly, a meta-analysis by Burgers et al. suggested that, in adults, both decreased IGF-1 and increased IGF-levels are linked to increased all-cause mortality. Their research included 12 studies enrolling 14,906 subjects and demonstrated, using a dose–response meta-regression, that there is a U-shaped relationship between all-cause mortality and IGF-1 levels (P = 0.003), as well as cardiovascular and cancer-related mortality (Burgers et al., 2011; Gunawardane et al., 2015). In adults, the effects of zinc on IGF-1 seem to have a different outcome versus children. These observations might be attributed to the installment of the somatopause during which GH and IGF-1 levels gradually decrease (Maggio et al., 2013). Thus, further studies are necessary to elucidate the roles of IGF-1 in health and disease, as well as the crosstalk between zinc supplementation and IGF-1 in humans.
Our study has several limitations, however; for instance, there was large heterogeneity in study design, where a wide array of zinc dosages, and study durations were employed. Moreover, the studies included a range of subjects and ages, including women with noninsulin-dependent diabetes mellitus, frail elderly, children and preterm infants, all of whom may conceivably demonstrate differing concentrations of IGF-1, although this issue is, at least in part, moderated by considering modifications from baseline. Moreover, despite these heterogeneities, the random-effects model methodology used in the present study represents a significant benefit in being able to control for such factors. In addition, according to the results of current study, more studies are needed in relation to zinc intake in the elderly, because zinc supplementation may be associated with prolonged life and aging process.
5 Conclusion
The present study highlighted that zinc supplementation leads to a significant increase in IGF-1 in humans. In addition, greater increments were observed when zinc intake dosage was ≤10 mg/day and intervention duration ˃8 weeks.
Acknowledgment
The authors reported no funding received for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effect of adding zinc to vitamin A on IGF-1, bone age and linear growth in stunted children. J. Trace Elem. Med Biol.. 2014;28(4):431-435.
- [CrossRef] [Google Scholar]
- Placental IGF-I, IGFBP-1, zinc, and iron, and maternal and infant anthropometry at birth. Acta Paediatr.. 2011;100(11):1504-1509.
- [Google Scholar]
- GH/IGF-1 signaling and current knowledge of epigenetics; a review and considerations on possible therapeutic options. Int. J. Mol. Sci.. 2017;18(10)
- [CrossRef] [Google Scholar]
- Positive effects of zinc supplementation on growth, GH, IGF1, and IGFBP3 in eutrophic children. J. Pediatr. Endocrinol. Metab.. 2012;25(9–10):881-887.
- [CrossRef] [Google Scholar]
- Effects of zinc alone versus zinc-containing multiple micronutrient powder on insulin-like growth factor 1 (IGF1) and IGF binding protein-3 (IGFBP3) in laotian children (OR07-05-19) Curr. Dev. Nutr.. 2019;3(Suppl. 1)
- [CrossRef] [Google Scholar]
- Zinc supplementation increases procollagen type 1 amino-terminal propeptide in premenarcheal girls: a randomized controlled trial. J. Nutr.. 2015;145(12):2699-2704.
- [CrossRef] [Google Scholar]
- Prophylactic subacute administration of zinc increases CCL2, CCR2, FGF2, and IGF-1 expression and prevents the long-term memory loss in a rat model of cerebral hypoxia-ischemia. Neural Plasticity. 2015;2015:375391
- [CrossRef] [Google Scholar]
- Short-term zinc supplementation in women with non-insulin-dependent diabetes mellitus: effects on plasma 5'-nucleotidase activities, insulin-like growth factor I concentrations, and lipoprotein oxidation rates in vitro. Am. J. Clin. Nutr.. 1997;66(3):639-642.
- [CrossRef] [Google Scholar]
- Borenstein, M., Hedges, L. V., Higgins, J., & Rothstein, H. R. (2009). References: Wiley Online Library.
- Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J. Clin. Endocrinol. Metab.. 2011;96(9):2912-2920.
- [Google Scholar]
- Effects of oral zinc supplementation on zinc status and catch-up growth during the first 2 years of life in children with non-organic failure to thrive born preterm and at term. Pediatrics Neonatol.. 2019;60(2):201-209.
- [CrossRef] [Google Scholar]
- Zinc supplementation and bone growth in pubertal girls. Lancet. 1999;354(9177):485.
- [CrossRef] [Google Scholar]
- The effect of zinc supplementation on linear growth, body composition, and growth factors in preterm infants. Pediatrics. 2003;111(5):1002-1009.
- [CrossRef] [Google Scholar]
- Zinc intake and status and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Nutrients. 2019;11(5)
- [CrossRef] [Google Scholar]
- Gunawardane, K., Hansen, T.K., Christiansen, J.S., Jorgensen, J.O.L. (2015). Normal physiology of growth hormone in adults. In: Endotext [Internet]: MDText. com, Inc.
- Effect of zinc supplementation on growth hormone-insulin growth factor axis in short Egyptian children with zinc deficiency. Italian J. Pediatrics. 2012;38:21.
- [CrossRef] [Google Scholar]
- Role of insulinlike growth factor 1 in fetal development and in the early postnatal life of premature infants. Am. J. Perinatol.. 2016;33(11):1067-1071.
- [CrossRef] [Google Scholar]
- Zinc supplementation increases the level of serum insulin-like growth factor-I but does not promote growth in infants with nonorganic failure to thrive. Horm. Res.. 1999;52(4):200-204.
- [Google Scholar]
- The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928
- [Google Scholar]
- Associations between zinc deficiency and metabolic abnormalities in patients with chronic liver disease. Nutrients. 2018;10(1)
- [CrossRef] [Google Scholar]
- Effect of zinc supplementation on growth hormone secretion, IGF-I, IGFBP-3, somatomedin generation, alkaline phosphatase, osteocalcin and growth in prepubertal children with idiopathic short stature. J. Pediatr. Endocrinol. Metab.. 2005;18(1):69-74.
- [Google Scholar]
- Janssen, J.A., 2018. IGF-I and the Endocrinology of Aging. Current Opinion in Endocrine and Metabolic Research.
- The role of zinc in growth and cell proliferation. J. Nutr.. 2000;130(5S Suppl):1500s-1508s.
- [CrossRef] [Google Scholar]
- IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients. 2013;5(10):4184-4205.
- [CrossRef] [Google Scholar]
- Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res. Rev.. 2017;30(1):50-72.
- [Google Scholar]
- Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. System. Rev.. 2015;4(1):1.
- [Google Scholar]
- Trajectories of function and biomarkers with age: the CHS All Stars Study. Int. J. Epidemiol.. 2016;45(4):1135-1145.
- [Google Scholar]
- Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am. J. Clin. Nutr.. 1996;63(4):514-519.
- [CrossRef] [Google Scholar]
- Zinc and IGF-I concentrations in pregnant women with anemia before and after supplementation with iron and/or zinc. J. Am. Coll. Nutr.. 1999;18(3):261-267.
- [Google Scholar]
- Effects of zinc supplementation on catch-up growth in children with failure to thrive. Nutrit. Res. Practice. 2017;11(6):487-491.
- [CrossRef] [Google Scholar]
- Linear growth and zinc supplementation in children with short stature. J. Pediatr. Endocrinol. Metab.. 2000;13(8):1121-1128.
- [Google Scholar]
- Molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants (Basel). 2019;8(6)
- [CrossRef] [Google Scholar]
- The influence of fasting and energy restricting diets on IGF-1 levels in humans: a systematic review and meta-analysis. Age. Res. Rev.. 2019;53:100910
- [CrossRef] [Google Scholar]
- Effect of zinc supplementation on GH, IGF1, IGFBP3, OCN, and ALP in non-zinc-deficient children. J. Am. Coll. Nutr.. 2015;34(4):290-299.
- [CrossRef] [Google Scholar]
- Zinc increases the effects of essential amino acids-whey protein supplements in frail elderly. J. Nutrit., Health Aging. 2009;13(6):491-497.
- [CrossRef] [Google Scholar]
- Zinc and its importance for human health: an integrative review. J. Res. Med. Sci.. 2013;18(2):144.
- [Google Scholar]
- ROLE of IGF-1 system in the modulation of longevity: controversies and new insights from a centenarians’ perspective. Front. Endocrinol.. 2019;10:27.
- [CrossRef] [Google Scholar]
- Trajectories of plasma IGF-1, IGFBP-3, and their ratio in the Mayo Clinic Study of Aging. Exp. Gerontol.. 2018;106:67-73.
- [Google Scholar]
- Zinc and its regulators in pancreas. Inflammopharmacology. 2019;27(3):453-464.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.01.018.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







