Translate this page into:
The highly efficient green synthesis of nanostructured ZnFe2O4 photocatalysts by using Ziziphus mauritiana and Salvadora persica extracts for the photocatalytic degradation of crystal violet in sunlight
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The current study is aimed to investigate the photocatalytic degradation of crystal violet dyes solution in the presence of modified ZnFe2O4 photocatalysts by Ziziphus mauritiana and Salvadora persica extracts in sunlight. Regardless of the extracts used, the modified ZnFe2O4 catalysts demonstrated excellent photocatalytic degradation. However, the catalysts modified using Salvadora persica extract achieved superior photocatalytic degradation of crystal violet dye. ZnFe2O4 with Salvadora persica yielded smaller particles with a larger surface area, smaller band gap energy and a better distribution of differently sized particles than by using Ziziphus mauritiana. This photocatalyst also demonstrated stability, being able to be reused at least five times with minimal loss of catalytic ability, falling from 91% at the first iteration to 85% by the fifth reuse. These materials were prepared easily using an environmentally friendly method.
Keywords
ZnFe2O4
Extracts
Degradation
Crystal violet
Sunlight
1 Introduction
Today, the water contamination and the energy crisis are the major concerns and they lies in the remarkable processes of photocatalysis (Mehtab et al., 2022a). The methods of synthesis of nanoparticles along with the photocatalytic degradation strategy have been broadly used to eliminate of harmful industrial pollutants and colour in the wastewater (Haseena et al., 2022) by elimination of recombination of electron and hole to their utilization in the photocatalytic degradation and water splitting reactions (Khan et al., 2022; Mehtab et al., 2022b; Shanavas et al., 2022). One is the most important and promising development is green synthesis, as it enables the creation of photocatalysts, antibacterial compounds and biofuels (Ali et al., 2021; Mehtab et al., 2022b), in a manner that is non-toxic and environmentally friendly, using water as the solvent and compounds extracted from leaves (Alhalili, 2022). Furthermore, these methods of synthesis require lower pressures and temperatures than traditional synthesis methods (Álvarez-Chimal et al., 2022; Dave et al., 2021).
For example, by using leaf extracts obtained from various plant species, M2+Fe2O4 nanoparticles can be prepared, where M2+ can represent gold (Au), cobalt (Co), nickel (Ni), copper (Cu), or silver (Ag) among other metals (Elumalai et al., 2021; Jaast and Grewal, 2021).
Using zinc to create ZnFe2O4 catalysts is attractive, as these nanoparticles are highly magnetic and electrically stable. Furthermore they have biomedical properties, can be prepared using diverse methods and come in a range of sizes, shapes and purities. These qualities lend these materials to be used in a broad range of applications (Din et al., 2020).
To improve the activity of ZnFe2O4 photocatalysts, different types of plant extract were used such as Indigofera tinetoria (Priya et al., 2020) and Iresine herbstii (Dwivedi et al., 2021). The products were calcined at different calcination temperatures, and the increasing of calcination temperatures led to reduce the particle sizes (Balasubramanian and Murali, 2020) and their photodegradation performances of dyes were increased in sunlight. The extracts were acted as both chelating and reducing agents, yielding products with high catalytic activity, pure phases and good crystallinity and size dispersion. The product also showed good voltammetric responses, having a high electrochemical performance, making it a promising material for use in electrochemical applications (Bishnoi et al., 2018).
Drawing upon the research described in (Khan et al., 2015) and (Asimuddin et al., 2020), and building upon our earlier work (Bayahia, 2022a), ZnFe2O4 was prepared and calcined using 500–900 °C. Maximal dye degradation was achieved 600 °C. In the current study, ZnFe2O4 that had been calcined at 600 °C was modified using leaf extracts from Ziziphus mauritiana and Salvadora persica. The materials were characterized and their activities were investigated in the degradation of CV dye. The findings in this work reveal that the performance of these bionanomaterials is more efficient than that of other nanomaterials modified by plant extracts.
2 Experimental
2.1 Materials and methods
All chemicals were of analytical grade and used in the state as purchased, and did not undergo addition or modification. p-Toluenesulfonic acid (C7H8O3S·H2O) was purchased from LOBA CHEMIE PVT.ltd, whilst Fe(NO3)3·9H2O, Zn(NO3)2·6H2O and NaOH were obtained from Sigma-Aldrich. Ziziphus mauritiana and Salvadora persica leaves were collected from the Albaha region of Saudi Arabia. The CV dye was purchased from BHD.
2.2 Preparation of Ziziphus mauritiana and Salvadora persica leaf extracts
Fresh and healthy leaves were rinsed in distilled water then left to dry for 7–10 days at room temperature. Once dry, 10 g of leaves was immersed in 100 mL of 65 °C distilled water and left to infuse for 1 h. The infusion was cooled to room temperature then it was filtered to remove the leaves, which were discarded and used to prepare the ZnFe2O4 photocatalysts.
2.3 Preparation of ZnFe2O4 using leaf extracts
Appropriate quantities of Zn(NO3)2·6H2O and Fe(NO3)3·9H2O were weighed out then added to the plant extract solution. The solution was stirred until the temperature reached 65 °C, whereupon 1 g of C7H8O3S·H2O was added, and stirred continuously for 1 hr; the temperature was kept constant at 65 °C. Co-precipitation was instigated by adding 1 M of NaOH solution in a dropwise manner until pH 12. the precipitate was retrieved, filtered and rinsed in distilled water before rinsing once in ethanol. The solids were dried overnight at 120 °C then annealed at 600 °C for 24 h.
2.4 Characterisation techniques
FT-IR Spectrometry (PerkinElmer Spectrum 100) was conducted using the KBr pellet method, in the range of 400–4000 cm−1. X-ray diffraction analysis was performed using a Brucker D8 advanced powder diffractometer instrument; the diffractometer used Cu Kα radiation (λ = 1.5416 Å) at the 0.2θ scale, in the range of 10°–80°, with a scanning step of 0.2° at 45 kV and 40 mV. Scanning electron microscopy (SEM) analysis was conducted using a Hitachi S-4800 device (Tokyo, Japan). Also, a JEOL (JEM-2100F) transmission electron microscope (TEM) was used. A UV–vis spectrophotometer (Philips 8800). The Micromeritics Tristar II 2030 analyzer was used for measuring surface area and porosity.
2.5 Photocatalytic reactions
CV dye solution was using batch reactor at neutral media and under sunlight. 15 mg of photocatalyst was added to 20 mL. The mixture was kept in darkness for 30 min to reach the equilibrium. Then the mixture was exposed to sunlight. Repeated reactions were conducted every-five minutes until all the dye had completely degraded. Then the separated and clear solution was analysed using UV–vis spectroscopy. Equation (1) was used to calculate the percentage of degradation.
3 Results and discussion
3.1 FT-IR analysis
FT-IR was used to identify the functional groups that contributed to the reduction and stabilisation of the synthesized photocatalytic materials (Shanavas et al., 2021; Nasiri et al., 2022). Fig. 1 shows the IR spectra for Salvadora persica and Ziziphus mauritiana extracts to be in the range of 500–4000 cm−1, it also shows the spectra for the extract-modified ZnFe2O4 nanoparticles. The peaks reflect the OH, C—O, CH and C⚌O functional groups. Salvadora persica presents broad peaks of free O—H at 3400, C—H at 2369, the bands at 1632 and 1063 cm−1 donate the C⚌O and C—O respectively and stretching vibrations of C—H at 1439 cm−1 (Elumalai et al., 2021).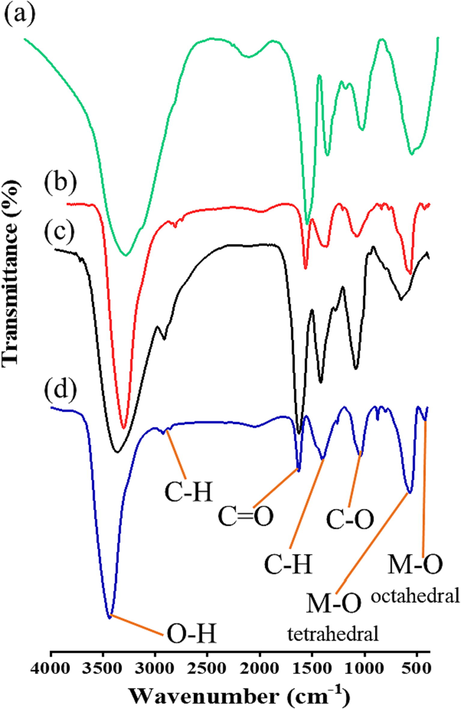
FT-IR spectra of (a) Salvadora persica extract; (b) ZnFe2O4 modified by Salvadora persica extract; (c) Ziziphus mauritiana extract; (d) ZnFe2O4 modified by Ziziphus mauritiana extract.
The spectra of modified ZnFe2O4 show peaks that reflect C—H stretch at 2915 cm−1 and C⚌O stretching band at 1632 cm−1. A broad peak at 3440 cm−1 is attributed to the stretching vibrations of O—H bonded and —NH2 functional groups (Cheperli et al., 2022). Meanwhile, the band at 400–850 cm−1 is assigned to M—O stretching vibrations (Din et al., 2020).
These results indicate that the extract contains phytogenic such as alkaloids, flavonoids, glycosides, tannins and phenolics (Priya et al., 2020). These functional groups are reported cap ZnFe2O4, which facilitates the particles’ photocatalytic activityThese functional groups are reported cap ZnFe2O4, which facilitates the particles’ photocatalytic activity. These results are consistent with those published elsewhere (Xiu et al., 2022).
3.2 XRD analysis
Fig. 2 presents the XRD results of modified and pure ZnFe2O4 photocatalyst. Pure ZnFe2O4 has a crystalline structure and sharp XRD peaks and modifying ZnFe2O4 with plant extracts reduced the crystallinity of ZnFe2O4. This can be explained by carbon annealing at 600 °C, resulting in an amorphous structure. The diffraction patterns at 30.14, 35.54, 36.53, 43.15, 53.41, 56.93 and 62.35, which are indexed to (2 2 0), (3 1 1), (2 2 2), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) lattice planes indicate that pure ZnFe2O4 nanostructures have a face-centred cubic structure. The assigned diffraction lines are in excellent agreement with corresponding JCPDS No: 00–022-1012 (Bayahia, 2022a). The peaks at 22.91 and 24.09, which are assigned to Fe2O3 impurities that exhibited in the structure of ZnFe2O4 treated by Ziziphus mauritiana extract. The observed pattern of ZnFe2O4 and Fe2O3 are consistent with JCPDS cards of # 22-1012 and #87–1166 respectively (Pakzad et al., 2019).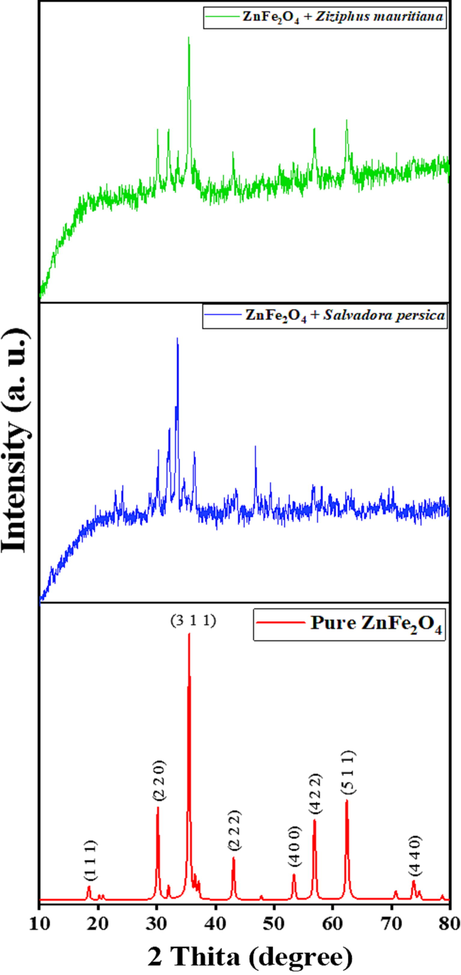
XRD patterns of pure and modified ZnFe2O4 photocatalysts.
On the basis of the half-width of the 3 1 1 reflection in the powder pattern, the average grain size, which was calculated using the Debye-Scherer formula: D = 0.9λ/β cos θ, where D is the crystalline size, λ is the wavelength (1.54, β is the width of the XRD peak posted at 35.54° at half maximum height (FWHM)) and θ is the Bragg diffraction angle (Bayahia, 2022b). Table 1 presents the XRD results of the nanoparticles crystalline grain sizes.
Sample
Position of highest peak (deg.)
Crystalline Size (nm)
Average particle size (nm)
Optical Band Gap energy (eV)
ZnFe2O4 Ziziphus mauritiana
35.49
18.39
7.59
1.37–2.12
ZnFe2O4 Salvadora persica
33.59
17.30
5.95
1.43–1.82
3.3 Morphological analysis
3.3.1 SEM and EDS analysis
The results of the SEM analysis of the modified ZnFe2O4 are shown in Fig. 3(a) and (b). This reveals that all of samples have broadly spherical morphologies with numerous agglomerations of flakes. Due to the low magnification of the SEM images, it was difficult to obtain measurements of the size of the particles. Treating the ZnFe2O4 nanoparticles with different plant extracts yielded products that differed in their morphologies. As Fig. 3(a) demonstrates, treating ZnFe2O4 with Ziziphus mauritiana extracts produced coarser particles than treating the nanoparticles with Salvadora persica, which were finer and more spherical in shape (Fig. 3(b)).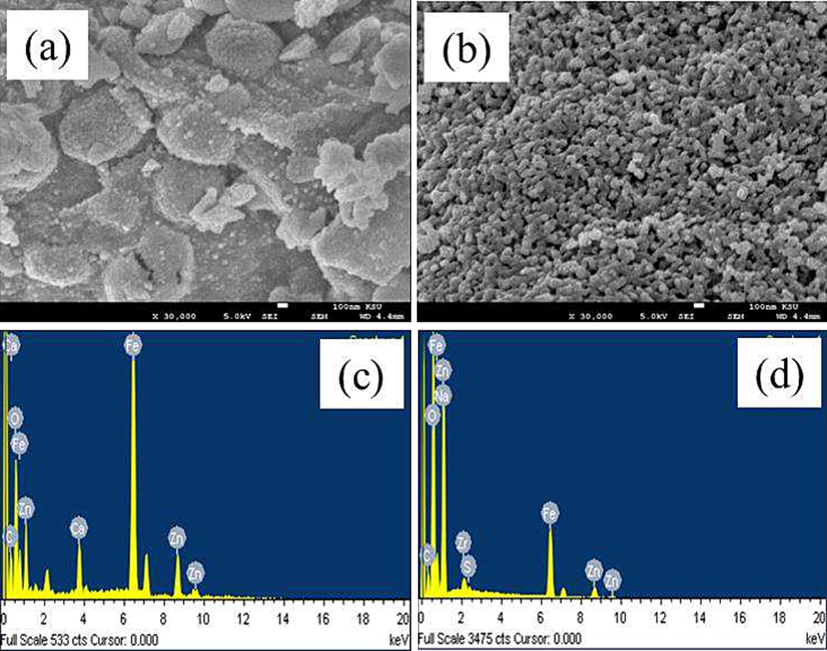
SEM images of ZnFe2O4 modified with (a) Ziziphus mauritiana (b) Salvadora persica; and EDS images of ZnFe2O4 modified with (c) by Ziziphus mauritiana (d) Salvadora persica.
To identify the elemental compositions of the plant-extract modified samples, EDS was used (Fig. 3(c) and (d)). From these images, it can be seen that the samples were pure; the small amount of carbon present was derived from the plant extracts.
The EDS results show that Zn, Fe and O were the only elements present in all of the samples. The plant extracts modified a reasonable percentage of the Zn, Fe and O elements of the ZnFe2O4. The other signals are consistent with the absorption of carbon, confirming the presence of plant extract-derived organic compounds, which act as capping ligands for the photocatalysts. The EDS results presented in Fig. 3(c) and (d) are aligned with others reported elsewhere (Gupta et al., 2020).
3.3.2 TEM analysis
The particle sizes of both pure and modified ZnFe2O4 NPs were measured using TEM (Fig. 4(a) and (b)). The sizes of modified and unmodified photocatalyst were 7.59 nm and 5.95 nm for Pure ZnFe2O4. ZnFe2O4 by Ziziphus mauritiana and ZnFe2O4 by Salvadora persica respectively. The morphologies of the nanoparticles in the images are spherical, indicative of organic compounds being present, which would have come from the plant extracts.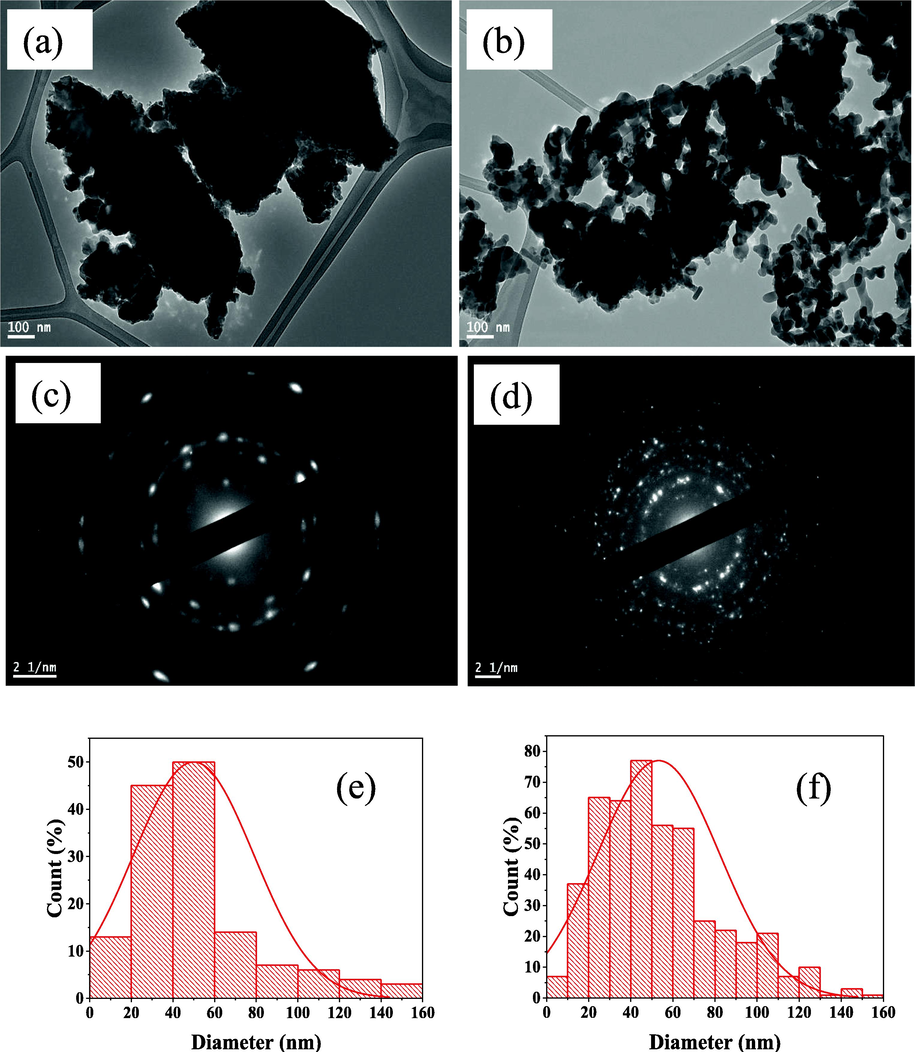
TEM images of ZnFe2O4 modified with (a) Ziziphus mauritiana (b) Salvadora persica. SAED images of ZnFe2O4 modified with (c) Ziziphus mauritiana (d) Salvadora persica. Distribution of different particle sizes for ZnFe2O4 modified by (e) Ziziphus mauritiana (f) Salvadora persica.
The results show that the agglomerations of NPs were excessed in all of the samples. This confirms that the residual organic compounds from the plant extracts were acting as capping ligands for the ZnFe2O4 photocatalysts. Using plant extracts reduced the amount of agglomeration. Using Salvadora persica extract resulted in small diameter particle sizes, which was expected when the capping method was used for ZnFe2O4 synthesis.
Fig. 4(c) and (d) present the results of the selected area electron diffraction (SAED) pattern. The SAED patterns reveal that the prepared materials are polycrystalline structures (Balasubramanian and Murali, 2020). The rings correspond to the crystal planes of (2 2 0), (3 1 1), (4 0 0) (5 1 1) and (4 4 0). As confirmed by analysing the XRD patterns, the prepared nanoparticles were crystalline.
The histograms plots shown in Fig. 4(e) and (f) depict the distribution of the size of the particles created, which was determined by analysing TEM images (Asimuddin et al., 2020). Comparing the two histograms shows that the distribution of ZnFe2O4 modified with Ziziphus mauritiana is narrower in scope than that of ZnFe2O4 modified with Salvadora persica. The difference in the distribution of particle sizes might play a role in photodegradation performance.
3.4 Optical properties analysis
Fig. 5(a) shows that, the prepared materials absorbed light radiation in the range of 480–850 nm, indicating they were active in sunlight. To calculate the band-gap energy the following equation was used: Eg (eV) = 1240/λ (Bayahia, 2022b), where, Eg is the band gap energy, and λ is the incident light wavelength (nm).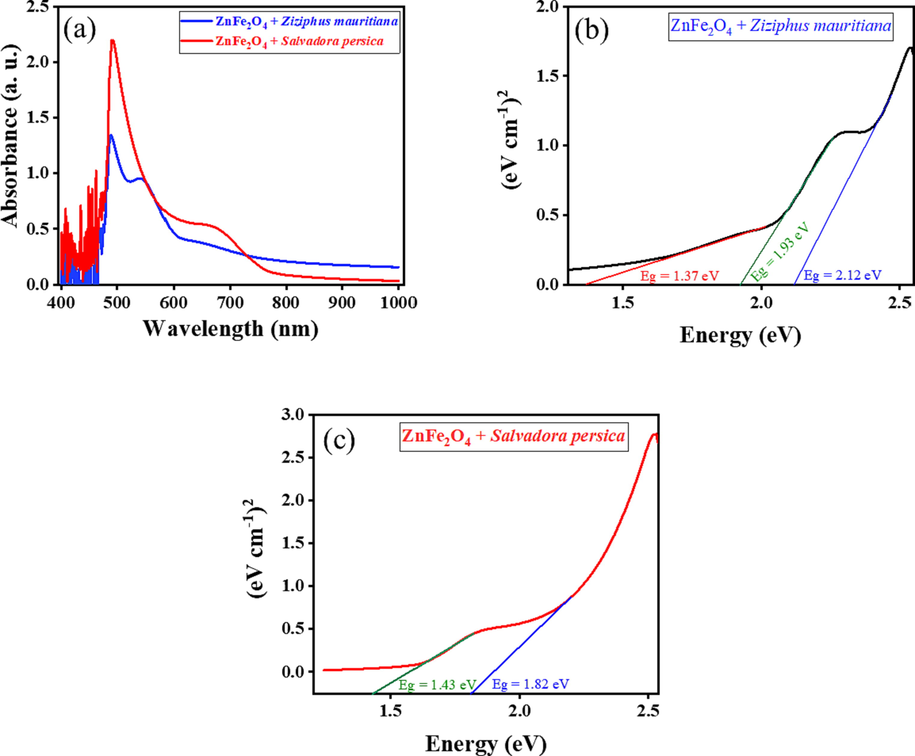
UV–vis absorption spectra (a) and plots of (Ahν)n as a function of photon energy (hν) of ZnFe2O4 treated by plant extracts (b, c).
Based on the results in Fig. 5(a), Tauc plots were used to estimate the value of band gap energies of the ZnFe2O4 modified nanopartilces. The following equation was used: (Ahν)n = B (hν – Eg) where, A is the light absorbance, hν is the photon energy, B is the constant related, and n can be ½ or 2 for direct or indirect transitions, respectively. Table 1 and Fig. 5(b and c) show that the band gaps for ZnFe2O4 modified with plant extracts are lower than the band gaps for pure ZnFe2O4 (Bayahia, 2022a). The band gaps were affected by doping ZnO with Fe; the capping activity of the plant extracts might have reduced the band gap values and Fermi level was achieved due to the excitation of electrons was from valance band (VB) to conduction band (CB).
3.5 Textural properties
The specific BET surface areas, pore volumes and pore diameters of the modified ZnFe2O4 nanoparticles are presented in Table 2. The different extracts resulted in ZnFe2O4 adopting different textural properties. Using the Salvadora persica extract gave the nanoparticles a significantly larger surface area than using the Ziziphus mauritiana extract. Fig. 6 shows that the modified photocatalysts presented as mesoporous structures. The prepared biomaterials in this study are matched the fact of materials with greater surface area and pore volume have great numbers of active site located on the surfaces (Algarni et al., 2022).
Photocatalysts
BET surface area (m2/g)
Pore Volume
(cm3/g)Pore Size (Å)
[CV]
(ppm)Degradation time (min)
kapp (min−1)
t1/2 (min)
ZnFe2O4 + Salvadora persica
3.583
0.0317
533.53
10
12
0.65
1.07
30
25
0.16
4.33
ZnFe2O4 + Ziziphus mauritiana
0.965
0.0079
570.55
10
19
0.13
5.33
30
30
0.10
6.93
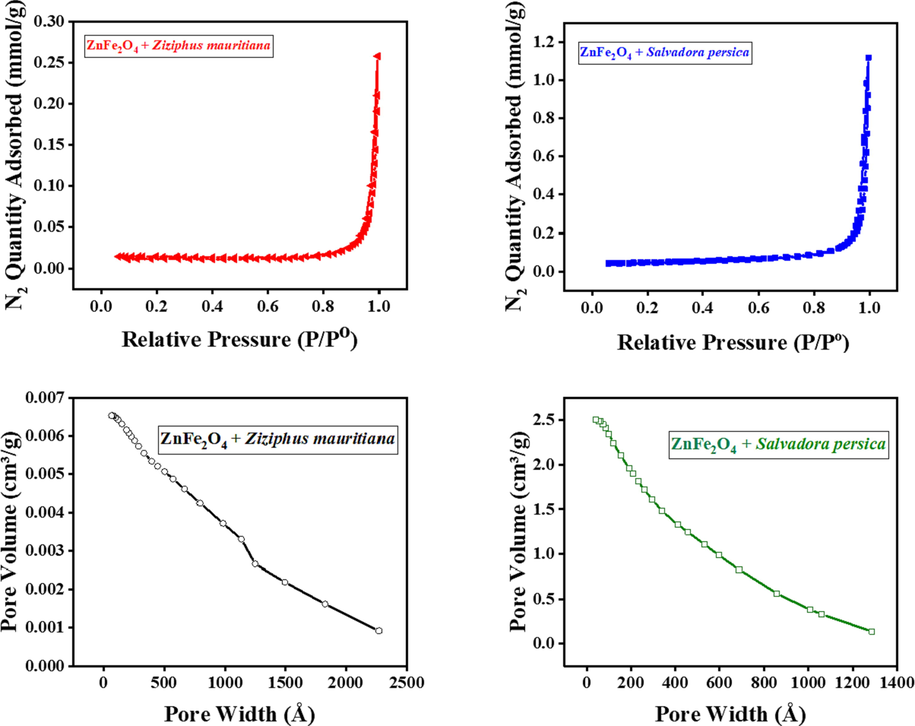
BET results of ZnFe2O4 modified by plant extracts.
3.6 Photocatalytic dye degradation studies
In our previous study (Bayahia, 2022a), the ability of ZnFe2O4 nanoparticles to degrade CV dye in solution was compared against the abilities of CuFe2O4, CoFe2O4 and NiFe2O4 nanoparticles. The photocatalysts were prepared using the co-precipitation method; these were then calcined at temperatures ranging between 500 °C and 900 °C. Maximum catalytic performance was achieved when catalysts were calcined at 600 °C. Consequently, for this study, ZnFe2O4 was selected for modification with Salvadora persica and Ziziphus mauritiana extracts, and calcined at 600 °C.
The ability of ZnFe2O4 treated with to photodegrade CV dye under the visible light conditions was explored. The different plant extracts exhibited different degradative abilities. The plant extracts affected the photocatalytic activity of the nanoparticles. In this instance, treating ZnFe2O4 with Ziziphus mauritiana resulted in poorer elimination of CV dye compared to ZnFe2O4 treated with Salvadora persica; the reaction and sunlight conditions were consistent for both samples.
Fig. 7(a) and (b) shows the dark and in sunlight conditions using 10 ppm of CV dye solutions. No dye was degraded in the blank condition, but there was some degradation of dye in the darkness condition. The results are similar to those described previously (Algarni et al., 2022).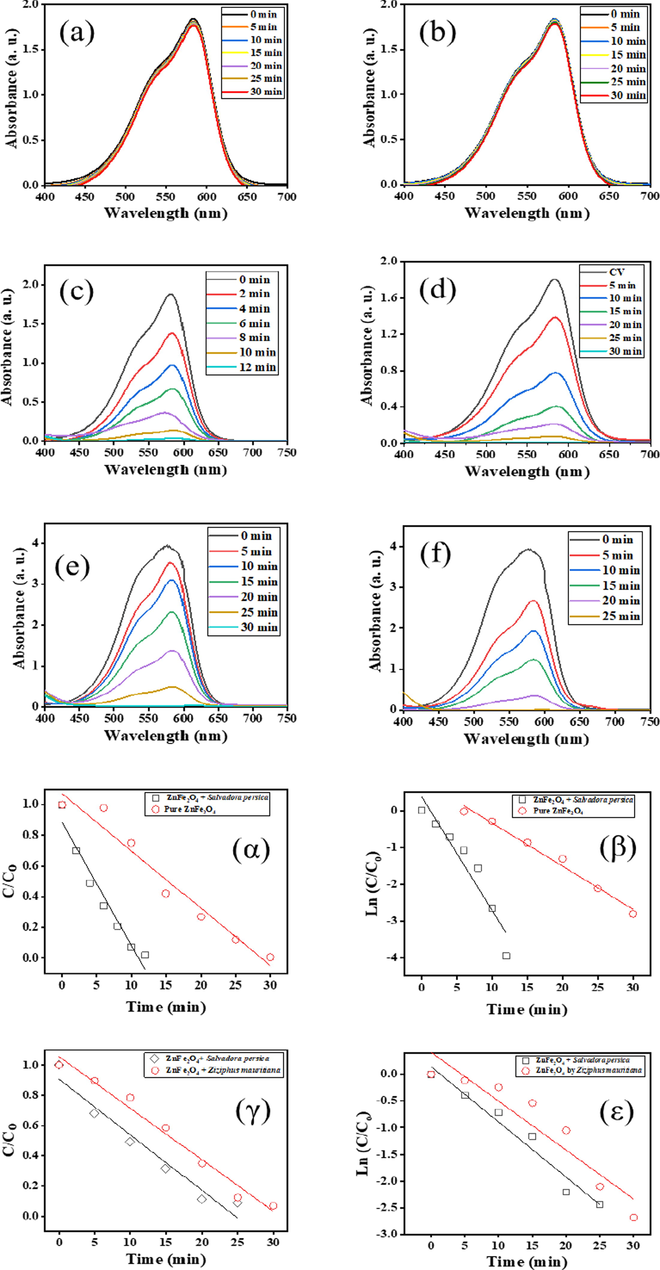
UV–vis spectra of photocatalytic degradation of (a) catalysts in darkness (b) blank (no catalyst) (c) Salvadora persica-modified ZnFe2O4 in 10 ppm CV (d) Pure ZnFe2O4 in 10 ppm CV (e) Ziziphus mauritiana-modified ZnFe2O4 in 30 ppm CV dye (f) Salvadora persica-modiied ZnFe2O4 in 30 ppm CV and C/Co vs time (α, γ) and ln (C/Co) vs time (β, ε) for pure and modified ZnFe2O4 with a CV dye concentration of 10 ppm (top) and 30 ppm (bottom).
For the comparative photocatalytic activity, 15 mg of modified ZnFe2O4 was used under the sunlight conditions. The photocatalytic activities of the modified ZnFe2O4 photocatalysts were improved by using neutral pH and sunlight (Fig. 7c–f). Two concentrations of CV dye (10 and 30 ppm) were loaded with 15 mg of plant extract-modified ZnFe2O4 nanoparticles. ZnFe2O4 with Salvadora persica extract achieved maximum photocatalytic degradation of 10 ppm of CV dye in 12 min (Fig. 7c). For pure ZnFe2O4, the maximum photocatalytic degradation of dye was reached in 30 min (Fig. 7d). This is consistent with earlier studies reported in the literature (Bayahia, 2022a).
The photodegradative ability of Salvadora persica modified photocatalysts declined when the concentration of CV rose to 30 ppm. It reached maximum photodegradation within 25 min, which is longer than when the concentration of dye was 10 ppm. However, the performance of Salvadora persica modified photocatalysts was superior to the catalysts modified with Ziziphus mauritiana at the same concentration and exposed to the same conditions (Fig. 7(e) and (f)).
Several factors influence the photocatalytic degradation of dye in solution, including the duration of exposure, the concentration of the dye and the intensity of the sunlight (Oliveira et al., 2020).
This work shows that the type of plant extract used to modify the photocatalysts is also influential. Compared to ZnFe2O4 modified with Ziziphus mauritiana, samples treated with Salvadora persica were more effective at degrading the dye. The plant extracts have several qualities, acting as stabilisers, reducing agents and the caps for the metals (Lau et al., 2020).
3.7 Kinetics studies
This study explored the photocatalytic rate of ZnFe2O4 modified with plant extract exposed to different concentrations of CV dye. The reactions followed pseudo-first-order kinetics and the initial decomposition reaction was calculated using the pseudo-first-order kinetic law (Lau et al., 2020). where kapp is the rate constant of the pseudo-first-order reaction and t is the reaction time.
Table 2 presents the half-life and rate constant results. Compared to unmodified ZnFe2O4, modified nanoparticles displayed greater photodegradative ability, which is attributed to their morphological, structural and textural properties. Photocatalysts with high crystallite sizes and small particle sizes have fewer surfaced defects, which typically acts as recombination centres for the electron-hole pairs.
The dye concentration significantly affected the photocatalytic degradation reaction undertaken by the nanoparticles. Modified photocatalysts were able to degrade dye concentrations of 10 and 30 ppm in less time than unmodified photocatalysts. ZnFe2O4 modified by Salvadora persica has smaller particle size with lower band gap and higher performance of photocatalytic degradation than ZnFe2O4 modified by Ziziphus mauritiana. Materials with smaller particle sizes have lower band gap and the decreasing of band gap can be increased the oxygen vacancy and the energy level (Deotale and Nandedkar, 2016), which can be decreased recombination of ē and h+ (Yang et al., 2022). The results of C/Co vs time and ln C/Co) vs time plots are presented in Fig. 7(α-ε).
3.8 Mechanism of photocatalytic degradation of CV dye
The proposed mechanisms by which modified ZnFe2O4 nanoparticles photocatalytically degrades CV dye are as follows. First, CV dye adsorbs to the surface of the ZnFe2O4 photocatalysts. When exposed to sunlight, the electrons (ē) in VB gets excited to the CB and hole ions (h+) was generate in the VB of the photocatalysts. Oxygen was reduced by electrons in the CB to become superoxide radicals (•O2) (Álvarez-Chimal et al., 2022; Dwivedi et al., 2021). The h+ attack the water molecules, releasing hydroxyl radicals (•OH). Radicals were attacked CV dye and degraded into CO2 and H2O (Rahmayeni et al., 2021) as equations (3)–(7) and Fig. 8 (left) show.
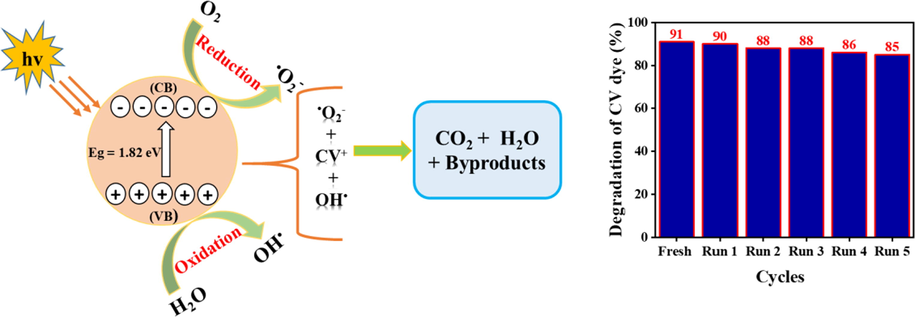
Schematic diagram of the photocatalytic degradation of CV solution (left) and percentage of decline in photodegradation of 30 ppm of CV dye exposed to recovered ZnFe2O4 photocatalysts modified with Salvadora persica extract (right).
3.9 Reusability test
To evaluate the reusability of the ZnFe2O4 photocatalysts created in this study, five iterations of recycling experiments were conducted under the same conditions. Fig. 8 (right) shows the decline in degradative ability of one set of photocatalysts after each cycle of use. After completing a reaction, the photocatalysts were retrieved, rinsed in distilled water and acetone, dried overnight in an oven set at 120° C and tested at the same conditions of fresh one. With each iteration, the photocatalytic degradation of CV dye declined, falling from 91 % in the first cycle, to 85 % in the fifth cycle. This small decline reflects changes to the morphological, optical and textural properties of the photocatalysts. The results of the reusability experiments are similar to those reported by other researchers (Rahmayeni et al., 2021; Malik et al., 2022).
4 Conclusions
This study shows that modifying ZnFe2O4 nanoparticles with Salvadora persica and Ziziphus mauritiana extracts creates nanoparticle products with superior ability to photodegrade CV dye solution in sunlight. The method used to synthesise the materials, their morphological, optical and textural properties and the reaction duration significantly influences the surface structure, optical properties, particle size and particle size distributions of ZnFe2O4 treated with Salvadora persica. The photocatalyst was stable, being able to be used five times without experiencing marked decline in photocatalytic performance.
Acknowledgment
The author would like to thank the Deanship of Scientific Research, Albaha University, Saudi Arabia for the project fund (1442/29).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Algarni, T., Abduh, N. A. Y., al Kahtani, A., & Aouissi, A. (2022). Photocatalytic degradation of some dyes under solar light irradiation using ZnO nanoparticles synthesized from Rosmarinus officinalis extract. In: Green Chemistry Letters and Reviews, vol. 15, issue 2, Taylor and Francis Ltd. pp. 460–473, https://doi.org/10.1080/17518253.2022.2089059.
- Green synthesis of copper oxide nanoparticles CuO NPs from Eucalyptus Globoulus leaf extract: Adsorption and design of experiments. Arab. J. Chem.. 2022;15(5)
- [CrossRef] [Google Scholar]
- Coupling azo dye degradation and biodiesel production by manganese-dependent peroxidase producing oleaginous yeasts isolated from wood-feeding termite gut symbionts. Biotechnol. Biofuels. 2021;14(1)
- [CrossRef] [Google Scholar]
- Influence of the particle size on the antibacterial activity of green synthesized zinc oxide nanoparticles using Dysphania ambrosioides extract, supported by molecular docking analysis. Arab. J. Chem.. 2022;15(6):103804
- [CrossRef] [Google Scholar]
- Study of antibacterial properties of ziziphus mauritiana based green synthesized silver nanoparticles against various bacterial strains. Sustainability (Switzerland). 2020;12(4)
- [CrossRef] [Google Scholar]
- Biosynthesis of zinc ferrite (ZnFe2O4) nanoparticles using flower extract of nyctanthes arbor-tristis and their photocatalytic activity. Ferroelectrics. 2020;555(1):1-14.
- [CrossRef] [Google Scholar]
- High activity of ZnFe2O4 nanoparticles for photodegradation of crystal violet dye solution in the presence of sunlight. J. Taibah Univ. Sci.. 2022;16(1):988-1004.
- [CrossRef] [Google Scholar]
- Green synthesis of activated carbon doped tungsten trioxide photocatalysts using leaf of basil (Ocimum basilicum) for photocatalytic degradation of methylene blue under sunlight. J. Saudi Chem. Soc.. 2022;26(2)
- [CrossRef] [Google Scholar]
- Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater. Res. Bull.. 2018;97:121-127.
- [CrossRef] [Google Scholar]
- Cheperli, A.-M., Mokaber-Esfahani, M., Farhad, A.T., 2022. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf & seed extracts of Malva neglecta Wallr. https://doi.org/10.21203/rs.3.rs-1286975/v1.
- Application of Green Synthesized Metal Nanoparticles in the Photocatalytic Degradation of Dyes and Its Mathematical Modelling Using the Caputo-Fabrizio Fractional Derivative without the Singular Kernel. J. Math. (Wuhan). 2021;2021
- [CrossRef] [Google Scholar]
- Correlation between particle size, strain and band gap of iron oxide nanoparticles. Mater. Today: Proc.. 2016;3(6):2069-2076.
- [CrossRef] [Google Scholar]
- Green synthesis of zinc ferrite nanoparticles for photocatalysis of methylene blue. Int. J. Phytorem.. 2020;22(13):1440-1447.
- [CrossRef] [Google Scholar]
- Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4 nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study. Nanotechnol. Rev.. 2021;10(1):1912-1925.
- [CrossRef] [Google Scholar]
- Investigation on synergistic effect of rGO and carbon quantum dots-embedded ZnO hollow spheres for improved photocatalytic aqueous pollutant removal process. J. Mater. Sci. Mater. Electron.. 2021;32(24):28633-28647.
- [CrossRef] [Google Scholar]
- Gupta, N.K., Ghaffari, Y., Kim, S., Bae, J., Kim, K.S., Saifuddin, M., 2020. Photocatalytic degradation of organic pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) nanoparticles at neutral pH. Sci. Rep. 10(1), 1–0. https://doi.org/10.1038/s41598-020-61930-2.
- Bio-synthesize of photocatalytic Fe2O3 nanoparticles using Leucas aspera and Jatropha podagrica leaf extract for an effective removal of textile dye pollutants. Optik. 2022;249
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles, characterization and evaluation of their photocatalytic dye degradation activity. Curr. Res. Green Sustainable Chem.. 2021;4
- [CrossRef] [Google Scholar]
- Green approach for the effective reduction of graphene oxide using Salvadora persica L. root (Miswak) extract. Nanoscale Res. Lett.. 2015;10(1):1-9.
- [CrossRef] [Google Scholar]
- Exploiting multiferroicity of TbFeO3 nanoparticles for hydrogen generation through photo/electro/photoelectro-catalytic water splitting. Int. J. Hydrogen Energy 2022
- [CrossRef] [Google Scholar]
- Eco-friendly photocatalysts for degradation of dyes. Catalysts. 2020;10(10):1-16.
- [CrossRef] [Google Scholar]
- Green synthesis of RGO-ZnO mediated Ocimum basilicum leaves extract nanocomposite for antioxidant, antibacterial, antidiabetic and photocatalytic activity. J. Saudi Chem. Soc.. 2022;26(2)
- [CrossRef] [Google Scholar]
- Mehtab, A., Ahmed, J., Alshehri, S.M., Mao, Y., Ahmad, T., 2022a. Rare earth doped metal oxide nanoparticles for photocatalysis: A perspective. In: Nanotechnology, vol. 33, Issue 14, IOP Publishing Ltd. https://doi.org/10.1088/1361-6528/ac43e7.
- Type-II CuFe2O4/Graphitic Carbon Nitride Heterojunctions for High-Efficiency Photocatalytic and Electrocatalytic Hydrogen Generation. ACS Appl. Mater. Interfaces. 2022;14(39):44317-44329.
- [CrossRef] [Google Scholar]
- CoFe2O4@Methylcellulose/AC as a New, Green, and Eco-friendly Nano-magnetic adsorbent for removal of Reactive Red 198 from aqueous solution. Arab. J. Chem.. 2022;15(5)
- [CrossRef] [Google Scholar]
- Synthesis and photocatalytic investigation of ZnFe2O4 in the degradation of organic dyes under visible light. J. Mater. Res. Technol.. 2020;9(6):15001-15015.
- [CrossRef] [Google Scholar]
- Green synthesis of Ni@Fe3O4 and CuO nanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceram. Int.. 2019;45(14):17173-17182.
- [CrossRef] [Google Scholar]
- Phyto-synthesis of CuO nano-particles and its catalytic application in C-S bond formation. Mater. Lett.. 2020;266
- [CrossRef] [Google Scholar]
- Rahmayeni, Febrialita, R., Stiadi, Y., Putri, Y.E., Sofyan, N., Zulhadjri, 2021. Simbang Darah (Iresine herbstii) extract mediated hydrothermal method in the synthesis of zinc ferrite spinel nanoparticles used for photocatalysis and antibacterial applications. J. Environ. Chem. Eng. 9(2). https://doi.org/10.1016/j.jece.2021.105140.
- A facile microwave route for fabrication of NiO/rGO hybrid sensor with efficient CO2 and acetone gas sensing performance using clad modified fiber optic method. Optik. 2021;226
- [CrossRef] [Google Scholar]
- Development of high efficient Co3O4/Bi2O3/rGO nanocomposite for an effective photocatalytic degradation of pharmaceutical molecules with improved interfacial charge transfer. J. Environ. Chem. Eng.. 2022;10(2)
- [CrossRef] [Google Scholar]
- Facile preparation of Fe2O3 nanoparticles mediated by Centaurea alba extract and assessment of the anti-atherosclerotic properties. Arab. J. Chem.. 2022;15(1)
- [CrossRef] [Google Scholar]
- Oxygen-vacancy-induced O2 activation and electron-hole migration enhance photothermal catalytic toluene oxidation. Cell Reports Phys. Sci.. 2022;3(8)
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102584.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







