Translate this page into:
Targeting FoxP3 gene to check out the impairment of tolerance in breast cancer patients
⁎Corresponding author. nageen.mmg@pu.edu.pk (N. Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
FoxP3 gene is a potential gene in the development of Breast Cancer (BC). Mutated expression of this gene shows some correspondence with tumorigenic activity for this reason it is important to understand. The principal goal of this study was the mutational analysis of the FoxP3 gene in BC patients. This research also provides the basic functions and genetic details on the FoxP3 gene in the Lahore population, especially how the FoxP3 gene acts as a risk factor in BC development. The study design was Cross-sectional.
Methods
Genomic DNA was extracted from peripheral blood samples of BC patients. Amplified products were electrophoretically separated and target PCR products were sequenced with a Sanger sequencing analyzer. The main purpose is to determine the order of nucleotides in the FoxP3 gene. Further mutations were analyzed by the chromatograms using Chromas, NCBI nucleotide BLAST X, and the Mutation Surveyor. After that, different protein-coding sequences were studied.
Results
The substitution occurs at location 324 where T is replaced by A and in another sample G is replaced by A at position 328 while in the third sample InDel mutation was observed at position 28.
Conclusion
In the current study, intronic variants typically in the FoxP3 gene exon 2 region were confirmed by computational analysis using a gene database. We investigated nucleotide mutations in FoxP3 in patients with malignant breast tumors, along with their association with various characteristics such as marriage status, age of menopause, miscarriage and lactation history, and family history. A better understanding of the FoxP3 gene in the etiology and progression of breast cancer can reveal many explorable problems, including the clinical implications of the FoxP3 gene. However, these intronic variations are of massive importance and demonstrated that this enhancer can establish a physical interaction with a promoter site binding nuclear factor-κB components necessary for T cell regulation.
Keywords
FoxP3
Mutational analysis
Breast cancer
Electrophoresis
PCR products
1 Introduction
As stated by the National Alliance of Breast Cancer Organizations, Breast Cancer is the second most frequent type of cancer among women, comprising approximately one-fourth of female cancers worldwide (Cheng et al., 2018). Cancer is a worldwide general medical condition, and the quantity of impacted individuals is significantly more. Since the high pace of repeat and metastasis, the etiology of malignant growth is as yet poor. The beginning of tumors came about because of changes in numerous ecological elements and qualities (Gao et al., 2013). Pakistan is the most reported country in Asia, with the highest incidence of breast cancer, accounting for 40,000 deaths annually and 90,000 positive breast cancer cases. Breast cancer can occur in either gender, males and females, but it is very unlikely in males. This study is based solely on females due to their high propensity for illness, but the incidence of less than 1% in males cannot be ignored. This is rare. Of the 100 people diagnosed with breast cancer, only one is male, so this work is intended for females only. Both epidemiological and cell biology studies have documented the contribution of multiple susceptibility loci to the progression of BC (Wang et al., 2016; Forte et al., 2012). There are a lot of reports that solitary nucleotide polymorphisms (SNPs) are related to malignant cancer risk. A few investigations have shown that polymorphic qualities assume crucial parts in the turn of events and pathogenesis of cancer (Leo et al., 2017; Sakai et al., 2017; Zheng et al., 2017). However, the particular component of various polymorphic qualities stays to be obscure. Regulatory T cells (Tregs), which help in the resistant reaction and auto-tolerance, are described by CD4 + Foxp3 + expression (Pesu et al., 2008). There are more than 100 genes on Major Histocompatibility Complex (MHC) and one of them is Forkhead box protein 3 (FoxP3) genes, which more recently has been implicated in the development of BC. The cytogenetic localization of the gene (FoxP3) by genome sequence map analysis revealed its presence on the p-arm of the X chromosome, specifically especially at position Xp11.23 (Bennett et al., 2000). While the molecular position on the X chromosome of Homosapiens is 49,250 43649,270,477 base-inverted strand pairs according to the genomic coordinates of the April 96, 2019 publication Ensembl annotation (GRCh38:CM000685.2). Recent work conducted to characterize the FoxP3 gene as an X-linked tumor suppressor is also implicated in the pathogenesis of British Columbia (Arabpour et al., 2018). The strong expression of Foxp3 protein in the tumor microenvironment suggests that the transcription factor Foxp3 may be a promising marker of human breast cancer pathogenesis and prognosis (Lopes et al., 2014). Overactivity of CD4 + CD25 + leads to immunodeficiency and prevent the dismantling of cancer cells. FoxP3 proteins are thought to be controlled by DNA-like interactions during transcription. The transcription factor FoxP3 is located in the promoter genes involved in the activity of regulatory T cells (Treg). It can halt the conversion of DNA to RNA of important genes, followed by the activation of T-cell receptors (Marson et al., 2007). The gene encoding the FoxP3 protein located on human chromosome Xp11.23 is involved in the tolerance mechanism. FoxP3 was initially identified for causing X-linked autoimmune diseases in species of mice alongside humans. It was also identified as a leading regulator for determining the developmental and functional characteristics of Treg. (Chandira et al., 2019). Subsequent studies have revealed that FoxP3 exerts the activity of suppressing tumors in some cancers including breast and prostate cancer (Hayes et al., 2013; Colditz et al., 2012). Many studies now establish with evidence that FoxP3 has a role in the metastatic spread of BC like the broad expression of the FoxP3 gene in breast epithelial cells and its downregulation in the mammary cancer tissues. Second, the high rate of FoxP3 mutations or deletion in the majority of BC samples. Third, the high incidence of BC in FoxP3 mutant mice. Forth, tumor growth inhibition following transfection of FoxP3 cDNA into BC cells (Gage et al., 2014). FoxP3 was able to regulate the activation and repression of key target genes and alter histone modifications by binding to promoters (Katoh et al., 2011). FoxP3 is a potent transcriptional repressor of several oncogenes (Oda et al., 2013) which all play an important role in BC development. Although recent researches have pointed out carcinogenic effects of FoxP3 gene polymorphisms, few studies addressing the FoxP3 polymorphisms in BC (Marson et al., 2007). The main aims and objectives of this study were mutational analysis of the FoxP3 gene in Breast Cancer patients and to provide the basic functions and genetic details on the FoxP3 gene in the Lahore population.
2 Materials and methods
A total of 100 samples were selected for this research to produce reliable results of known malignant Breast Cancer patients from different hospitals in Lahore by https://goodcalculators.com/samplesize-calculator. In Pakistan, the prevalence of Breast Cancer is 31% (Idrees et al., 2018). Demographic information was collected for all patients and is published elsewhere. A total of 100 samples were selected to produce reliable results on age and gender distribution, family history, and current health status were analyzed. It was not easy to have a high number of samples as a study has been conducted in Lahore city of Pakistan. This project has been evaluated by Departmental Research Ethics and Biosafety Committee. The committee could not find any Biosafety and Ethical concerns related to the proposed work and its execution at the Institute of Microbiology and Molecular Genetics, University of the Punjab, Lahore (D/2302/MMG Dated 6th December 2019). Blood samples that were obtained from the BC participants were stored at 4–8 °C soon after collection. Genomic DNA that was hauled out from the peripheral blood samples of the patients was preserved at −20 °C for further analysis. Annealing was done at 60.4 °C (Fig. 1) and the amplified DNA products were visualized on a 1.2% agarose gel using the electrophoresis technique. A DNA ladder of size 100 bp-1 kb was introduced into the gel to determine the size of PCR products.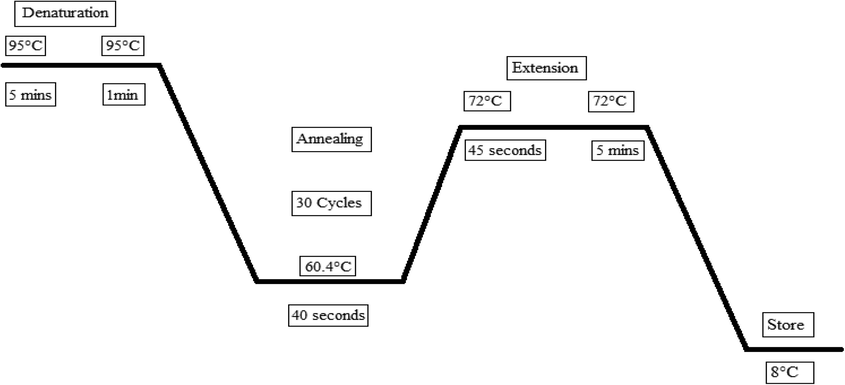
PCR cycle for exon 2 of the FOXP3 gene showing denaturation, annealing and elongation.
The target PCR products were sequenced using an Applied Biosystems Sanger Sequencing analyzer. The chromatograms that were obtained as a result were analyzed using Chromas, RRID: SCR_000598. NCBI nucleotide BLAST X (Basic Local Alignment Sequencing Tool), RRID: SCR_001653. This analysis was done to match and validate the sequence results with a reference sequence. The Mutation Surveyor, RRID: SCR_000598 detected mutations. After that, different protein-coding sequences were studied. Univariate analysis was done by SPSS statistics 25.
3 Results
To determine the effects of the FoxP3 gene on the risk of BC, a cross-sectional study was conducted involving 100 patients with malignant Breast Cancers from the population of Lahore, Pakistan. The majority of samples were collected at Lahore Service Hospital, Inmol Hospital in Lahore and Jinnah Hospital in Lahore. This is a novel work reported from this region of Pakistan concerning FoxP3 and BC. Aging; being female; family history were the leading factor in Breast Cancer. Briefly, the mean age at onset of disease was 46.38 ± 9.58 years, this is in line with several studies that showed Asian females have a low age at onset of this condition and above 50 years onset is low (Fig. 3.1).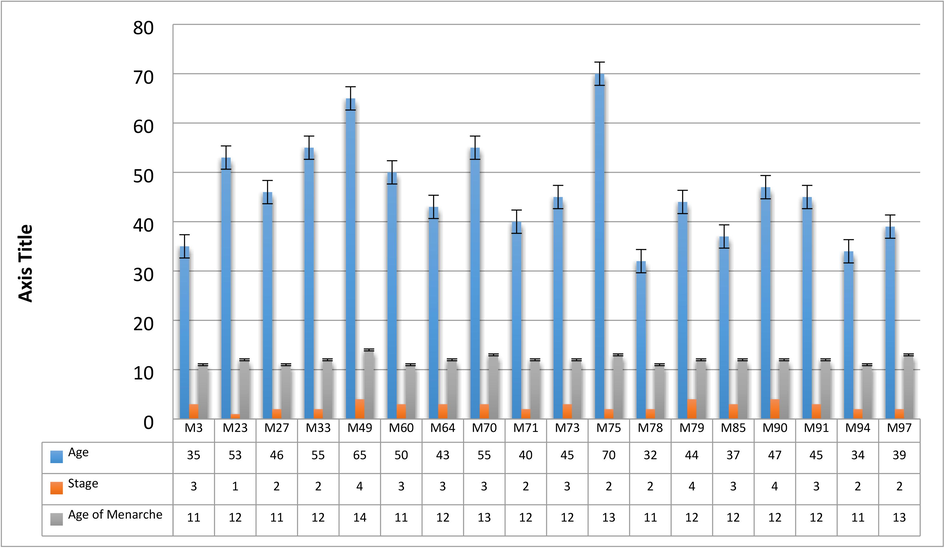
Correlation with age distribution, age of menarche, and stage of malignancy.
Gradient PCR helps to amplify FoxP3 gene exon 2as shown in Fig. 3.2. The primers used for exon 2 of the FoxP3 gene were F5′-CGTGTGACTCCTTTCCCCT-3′ R5′-ACAGTAAAGGTCGGCACCT-3′. DNA bands having 335 base pairs were separated based on their size on a 1.3% agarose gel. To confirm results, PCR Low range DNA ladder was implemented (Fermentas Cat # SM0313).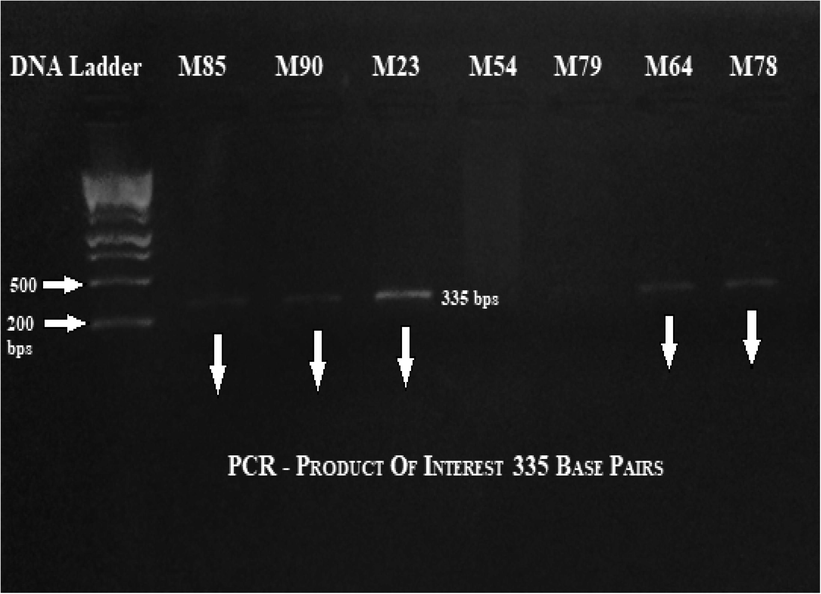
Gradient PCR thermal profile for FOXP3 gene – exon 2.
The target PCR products were sequenced using an Applied Biosystems Sanger Sequencing analyzer. The chromatograms hence obtained were carefully examined by using the Chromas, RRID: SCR_000598. NCBI nucleotide BLAST X (Basic Local Alignment Sequencing Tool), RRID: SCR_001653. This helped to compare and verify the sequence results that were obtained in the experiment with a reference sequence. Intronic variants were also identified with the help of the Mutation Surveyor software. Finally, the protein-coding sequences were also studied. The substitution occurred at position 324 where thiamine is swapped with adenine. In another sample, the substitution occurred at location 328 where G is replaced by A (Fig. 3.3) while in the third sample InDel Mutation was observed at position 28 (Fig. 3.4). Point mutations were found at two positions in patient M71, suggesting that the nucleotide base (T) at position 11,600 of the FoxP3 gene was replaced by (A). Another substitution is at position 11,596 of exon 2, where (G) is replaced by (A) as shown in (Fig. 3.5). In particular, these two positions occurred at the junction of the exon–intron and produced intron variants that did not correspond to translation-induced amino acid changes. The sequencing results suggest that heterozygous SNPs are present at positions 294 and 298 of the forward primer sequence.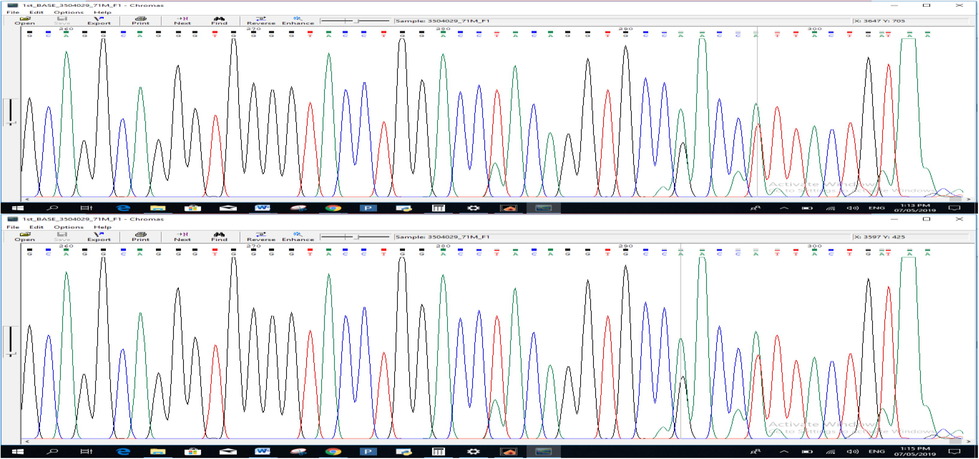
Variants where T is replaced by A at 324 position and in the second sample G is replaced by A at position 328.
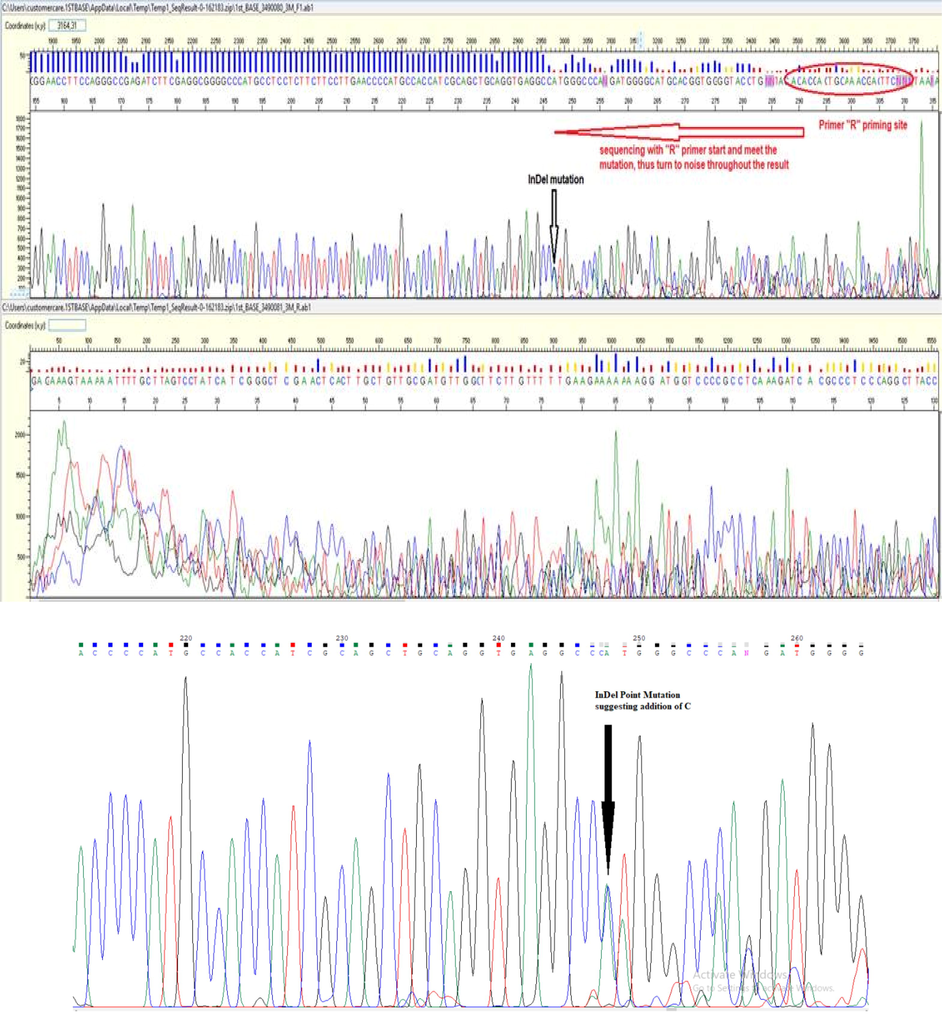
InDel mutations near the priming site of forward primer.
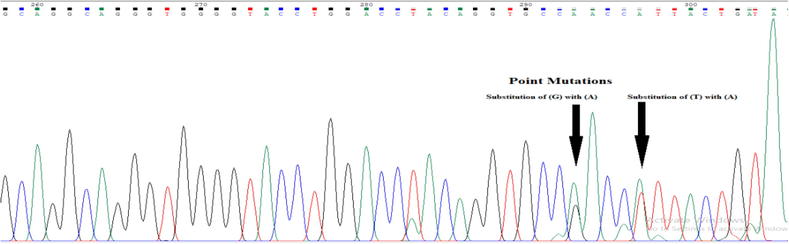
The recording illustrates substitution wherever T is replaced by A and G by A.
4 Discussion
Breast Cancer is identified as the second main reason for death in females worldwide and is the most frequent malignancy concerning females. Almost 1,000,000 women develop BC each year, with 23% of the incidence reported in women in developed countries. A small proportion of BC at a young age is due to the widespread susceptibility to autosomal dominant. A variety of prognostic and pathologic variables have been utilized to foresee the endurance in breast carcinoma patients, including cancer size and grade, nodal status, growth corruption, and protein markers. Although the current prognostic elements foresee backslide in the initial 5 years after treatment, it is unreliable whether these boundaries are valuable in anticipating long-term endurance (Schreiber et al., 2011). Several factors lead to the development of this condition including immunological, genetic, and environmental, so it is one of the complex and multifactorial diseases. The tumor microenvironment (TME) assumes a significant part in disease concealment and advancement (Nishikawa and Sakaguchi, 2010) and as a crucial part of TME, Tregs are liable for down-directing persistent aggravation, obstructing immune system responses, and keeping up with fringe immunological resistance. Recently published data distributed information exhibited that Tregs-interceded immunosuppression was a crucial growth resistant avoidance system, and might add to the cancer immunotherapy (Sakaguchi, 2004). A prominent trait of Tregs is their appearance of the record factor Forkhead box P3. The FoxP3 protein has capacities as a transcriptional repressor to down-regulate cytokine secretions of Tregs (Yamagami et al., 2011). A few examinations announced that FoxP3+ Tregs could penetrate cancers at higher proportions than other types of T cells. The aggregation of FoxP3+Tregs in growths and neighborhood lymph nodes could repress invulnerable reactions and in this way result in tumorigenesis with a less ideal forecast (Fu et al., 2007; Tang and Bluestone, 2008). While Tregs are the significant cell type communicating FoxP3 under normal conditions (Curiel et al., 2004). It has recently been observed that FoxP3 was likewise communicated in an assortment of diseases, for example, pancreatic, ovarian, thyroid, and hepatocellular (Hinz et al., 2007; Shevach et al., 2006; Ugolini et al., 2014). The present work comprised 100 samples of known malignant Breast Cancer patients from different hospitals in Lahore. Demographic information was collected for all patients and is published as Khan et al. (2018). Briefly, the mean age at onset of disease was 46.38 ± 9.58 years. This is in line with several studies that show that Asian females have a low age at onset of this condition and above 50 years onset is low. Specifically, Malaysian and Indonesian females have onsets between 40 and 50 years (Rudensky, 2011). All subjects selected were genetically unrelated and Breast Cancer was observed in higher numbers if any of the family members were affected by the same condition (especially first-degree relatives) emphasizing the involvement of genetic factors in disease development and progression. FoxP3 gene Exon 2 skipping is a phenomenon observed in cancer cells that leads to impaired folding and secretion of protein (Bennett et al., 2000) Genotyping of all subjects was performed after extraction of DNA from whole blood, and sequences were analyzed. The sequence selected for the present study was exon 2 of the FoxP3 gene. It contains a transcription start site and is studied extensively in many immunological disorders. The present study showed the failure of the tolerance mechanism involving the FoxP3 gene in BC patients. Intron 2 of the gene has a bonafide enhancer element (Smith et al., 2006). Some factors including Smad3 and NFAT are essentially needed for the functioning of this Foxp3 enhancer in the process of transcription. They are integral for the acetylation of histone in the enhancer region and the induction of tolerance. An analysis was performed by to examine the polymorphism of the FoxP3 gene, then the effect of frameshift mutations in the FoxP3 gene, specifically the FKH forkhead region, resulted in the death of mice of homozygous males from birth to one month old. This is similar to a human mutation in patients in which initial translation completes beyond a frame shift (Alvarado-Sánchez et al., 2006, Wang et al., 2016). In the Indian population, many research studies can be found concerning a frameshift mutation. Several types of research support an association of the FoxP3 gene with BC. In addition to the influence of the above factors on breast malignancy, SNPs also play an important role in cancer spread. In the current study, the substitution occurred at position 324 where thiamine is swapped with adenine. In another sample, the substitution occurred at location 328 where G is replaced by a while in the third sample InDel Mutation was observed at position 28. Point mutations recorded in patient M71 at two sites indicate a substitution at position 11,600 of the FoxP3 gene where one nucleotide base (T) is replaced by (A). Another replacement is located at position 11,596 of exon 2 where (G) is replaced by (A). Notably, these two sites originate from the junction of exon-introns and induce intron variants that do not correspond to any amino acid changes due to translation. Sequencing results showed that dissimilar alleles were present in 294 and 298 in the forward primer sequence. It was noted that no mutation was observed in all cases regarding the gene of interest. One of the sequences observed had two mutations at positions 324 and 328 of the analyzed sequence. It had a substitution of A from T and G of both locations (figures and global BLAST in supp material). Both mutations were 55th and 58th bp from exon 2 and were part of intron 2. This intron appeared to be very significant and current studies revealed that this enhancer has some physical interaction with the components of promoter site binding nuclear factor-κB that is necessary for T cell regulation. According to this study, the FoxP3 gene is anti-oncogenic.
5 Conclusions
The substitution occurs at location 324 where T is replaced by A and in another sample G is replaced by A at position 328 while in the third sample showed InDel mutation was observed at position 28. This study provides genetic data on the FoxP3 gene in the Lahore population and showed intronic variants typically in the FoxP3 gene exon 2 which proved that FoxP3 gene is anti-oncogenic. However, this intron is of massive importance and demonstrated that this enhancer has the ability to establish a physical interaction with a promoter site binding nuclear factor-κB components necessary for T cell regulation.
Acknowledgments
The authors are obliged to acknowledge the Institute of Microbiology and Molecular Genetics, University of the Punjab for giving the working space and amenities. The authors are also thankful to all the participants of the study to provide their consent.
Conflict of interest
Authors have no conflict of interest.
Disclaimer
The current manuscript is a part of MPhil thesis.
Funding disclosure
University of the Punjab, Lahore-Pakistan (Fiscal Year 2018-2019).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Regulatory T cells in patients with systemic lupus rythematosus. J. Autoimmun.. 2006;27:110.
- [Google Scholar]
- FoxP3 gene polymorphism is associated with breast cancer in Iranian patients. Exp. Oncol.. 2018;40:309.
- [Google Scholar]
- Bennett CL, Yoshioka R, Kiyosawa H, Barker DF, Fain PR, Shigeoka AO et al. (2000). X-Linked syndrome of polyendocrinopathy, immune dysfunction, and diarrhea maps to Xp11. 23-Xq1. Am. J. Human Genet. 66:461.
- Functional Foxp3 polymorphisms and the susceptibility to cancer: An update meta-analysis. Medicine. 2018;97(34):e11927
- [Google Scholar]
- Family history a risk of breast cancer: nurses’ health study. Breast Cancer Res. Treat.. 2012;133:1097.
- [Google Scholar]
- Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med.. 2004;10(9):942-949.
- [Google Scholar]
- Obesity, diabetes, the cardiorenal syndrome, and risk for cancer. Cardiorenal Med.. 2012;2:143.
- [Google Scholar]
- Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132(7):2328-2339.
- [Google Scholar]
- Translational advances regarding hereditary breast cancer syndromes. J. Surg. Oncol.. 2014;105:444.
- [Google Scholar]
- Insertion/deletion polymorphisms in the promoter region of BRM contribute to risk of hepatocellular carcinoma in Chinese populations. PLoS ONE. 2013;8:e55169
- [Google Scholar]
- Population attributable risks for modifiable lifestyle factors and breast cancer in New Zealand women. Internal Med. J.. 2013;43:1198.
- [Google Scholar]
- Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res.. 2007;67:8344-8350.
- [Google Scholar]
- Cancer prevalence in Pakistan: meta-analysis of various published studies to determine variation in cancer figures resulting from marked population heterogeneity in different parts of the country. World. J. Surg. Oncol.. 2018;16:129.
- [Google Scholar]
- FOXP3 orchestrates H4K16 acetylation and H3K4 trimethylation for activation of multiple genes by recruiting MOF and causing displacement of PLU-1. Mol. Cell. 2011;44:770-784.
- [Google Scholar]
- Defining the genetic susceptibility to cervical neoplasia-A genome-wide association study. PLoS Genet.. 2017;13:e1006866
- [Google Scholar]
- FOXP3 transcription factor: a candidate marker for susceptibility and prognosis in triple negative breast cancer. BioMed Res. Int.. 2014;14:341-654.
- [Google Scholar]
- Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931.
- [Google Scholar]
- Genetic polymorphism in FOXP3 gene: imbalance in regulatory T-cell role and development of human diseases. J. Genet.. 2013;92:163.
- [Google Scholar]
- T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246-250.
- [Google Scholar]
- Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol.. 2004;22:531-562.
- [Google Scholar]
- Clonal composition of human ovarian cancer based on copy number analysis reveals a reciprocal relation with oncogenic mutation status. Cancer Lett.. 2017;405:22-28.
- [Google Scholar]
- Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565-1570.
- [Google Scholar]
- The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev.. 2006;212:60.
- [Google Scholar]
- Robinson MK: Splice variants of human FOXP3 are functional inhibitors of human CD4+ T-cell activation. Immunology. 2006;119:203.
- [Google Scholar]
- The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol.. 2008;9:239-244.
- [Google Scholar]
- FoxP3 expression in papillary thyroid carcinoma: a possible resistance biomarker to iodine 131 treatment. Thyroid. 2014;24:339-346.
- [Google Scholar]
- Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459.
- [Google Scholar]
- Immunofluorescence-detected infiltration of CD4+FOXP3+ regulatory T cells is relevant to the prognosis of patients with endometrial cancer. Int. J. Gynecol. Cancer. 2011;21:1628-1634.
- [Google Scholar]
- Functional miR-146a, miR-149, miR-196a2 and miR-499 polymorphisms and the susceptibility to hepatocellular carcinoma: an updated meta-analysis. Clin. Res. Hepatol. Gastroenterol.. 2017;41:664-676.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.101864.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







