Translate this page into:
Structure, physicochemical and toxicity properties of underused malaysian native Tuber’s starch (Dioscorea Pentaphylla)
⁎Corresponding author. azwanlazim@ukm.edu.my (Azwan Mat Lazim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
The objective of this study was to characterize the structure, physicochemical and toxicity properties of Dioscorea Pentaphylla tuber’s starch.
Methods
Starch was extracted from Dioscorea Pentaphylla tuber’s and analyzed to determine their properties such as pH, water binding capacity, solubility, swelling power, chemical composition, thermal properties, degree of crystallinity, morphology and toxicity.
Results
Results showed that more than half of Dioscorea pentaphylla starch consist of amylose (64.10 ± 1.15%). It has gelatinization temperature started at 76.90 ± 0.08 °C. This C-Type starch has a degree of crystallinity around 32.90 ± 2.59%. The SEM image showed that Dioscorea pentaphylla has oval-shaped particles with sizes ranging from 42 to 55 µm. The toxicity test demonstrated that the starch was safe and can be classified as non-toxic.
Conclusion
The physicochemical and toxicology properties of Dioscorea Pentaphylla starch recorded in this study indicate that this starch is applicable as biopolymer source for food and non-food purposes.
Keywords
Starch
Dioscorea
Biopolymer
Non-toxic
Tubers
1 Introduction
Starch or amylum refers to a complex polymeric carbohydrate that made of large quantity of glucose units connected by glycosidic bonds. This polysaccharide is produced in amyloplast by most of the green plants as energy reserved. Starch is widely used in food applications such as thickener, gelling agent, binders or nutriment stabilizer (Xian et al., 2020). It has been classified as one of the most versatile natural biopolymer macromolecules after cellulose (Azeredo et al., 2010; Pramodrao & Riar, 2014; Madzlan et al., 2012). The technology advancement has improved their functionality and extended to be used in food and non-food applications. As relevant inherent, this polysaccharide has widely been used in paper manufacturing, textile industry, adhesives, organic filler, drinks, bakery, plastics and pharmaceutical products (Azfaralariff et al., 2020; Airul Ashri et al., 2014; Elmi Sharlina et al., 2017). In point of fact, starch has highly demand from the industry because of its special characteristics such as hydrophilic, eco-rich, biodegradable and economical (Airul Ashri et al., 2014; Alinnor & Akalezi, 2010; Nand et al., 2008).
About 600 species of Dioscoreaceae family has been identified from different origin, either tropical or subtropical regions. In general, Dioscorea spp. is creeping, woody, thorny and slightly poignant plant that can grow up to 20 m in height and produces hairy bulky tubers (Kamaruddin et al., 2020; Nashriyah et al., 2010). It contains about 75–84% of starch including minor components such as proteins, vitamins, lipids and minerals (Shajeela, 2011). The active compounds in Dioscorea Spp. such as steroidal sapogenin, glycan, alkaloid, tannin including saponin give septic characteristic, acrid taste and bitterness (Prakash et al., 2014). In addition, some extracts from Dioscorea species have been used as traditional remedy to reduce blood sugar or blood lipid content, antimicrobial, inflammation, joint pain, diabetes, infections and dysmenorrhea (Araghiniknam et al., 1996; Poornima and Ravishankar Rai, 2007).
In food or non-food industries, details on the structure, physiological and physiochemical properties of established starch such as wheat, potatoes, corn and rice were sufficiently documented and vastly used. (Jiang et al., 2012). Although there are many applications of starch from Dioscorea species yet, there was no research reported on Dioscorea pentaphylla or locally known as “Ubi Pasir” that wildly grown in Malaysia. These tubers are usually found in hot and humid regions of Asia and some Pacific islands from the lowland up to 2000 m of altitude. Although this yam is classified as edible but it rarely consumed as food due to its bitterness and hard structure.
The demand for carbohydrate to be used in food and non-food industry increases yearly. As example, the global native starch market reached a volume of 90.6 million tons in 2019 (IMARC, 2020). Moreover, the international analysts expect that there will be approximately 25% of global market growth regarding starch processing and reach 1.191billions tons production by 2026. More than half (53%) of the demand is predicted for starch products in Asian countries by 2026 compared to similar indicators in 2016. While, the consumption of corn products in North and South America is estimated increase by 38% in 2026 (Tanklevska et al., 2020). Based on positive trending market, therefore, researchers have started to identify and focusing more on the underutilized crops that highly potential to be used as alternative of conventional cereal or tubers. Starch from D.pentaphylla hence possibly to be employed as alternative to the commercially established starch such as corn, tapioca and potato. In order to use new starch source for industry, it requires full understanding of the composition, structure and its functional properties. Therefore, this study aims to investigate the properties of D. pentaphylla starch especially on the physicochemical properties and their toxicity. A complete profiling is important in order to facilitate any further applications.
2 Materials and Methods
2.1 Materials
Fresh Dioscorea pentaphylla tubers were taken from Pasir Puteh, Kelantan, Malaysia. The scientific verification name of specimen WYA526 was identified and confirmed by a botanist, En. Sani Miran whom plant expert of National University of Malaysia, Bangi. Herbarium. Sodium Hydroxide, Sulfuric Acid and Müller–Hinton agar were purchased from SigmaAdrich (SigmaAldrich, Malaysia). All other solvents and chemicals used were analytical grade and purchased from R&M Chemicals (R&M Chemicals, Malaysia).
2.2 Starch extraction
The starch was extracted using the method reported by Elmi et al., (2017). The fresh tubers skin firstly removed, cleaned and cut into small cubes. The sample was blended with distilled water in a ration of (1:3) using a food mixer (Sharp EM110, Japan) for 60 s. The starch slurry was filtered by using a double layers cheesecloth. In a beaker, the filtrate was left overnight to allow starch submersed at the bottom. In an oven, starch was dried until a constant weight attained at 45 °C. The dried starch sample was ground into powder and kept in a dry container for later use. Starch content was calculated using equation (1).
2.3 pH of starch
Nand et al. (2008) procedure has been followed to determine the pH. Approximately 5 g of starch was added into a beaker containing 20 mL of distilled water, and stirred for 300 s using a magnetic stirrer. A pH meter (Thermo Scientific Orion 5 Star, USA) was used to determine the pH of starch solution.
2.4 Water binding capacity
Water-binding capacity (WBC) was calculated by using the protocols as reported by Jiang et al. (2012) and Airul Ashri et al. (2014). A mixture of 5 g of starch and 75 mL distilled water was stirred with a magnetic stirrer for 1 h before centrifuged at 3000 rpm for 10 min (Kubota 2420 tabletop centrifuge). The supernatant was discharged while the wet starch was filter off at ambience temperature (25 °C) before weighed. The WBC (%) was determined using equation (2).
2.5 Field emission scanning Electron microscopy
Starch morphology was investigated using an Electron-Radiation Scanner Microscope (FESEM-Supra Zeiss, German). The starch was thin spread on a metal stump and coated with platinum to create a conductive sample. All images were captured at a potential acceleration of 20 kV (Regina et al., 2016; Adil Hakam et al., 2015).
2.6 Elemental analysis
Elemental analysis such as carbon (C), hydrogen (H), nitrogen (N) and sulfur (S) of Dioscorea pentaphylla starch were determined using CHNS Thermo-Finnigan Analyzer series EA 1112 (Italy).
2.7 Proximate analysis
The standard method by the Association of Official Analytical Chemists (AOAC, 1990) was followed in order to analyze the proximal composition of Dioscorea pentaphylla starch such as moisture, ash, protein, lipids, fibers and carbohydrates. The moisture content of starch was obtained by drying 2 g of starch at 105 °C for 5 h and result was calculated using equation (3).
The ash content was determined by burning 2 g of starch in a furnace at 550 °C for 5 h and calculation made using equation (4).
The nitrogen content of the starch was determined using the Kjeldahl method using the Kjeltec ™ 2100 Distillation Unit apparatus (Foss, Denmark). The protein content was calculated using equation (5).
The lipid content was determined using the Soxhlet method (Soxtec ™ 2043 Fat Extraction System (Foss, Denmark) with n-hexane as solvent. Results were calculated using equation (6).
System of procedure FiberCap™ FC 221 (Foss, Denmark) was used to determine the content of fiber in starch. The content of fiber was calculated using equation (7).
Carbohydrate content and energy of metabolism were calculated as suggested by Alinnor & Akalezi (2010) as shown in equation (8) and (9).
2.8 Amylose and amylopectin content
Both amylose and amylopectin content were determined by using a method reported by Elmi Sharlina et al. (2017). In a beaker, a mixture of 100 mg of starch, 1 mL of ethanol (95%) and 9 mL of 1 N sodium hydroxide solution were prepared before heated in boiling water for 10 min. The sample was cooled and transferred into a 100 mL volumetric flask and topped up with deionized water to produce starch suspension (A). In another volumetric flask (100 mL) starch B suspension was made up by using starch A suspension (5 mL), 1 mL of 1 N acetic acid, 2 mL of 2.6% potassium iodide and deionized water before sonicated for 20 min. The sample absorbance was recorded at 620 nm using a UV–vis spectrophotometer UV-1800 Shimadzu ultra-light spectrophotometer (Shimadzu, Japan). As a reference, solutions using standard amylose of potato was prepared (0, 20, 40, 60, 80 and 100 mg of amylose). Standard amylose graphs were plotted using absorption result at 620 nm against standard amylose percent. Amylose content (%) was determined from the standard amylose graph. The amylopectin content has been calculated using equation (10).
2.9 Solubility and swelling power
The solubility and swelling power of starch were investigated by using the procedures described by Jiang et al. (2012), Ma et al. (2010) and Nattapulwat et al. (2009) with slight modifications. A mixture of starch and distilled water (1% in 15 mL mixture) was stirred for 30 min before heating at 35 °C for 1 h. The same steps were repeated with different temperature; 45 °C, 55 °C, 65 °C, 75, 85 °C and 95 °C respectively. Each sample was cooled until it reached about 25 °C before centrifuged at 3000 rpm for 15 mins. The supernatants were carefully separated and placed in a vial, dried at 110 °C in oven until a consistent weight was recorded. The wet starch precipitated was also dried and weighed. All experiments were performed in triplicate. The solubility and swelling power were calculated using equations as shown below (11 and 12).
2.10 X-Ray diffractometer
X-ray diffraction (XRD, Bruker D8 Advance, German) was employed to determine the degree of starch crystallization. The starch was inserted into the container and the XRD pattern has been recorded in the reflection 5–80 °C (2θ) at room temperature that operated at 40 kV and 40 mA with the radiation of Kα Cu, k = 0.5406 nm (Regina et al., 2016).
2.11 Differential scanning calorimeter (DSC) analysis
Starch gelatinatizaton study was carried out using a calorimeter differential scanner (DSC 822 Mettler Toledo, Switzerland), where all protocols followed as reported by Ma et al. (2010). In a small aluminum pan 10 mg starch was added with deionized water to form a starch suspension in a ratio of 1: 3. The sample was sealed and left at room temperature for 1 h. Before further application, calibration was made using reference (indium and empty aluminum pan). Sample was heated in range of 20 °C to 120 °C with a heating rate of 10 °C/min. Results obtained have been used to calculate the initial temperature (To), peak temperature (Tp), ending temperature (Tc), temperature range (ΔT), and gelatinization enthalpy (ΔHgel).
2.12 In vitro antimicrobial activities of starch
The starch antimicrobial activity using the disk diffusion susceptibility experiment was followed the protocol reported by Azman et al. (2016) which recommended by National Committee for Clinical Laboratory Standards (NCCLS) (Kiehlbauch et al., 2000). Four types of bacterial cultures: E. coli [ATCC 25922], S. aureus [ATCC 25923], Salmonella typhi. [ATCC 14028], and Saccharomyces cerevisiae [ATCC 9763] were placed on Müller–Hinton agar plates. Standard protocols were followed and the inhibition zone diameter (mm) for every starch samples were measured after 24-hour incubation.
2.13 Toxicity test
Starch toxicity was tested using the protocols suggested by Organization for Economic Cooperation and Development (OECD, 2006). The zebra fish (Danio rerio) embryos in E3 medium were obtained from the Molecular Biology Laboratory, School of Biology and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, Bangi, Malaysia. Starch was dissolved in deionized water at different concentrations (100–0.001 mg/mL). For each concentration, five embryos were tested and the experiments habe been repeated three times. The 96-well microplate was used to place embryos in each well 200 µL starch solution and stored in an incubator. Embryo observation was performed by using a light microscope at 10X magnification at 24, 48 and 72 h post fertilization (hpf) to determine the percentage of surviving embryos (Imran et al., 2015; Regina et al., 2016).
2.14 Statistical analysis
All studies were performed at least three times and data were examined using a one-sample t-test to determine significant differences at p < 0.05 using statistical software SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
3 Results and discussion
3.1 Properties and proximate composition of D. Pentaphylla starch
The isolated starch from D. Pentaphylla has been characterized to determine their properties and results obtained were compared with other Dioscorea species. Table 1 showed that D. Pentaphylla has the least amount of starch content, with 1.89 ± 0.44% compared to other species under diascorea family. Like most of the D. hispida sp family, D. Pentaphylla can be classified under acidic condition with pH-approximately 5.30 ± 0.00. Although the moisture content can be considered as low (1.55 ± 0.4%), but its water binding capacity (WBC = 2.25 ± 0.05) resembles other discorea tubers such as D. Bulbifera (2.53 ± 0.02%) and D. Pyrifolia (2.45 ± 0.02%). Results obtained also reflected the low interactions with water molecules and starch hydroxyl group to form hydrogen or covalent bonds (Airul Ashri et al., 2014; Hoover and Ratnayake, 2002).
Species
Starch content (%)
PH
Moisture content (%)
WBC (%)
D. Pentaphylla(This Research)
1.89 ± 0.44
5.30 ± 0.00
1.55 ± 0.4
2.25 ± 0.05
D. Hispida(Airul et. al 2014)
11.46 ± 0.08
4.48 ± 0.03
2.49 ± 0.6
1.07 ± 0.02
D. Opposita
D. Alata
D. Nipponica
D. Bulbifera
(Jiang et al. 2012)69.90 ± 0.02
41.90 ± 0.01
35.40 ± 0.03
62.70 ± 0.01-
-
-
-13.46 ± 0.86
14.35 ± 0.84
11.62 ± 0.33
12.26 ± 0.501.41 ± 0.01
1.56 ± 0.11
4.43 ± 0.0
2.53 ± 0.02
D. pyrifolia(Elmi Sharlina et al. 2017)
92.73 ± 0.48
3.43 ± 0.05
4.84 ± 0.29
2.45 ± 0.23
Fig. 1 shows SEM micrographs of the oval shaped starch granule isolated from D. pentphylla with sizes in ranged of 42 μm to 55 μm. In general, each species of Dioscorea tuber has unique shapes where significantly influenced by endemic origins and living environment (Airul Ashri et al., 2014; Svegmark & Hermansson, 1993). As example, due to different sampling sources, D. alata was reported to have three different starch forms; ellipsoid, polyhedral, triangular and rod-like shapes (Riley et al., 2006). Findings by Jiang et al. (2012) demonstrated that each Dioscorea sp. not limited to a congruent formed. Size diversity in granule as a result of the plant biochemistry and physical properties such as chloroplast, light transmittance and total amylose contest (Kaur et al., 2002).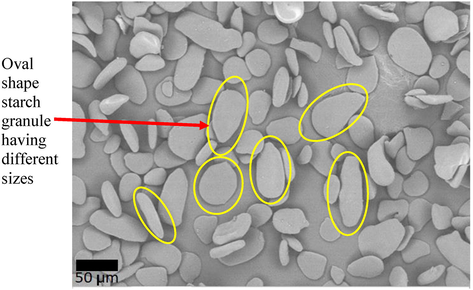
Granule morphology for starch from Dioscorea pentaphylla tuber.
Peroximate analysis was carried out to determine the relative amount of proteins, lipid, water, ash and carbohydrate of D. pentaphylla starch (Table 2). Similarly to other dioscorea species, D. pentaphylla highly contains with carbohydrate (85.65%) and slight amount of lipid (0.03%) without fiber. Vastly content of carbohydrate with less amount of nitrogen is also an indicator that the extracted starch is pure and good quality (Elmi Sharlina et al., 2017). This value is comparable to corn (Zea mays L.) and Sago (Metroxylon sagu) where their carbohydrate content about 85.42% and 86.28% respectively (Ahmad et al., 1999, Azfaralariff et al., 2020). It can also be seen that the ash content of D. pentaphylla is the third highest while its protein content second highest after D. dumetorum sp.
Starch source
Ash (%)
Lipid (%)
Protein
Fiber
Carbohydrate
D. pentaphylla(This research)
0.59 ± 0.10
0.03 ± 0.00
2.75 ± 0.03
0.00 ± 0.00
85.65 ± 0.16
D. pyrifolia( Elmi Sharlina et al. 2017)
0.88 ± 0.21
0.00 ± 0.00
1.34 ± 0.11
0.00 ± 0.00
92.73 ± 0.48
D. alata (Senanayake, 2012)
0.33 ± 0.01
0.43 ± 0.06
2.10 ± 0.09
Nd
86.81 ± 1.83
D.esculenta (Senanayake, 2012)
0.25 ± 0.01
0.42 ± 0.01
1.20 ± 0.01
Nd
86.30 ± 1.11
D. rotundata (Gbassi et al., 2014)
0.01 ± 0.00
0.10 ± 0.00
0.18 ± 0.00
0.00 ± 0.00
88.41 ± 0.00
D. dumetorum(Oyeyinka and Oyeyinka, 2018)
5.39 ± 0.01
0.64 ± 0.01
5.67 ± 0.01
1.97 ± 0.01
78.15 ± 0.01
As carbohydrate derivative, starch contains basic elements (carbon and hydrogen) that builds its structure. Results from the elemental analysis have shown that D. pentaphylla contained 38.45 ± 0.11% of carbon, 6.84 ± 0.14% hydrogen. Only 0.51 ± 0.03% of nitrogen can be traced however no sulphur was observed.
nd = not determine/The results are given as the mean of three determinations ± standard error of the mean.
3.2 Amylose and amylopectin content of starch
Both amylose and amylopectin functioning as one of the elements that provides energy to plant. They also responsible in developing starch characteristics. Starch with high amylose content easily produces gel (Vignaux, 2005). Due to its branching structure, amylopectin is able to reduce the viscosity and increases the slimy-like structure of hydrocolloid (Bertoft et al., 2016). The amylose content obtained for Dioscorea pentaphylla in this study was 64.10 ± 1.15 while amylopectin 35.90 ± 1.15. This result demonstrated that Dioscorea pentaphylla has the highest amylose content compared with D. pyrifolia (44.47 ± 1.86%) (Elmi Sharlina et al., 2017) or other Dioscorea sp. where their value was between 9 and 23% (Jiang et al., 2012). Amylose content plays vital role in establishing the chemical properties of starch and its further applications. As example starch that highly content with amylose and amylopectin are suitable to be used as film, membrane, gelling agent and coating including the industry that needs less absorptive and highly hydrophobic material (Joshi, 2013). This category of carbohydrate is also applicable for health purposes because according to the previous reports (Elmi Sharlina et al., 2017) it works in reducing insulin and the glycemic response, overweight problems, cardiovascular epidemic along with consequent of the type II diabetes.
3.3 Solubility and swelling power of Dioscorea pyrifolia starch
Both swelling power and solubility properties of Dioscorea pentaphylla starch are shown in Fig. 2a. As temperature increased, the swelling power was consistently rose from 4.95 to 18.50 g/g. This trend was paralleled with the starch solubility, where it quadrupled from 10% to 40%. Below 60 °C, the amylose starch is packed in crystals form consequently inhibits granules to swell (Coulibaly et al., 2013; Azfaralariff et al, 2020). However, when the temperature passed 65 °C, endothermic process occurred whereby more energy was absorbed resulting the crystallites melted, fracture the intermolecular bindings. This condition allows more hydroxyl groups (starch) to incorporate with water molecules via hydrogen bonding. As a result, the starch swelled. Both results demonstrated that the starch solubility has developed a rapid progress starting at 75 °C. The two progressions were also contributed by different aspects for example amylose and amylopectin content, chain length, type or structure of branches association molecules (Atuobi & Sakyi-Dawson, 2011, Elmi Sharlina et al., 2017).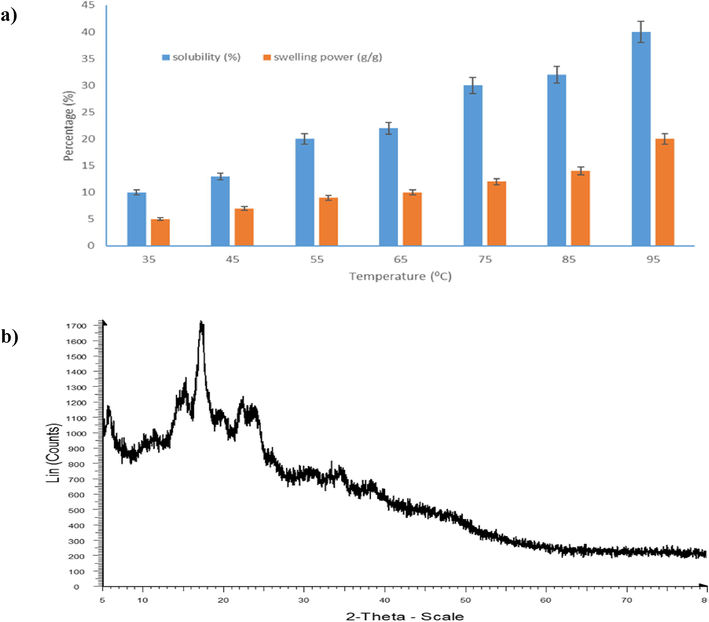
Properties of starch from Dioscorea pentaphylla tuber: a) Solubility (%) and swelling power (g/g); and b) Crystallinity.
3.4 X-ray diffraction studies
X-ray diffraction analysis of starch Dioscorea pentaphylla showed that the starch was semi-crystalline with an angular top of 2θ at 5.60°, 15.18°, 17.12° and 23.47° (Fig. 2b). Study reported by Wang et al. (2008) found that the peak at 5.60° and 15.18° were categorized as Type-B starch while peaks of 17.12° and 23.47° has been classified as Type-A starch. However, D. pentaphylla can be grouped as a Type-C starch because it has a mixture of Type-A and Type-B starch. The degree of crystallization of D. pentaphylla starch was 32.90 ± 2.59%. The crystalline content of starch indicates the presence of long-chain amylopectin molecules or parallel structures while amorphous content indicates the presence of short-chain amylose or amylopectin molecules in starch granules. (Cheetham & Tao, 1998).
3.5 Thermal properties
As shown in Table 3, Dioscorea pentaphylla started to gelatinize at 76.91 ± 0.08 ⁰C which was identical to other Dioscorea sp. While, the initial (To), peak (Tp) and complete (Tc) gelatinization temperature were, 76.91 ± 0.08 °C, 81.04 ± 0.02 °C and 84.80 ± 0.05 °C respectively. Besides, its gelatinization enthalpy (ΔHgel (J/g)) was 4.82 ± 0.03 and the range temperatures (R = Tc-To) was 4.82 ± 0.03. Starch gelatinization is a process where breaking down inter-molecules take place, allowing more interactions with water in the presence of heat. It affects the double helix de-coiling together with disintegrates of the crystalline zone resulting the particles swell during the endothermic process. Variance was observed between the gelatinization initial (To) and peak (Tp) mostly related to the starch molecular structure that prefabricated by crystal region such as lamellar, super-helix complex and amylopectin (Azfaralariff et al, 2020; Noda et al., 1998). The gelatinization enthalpy of Dioscorea pentaphylla was significantly higher compared with other Dioscorea sp. This result indicated the loss of double helical uniformity or the inclusive crystallinity of amylopectin (Oyeyinka and Oyeyinka, 2018; Elmi Sharlina et al., 2017; Noda et al., 1996).
Starch source
To (°C)
Tp (°C)
Tc (°C)
ΔHgel (J/g)
Tc - To
D. pentaphylla(This research)
76.91 ± 0.08
81.04 ± 0.02
84.80 ± 0.05
4.82 ± 0.03
7.89
D. pyrifolia(Elmi Sharlina et al. 2017)
71.51 0.07
75.05 ± 0.15
78.25 ± 0.18
3.86 ± 0.05
6.74
D. alata(Jiang et al., 2012)
71.26 ± 1.11
74.33 ± 1.73
82.11 ± 1.28
3.58 ± 0.29
10.86
D.nipponica(Jiang et al., 2012)
70.09 ± 0.92
77.63 ± 1.03
85.18 ± 0.74
0.64 ± 0.03
15.09
D. bulbifera(Jiang et al., 2012)
71.63 ± 0.91
75.98 ± 0.38
81.23 ± 0.95
2.05 ± 0.03
9.60
D. septemloba(Jiang et al., 2012)
75.06 ± 0.04
80.74 ± 0.90
84.74 ± 0.58
0.28 ± 0.02
9.67
To = Initial temperature, Tp = Peak temperature, Tc = finishing temperature, ΔHgel = gelatinization enthalpy
3.6 Antibacterial efficacy test
Antibacterial efficiency evaluation has been employed to investigate the inhibitory activity of Dioscorea pentaphylla starch. The study was evaluated based on the identification between growth conditions of all microorganism used (E. coli sp., S. aureus, S. cerevisiae, and Salmonella typhi). Observation made after 24 h found that the bottom of cultured disk was covered by bacteria and the growth evenly occurred around the plate. Therefore, it presumed there was no inhibition activity occurred. There is no remarkable disinfection effect of Dioscorea pentaphylla owing to the absence of antiseptic alkaloid such as dioscorine which contains carbon and nitrogen (C-N) that provides antioxidant and antibacterial properties (Airul Ashri et al., 2014; Lazim, 2016). This result gave negative respond unlikely Discorea hispida starch where it has performed as a good inhibitor towards the similar bacteria (Azman, 2016).
3.7 Toxicity
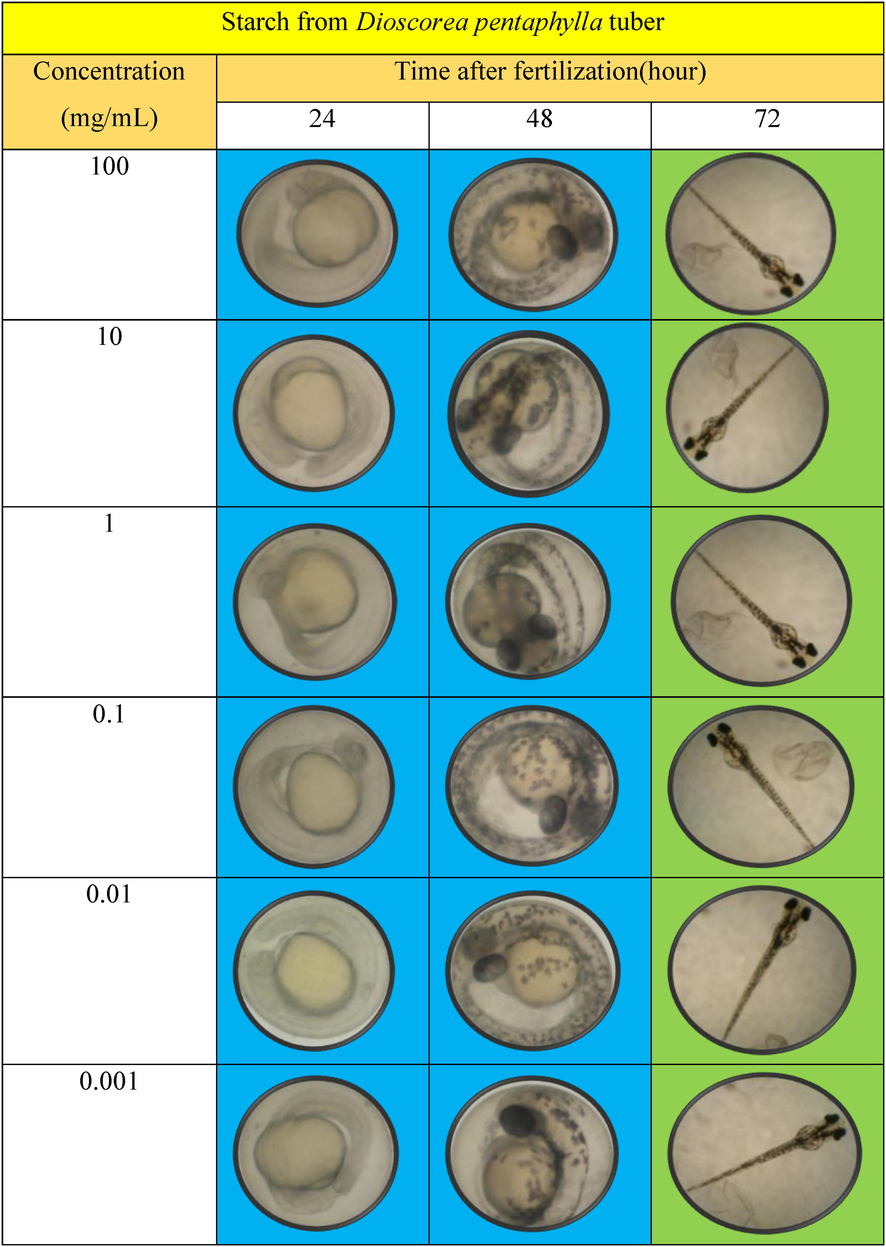
The in vitro toxicity assessment using fish embryo has been conducted to ensure that the Dioscorea pentaphylla starch consumed by the local community can be classified as non-toxic. Table 4 showed the progress fish embryo in a period of 24, 48 and 72 h. The observation made in a period of 48 h fish embryos exposed with the Dioscorea pentaphylla starch has normally developed. After 72 h, the percentage of embryos survived at different concentrations (0.001, 0.01, 0.1, 1, 10 and 100 mg/mL) were 100%, 93, 93, 87, 60, and 53%, respectively. The value of LC50 for Dioscorea pentaphylla starch was 29.27 mg/mL indicated at least about 50% of fish embryos survived. Thus, this starch can be considered as non-toxic and biocompatible because of its value of LC50 is categorized as toxic is when over 50% of embryos die at a concentration of 1 mg/mL equal to 1000 mg/L (Afidah Hassan, 2014). Blue box is for living embryo, green box is for hatching embryo.
4 Conclusions
Starch from Dioscorea pentaphylla tuber has been successfully extracted and characterized. Result showed these oval shaped starch granules represent 1.89 ± 0.44% of starch content in this tuber. The amylose content was 64.10 ± 1.15% and amylopectin 35.90 ± 1.15% respectively. The D. pentaphylla starch is classified as C-type with a degree crystallization of 32.90 ± 2.59%. Result showed that the starch gelatinization temperature was 81.04 ± 0.02 °C and no gelatinization occurred below 76 °C. There was no antimicrobial activity progress when the starch has been exposed to E. coli sp., S. aureus, S. cerevisiae, and Salmonella typhi. The toxicity tests have confirmed that this starch was non-toxic and potentially to be used for food and non-food industry.
Acknowledgements
This research was supported by research grant GUP-2020-038 and FRGS/1/2019/STG01/UKM/02/14. The authors would like to thank the Ministry of Higher Education (MOHE) Malaysia, Ministry of Education (MOE) Malaysia and Universiti Kebangsaan Malaysia (UKM), Malaysia for supporting this work through research grants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Removal of methylene blue dye in aqueous solution by Sorption on a bacterial-g-poly- (acrylic acid) polymer network hydrogel. Sains Malaysiana. 2015;44(6):827-834.
- [Google Scholar]
- Sintesis, pencirian dan penggunaan n-metilena fosfonik, oleoil dan oleoil metilena fosfonik kitosan sebagai penjerap minyak. Tesis Ijazah Doktor Falsafah: Fakulti Sains dan Teknologi, Universiti Kebangsaan Malaysia; 2014.
- Physicochemical characterisation of sago starch. Carbohydr. Polym.. 1999;38:361-370.
- [Google Scholar]
- Physicochemical characterization of starch extracted from Malaysian wild yam (Dioscorea hispida Dennst.) Emirates J. Food Agric.. 2014;26(8):652-658.
- [Google Scholar]
- Proximate and mineral compositions of Dioscorea rotundata (white yam) and Colocasia esculenta (white cocoyam) Pakistan J. Nutr.. 2010;9(10):998-1001.
- [Google Scholar]
- Official Methods of Analysis of the Association of Analytical Chemists (15th Ed.). Washington, D.C.: AOAC; 1990.
- Antioxidant activity of dioscorea and dehydroepiandrosterone (DHEA) in older humans. Life Sci.. 1996;59:147-157.
- [Google Scholar]
- Microstructural and physico-functional characterization of starches from selected cowpea (Vigna unguiculata L. Walp.) varieties developed for pest and disease resistance. J. Nutr. Food Sci.. 2011;01(02):1-5.
- [Google Scholar]
- Nanocellulose reinforced chitosan composite films as affected by nanofiller loading and plasticizer content. J. Food Sci.. 2010;75(1):N1-N7.
- [Google Scholar]
- Food-grade particle stabilized pickering emulsion using modified sago (Metroxylon sagu) starch nanocrystal. J. Food Eng.. 2020;280:109974
- [Google Scholar]
- Novel Dioscorea hispida starch-based hydrogels and their beneficial use as disinfectants. Journal of Bioactive and Compatible Polymers. 2016;31(1):45-59.
- [CrossRef] [Google Scholar]
- Small differences in amylopectin fine structure may explain large functional differences of starch. Carbohydr. Polym.. 2016;140:113-121.
- [Google Scholar]
- Solid state NMR studies on the structural and conformational properties of natural maize starches. Carbohydr. Polym.. 1998;36:277-284.
- [Google Scholar]
- Coulibaly et al., Coulibaly, S., F. A. Tetchi, M. Adou and G. N. Amani. 2013. Comparative characterization of some functional properties of flours of new plantin hybrids with the Orishele variety (Musa spp.) as control. Emir. J. Food Agric. 25:1-92013).
- Physicochemical properties of starch from Dioscorea pyrifolia tubers. Food Chem.. 2017;220:225-232.
- [Google Scholar]
- Granulometric structure, zeta potential and differential scanning calorimetry of native starch powders from Dioscorea spp. Mediterranean J. Chem.. 2014;2(5):632-638.
- [Google Scholar]
- Starch characteristics of black bean, chick bean, lentil, navy bean and pinto bean cultivars grown in Canada. Food Chem.. 2002;78:489–498)
- [Google Scholar]
- IMARC. 2020. Native Starch Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2020-2025. https://www.imarcgroup.com/native-starch-market.
- Novel Dioscorea hispida starch-based hydrogels and their benefial use as disinfectant. J. Bioactive Compatible Polym. 2015:1-19.
- [Google Scholar]
- Characterizations of starches isolated from five different Dioscorea species. Food Hydrocolloids. 2012;29(35–41)
- [Google Scholar]
- Physicochemical and functional characteristics of lentil starch. Carbohydrate polymers. 2013;92(2):1484-1496.
- [CrossRef] [Google Scholar]
- Rapid detection and identification of dioscorine compounds in dioscorea hispida tuber plants by LC-ESI-MS. BioResources. 2020;15(3):5999-6011.
- [Google Scholar]
- Some properties of potatoes and their starches II. Morphological, thermal rheological properties of starches. Food Chem.. 2002;79:183-192.
- [Google Scholar]
- Use of the national committee for clinical laboratory standards guidelines for disk diffusion susceptibility testing in New York State laboratories. J. Clin. Microbiol.. 2000;38(9):3341-3348.
- [Google Scholar]
- Synthesis and characterization of Dioscorea hispida sp. tuber starch-polyacrylamide wood coating and its facile inhibitory towards Pycnoporus sanguineus and Coptotermes curvignathus. Progress in Organic Coatings. 2016;99:182-190.
- [CrossRef] [Google Scholar]
- Characterization of new starches separated from several traditional Chinese medicines. Carbohydr. Polym.. 2010;82(148–152)
- [Google Scholar]
- Extraction of starch and enzymatic production of high amylose starch from sweet potato (Ipomea batatas) var. Telong. 2012;40(2):203-210.
- [Google Scholar]
- Isolation and properties of starch from some local cultivars of cassava and taro in Fiji. South Pacific J. Nat. Sci.. 2008;26(45–48)
- [Google Scholar]
- Nashriyah, M., N. Yusoff, S. Tajuddin, N. Ngah and M. R. M. Rejab. 2010. Dioscorea hispida Dennst (Dioscoreaceae): An overview. Buletin UniSZA, December.
- Preparation and application of carboxymethyl yam (Dioscorea esculenta) starch. AAPS PharmSciTech. 2009;10(1):193-198.
- [Google Scholar]
- Physicochemical properties of starches from purple and orange fleshed sweet potato roots at two levels of fertilizer. Starch-Stärke. 1996;48:395-399.
- [Google Scholar]
- Relationship between chain length distribution of amylopectin and gelatinization properties within the same botanical origin for sweet potato and buck wheat. Carbohydr. Polym.. 1998;37:153-158.
- [Google Scholar]
- OECD. 2006. Draft Proposal for A New Guideline - Fish Embryo Toxicity (FET) Test. Draft Guideline; Organization for Economic Cooperation and Development: Paris, France.
- A review on isolation, composition, physicochemical properties and modification of Bambara groundnut starch. Food Hydrocolloids. 2018;75:62-71.
- [Google Scholar]
- Afr. J. Biotechnol.. 2007;6(20):2348-2352.
- Antimutagenic effect of dioscorea pentaphylla on genotoxic effect induced by methyl methanesulfonate in the Drosophila wing spot test. Toxicol. Int.. 2014;21(3):258-263.
- [Google Scholar]
- Comparative study of effect of modification with ionic gums and dry heating on the physicochemical characteristic of potato, sweet potato and taro starches. Food Hydrocolloids. 2014;35:613-619.
- [Google Scholar]
- Transformation of crystalline starch nanoparticles into highly luminescent carbon nanodots: toxicity studies and their application. Carbohydr. Polym.. 2016;137(488–496)
- [Google Scholar]
- Proximate analysis and phytochemical and mineral constituents in four cultivars of yams and tuber crops in Sri Lanka. Trop Agric Res Ext. 2012;15(1):32-36.
- [Google Scholar]
- Nutritional and antinutritional evaluation of wild yam (Dioscorea spp.) Tropical and subtropical Agroecosystems. 2011;14(2):723-730.
- [Google Scholar]
- World corn market: analysis, trends and prospects of its deep processing. Agric. Resour. Econom. Int. Sci. E-J.. 2020;6(3):96-111.
- [Google Scholar]
- Quality of spaghetti made from full and partial waxy durum wheat. Cereal Chemistry. 2005;82(1):93-100.
- [CrossRef] [Google Scholar]
- Modified tuber starches as potential stabilizer for food-grade pickering emulsions. Food Res.. 2020;4(3):753-763.
- [Google Scholar]







