Translate this page into:
Rumex nervosus leaf extracts enhance the regulation of goblet cells and the inflammatory response during infection of chickens with Eimeria tenella
⁎Corresponding author at: Department of Zoology, College of Science, King Saud University, P.O. Box: 2455, Riyadh 11451, Saudi Arabia. squraishy@ksu.edu.sa (Saleh Al-Quraishy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Eimerian parasites are the main intestinal tract pathogens in many animals, which invade and damage the intestinal epithelium causing severe injuries and economic loss. Our study was planned to examine the ameliorative effect of Rumex nervosus leaf extracts (RNE) against Eimeria tenella-induced changes in caecal goblet cells and cytokines of chickens. The infected chickens with E. tenella were treated with 50, 100, 200 mg/Kg RNE, respectively. Amprolium was used as a reference drug. Our result showed that RNE contained 8 phytochemical components that were able to decrease the number of oocysts in bird’s faeces. Also, the number of goblet cells was decreased after infection. This number was increased after RNE treatment. In addition, RNE caused upregulation of the mucin gene, MUC2 after E. tenella infection. Moreover, the infection caused upregulation in the inflammatory cytokines IL1β, IL 6, INF-γ and LiTAF. This increase in mRNA expression of IL1β, IL 6, INF-γ and LiTAF was about 5, 5.6, 4 and 5.8 fold, respectively. Collectively, R. nervosus is a promising medicinal plant with anticoccidial, and anti-inflammatory properties and could be used for the treatment of eimeriosis.
Keywords
Eimeria tenella
Rumex nervosus
Goblet cells
Inflammation
Cytokines
1 Introduction
In recent decades, the demand for poultry meat has increased greatly by consumers especially broiler chickens, thus many countries use intensive breeding systems for increasing the level of production, where over 50 billion chickens are produced annually in allover world (Quiroz-Castañeda and Dantán-González, 2015). However, the huge production leads to increase of diseases either microbial or parasitic.
Coccidia is a renowned parasitic and infectious disease, caused by the eimerian parasites which represent the principal pathogens of the intestinal tract in poultry and many other domestic animals, caused major economic losses (Long, 1990; Yunus et al., 2007; Mehlhorn, 2014). Among poultry, Eimeria parasites infect numerous species-specific sites in the intestine, triggering intestinal coccidia except for Eimeria tenella, which invades caeca cells. (Daugschies and Najdrowski, 2005; Chapman et al., 2010; Blake and Tomley, 2014). E. tenella is the most dangerous chicken Eimeria sp because it is very virulent and leading to severe hemorrhage and very high mortality (Fossum et al., 2009).
The goblet cells represent an immunological defense line in the intestinal tract; it is responsible of secreting mucus, so when E. tenella invade the caecal epithelial cells, it increase the rate of mucus production and changes the constituents to protect the epithelium as immunological reaction against the parasite (Sharma and Schumacher, 1995; Meslin et al., 1999). In addition, immune cells trigger and generate cytokines that are proteins or peptides such as interferons (IFN), tumor necrosis factors (TNF), interleukins (IL), transforming growth factors (TGF) once infection occurs for chickens. (Wigley and Kaiser, 2003).
Notwithstanding effective control of avian coccidiosis with various anticoccidial chemical drugs (Sharma and Ranjan, 2015) such as Amprolium, Diclazoril, Monincine and others, the incidence of drug resistance from parasites and high costs as well as consumers prefer residue-free meats have prompted researchers to propose and establish alternative control programs (Chapman et al., 2010; Abbas et al., 2011; Wunderlich et al., 2014).
Today, researchers consider the medicinal plants (e.g., garlic, neem, moringa, pomegranate, and others) and their derivatives to be the main point of interest. They obtain bioactive phytochemical compounds against the induced eimeriosis and also innocuous of the consumers for chicken’s meat (Abbas et al., 2012; Wunderlich et al., 2014; Bauri et al., 2015; Muthamilselvan et al., 2016; Thagfan et al., 2017).
Rumex nervosus is a medicinal herb that belongs to the Polygonaceae family and has pharmacological and biological properties (Alwashli et al., 2012), used as a traditional therapy for many diseases, and functions as an antiparasitic (Awadh Ali et al., 2016). However, it is not yet tested as anticoccidial therapy in broiler chickens. Therefore, in this study, we assessed the efficacy of R. nervosus leaf extracts (RNE) as anticoccidial and anti-inflammatory agent in E. tenella- experimentally infected broiler chickens.
2 Material and methods
2.1 Collection, extract preparation and gas chromatography-mass spectrometry (GC–MS) analysis of R. nervosus
Fresh leaves of R. nervosus were harvested during the fully developed green stage of the plant from Taif region, Saudi Arabia. The plant was authenticated at the herbarium in Department of Botany, Faculty of Science, King Saud University. The leaves were air-dried, and milled into powder by using an electric blender then cold extracted in 70% methanol with mixing continuously by shaker for 24 h (Amer et al., 2015). The dissolved contents were filtered and evaporated at 50 °C in rotary evaporator. The solvent was evaporated to obtain dark hydro-alcohol extractions (Chikoto and Eloff, 2005), and to obtain liquid extract again, it was dissolved in dimethyl sulfoxide (DMSO) (Al Yahya et al., 2018) for inoculating chickens.
Thermo Scientific, Trace GC Ultra and ISQ Single Quadruple MS were used to analyze the extract of R. nervosus. The used flow rate was 1.5 mL/min. The identification of mass spectrum was conducted using the database of the Wiley9, replib, and National Institute Standard and Technology.
2.2 Infection of birds
Oocysts of E. tenella strain were collected from the cecum of naturally infected chickens. Identification of the species was based on the morphological characteristics described by Thienpont et al. (1979), as well as the site of lesions. The collected oocysts were sporulated (using 2.5% potassium dichromate at 25 °C 72 h), washed with distilled water, and passaged from two to three times in healthy chickens to confirm the site of lesions. After the confirmation of the parasite species, oocysts were also collected, sporulated, washed and dose was adjusted to 1 × 104/100 µL/bird.
3 Experimental design
Seventy broiler chicks of one-day-old were purchased from a commercial hatchery. They were bred until reaching 21 days’ age at the animal facility of Zoology Department, King Saud University. Then, all chickens were individually weighted and allocated into 7 groups (10 birds per group). The first two groups as are the non-infected and the non-infected-treated group with 200 mg/kg RNE, respectively. The third to the seventh groups were infected with 10,000 E. tenella oocysts (Lee et al., 2012). Groups 4, 5 and 6 were treated after infection with 50, 100, 200 mg/Kg RNE, respectively. The seventh group was treated with 25 mg/Kg Amprolium 60% (Veterinary and Agricultural Products Company (VAPCO), Jordan) (EMEA, 2001). The treated groups were medicated orally and daily with the extract until day 6 post infection (Wang et al., 2016).
3.1 Counting of goblet cells
In order to obtain cecum samples, 5 birds of each group were slaughtered on day 6 post infection with E. tenella. Small parts of cecum were freshly prepared and fixed in 10% neutral buffered formalin. Sections were stained with Alcian blue for counting of the caecal goblet cells. For each chicken, the number of goblet cells in the cecum was counted on at least ten well-orientated villous-crypt units (VCU). Results were expressed as the mean number of goblet cells per ten VCU (Allen et al., 1986).
3.2 Quantitative real-time PCR
Total RNA from caecum tissue was extracted by using Trizol reagent and then converted to cDNA using RevertAid™ H Minus Reverse Transcriptase (Fermentas, Thermo Fisher Scientific Inc., Canada) in accordance with the manufacturer’s instructions. For gene expression analysis, quantitative real-time PCR was employed by using QuantiFast SYBR Green RT-PCR kit (Qiagen, Hilden, Germany). Sense and antisense primers were obtained from Sigma-Aldrich and are listed in Table S1. All reactions were performed in duplicate by using ViiA™ 7 System (Thermo Fisher Scientific, CA, USA). The PCR cycling conditions were set as follows: initial denaturation at 95 °C for 2 min, followed by 45 cycles of denaturation at 94 °C for 60 s, annealing at 60 °C for 20 s, and extension at 70 °C for 20 s, with a final extension at 70 °C for 10 min. The relative differences in gene expression between different groups were determined by using Ct method (2−ΔΔct) (Livak and Schmittgen, 2001). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as the reference gene.
3.3 Statistical analysis
All obtained data were analyzed using computerized statistical program (SPSS version 17.0). Statistical comparison among the studied groups was carried out using one-way analysis of variance (ANOVA). The significant levels for means were tested by Duncan’s t-test when P ≤ 0.05.
4 Results
The phytochemical analysis carried out by GC-Mass (Fig. 1) showed that RNE contained 8 phytochemical components (Table S2) at different peak areas and retention time.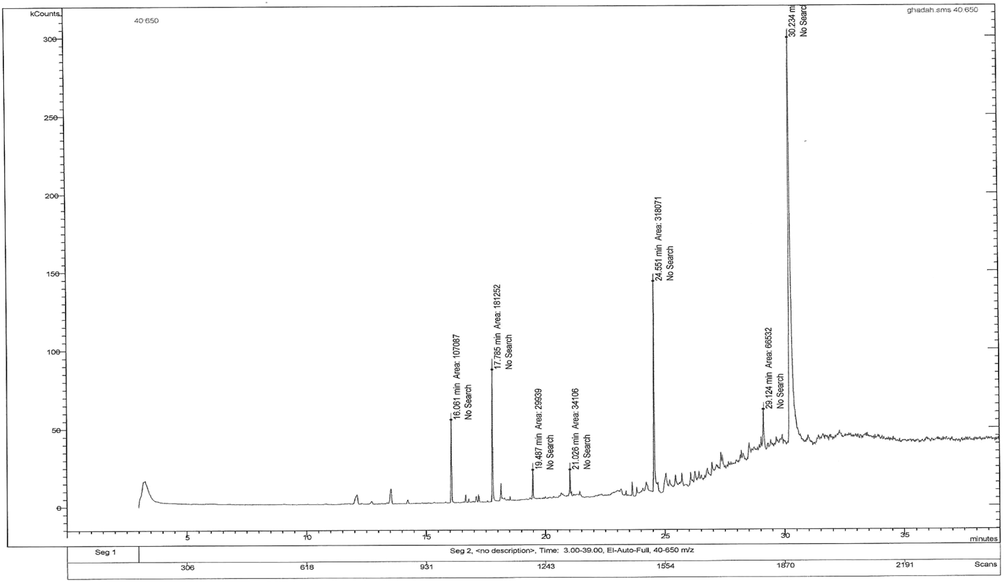
GC–MS chromatogram for phytochemical compounds of Rumex nervosous leaf extracts.
RNE was able to suppress the shedded oocysts by about 72% when treating the E. tenella infected chickens with 200 mg/kg (Fig. 2), while in the group treated with the reference drug Amprolium, the oocyst output was still high and reached 35.77 ± 8.69 × 106/g faeces.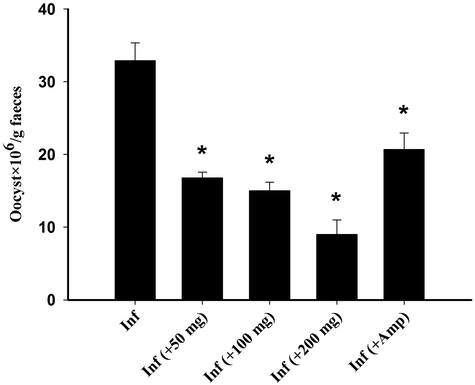
RNE treatment induced changes in the number of E. tenella oocysts. Different doses (50, 100 and 200 mg/kg) of RNE indicated changes in oocysts output in chickens’ faeces. Values are expressed as means ± SEM. *p < 0.05 with respect to the infected group.
Our results concerning the change in goblet cells number (Fig. 3) revealed that the infection of animals with E. tenella induced a decrease in the number of goblet cells (26.72 ± 2.27/10 VCU) when compared to the non-infected control chickens (60.60 ± 1.60). Treatment of the birds with RNE significantly increased (P ≤ 0.05) the number of goblet cells (53.90 ± 2.81) more than when the chickens treated with Amprolium (41.89 ± 3.70/10 VCU) (Fig. 4). These data were obtained from the examination of the caecum sections stained with alacian blue (Fig. 3). The goblet cells were arranged in the villi of chicken caecum.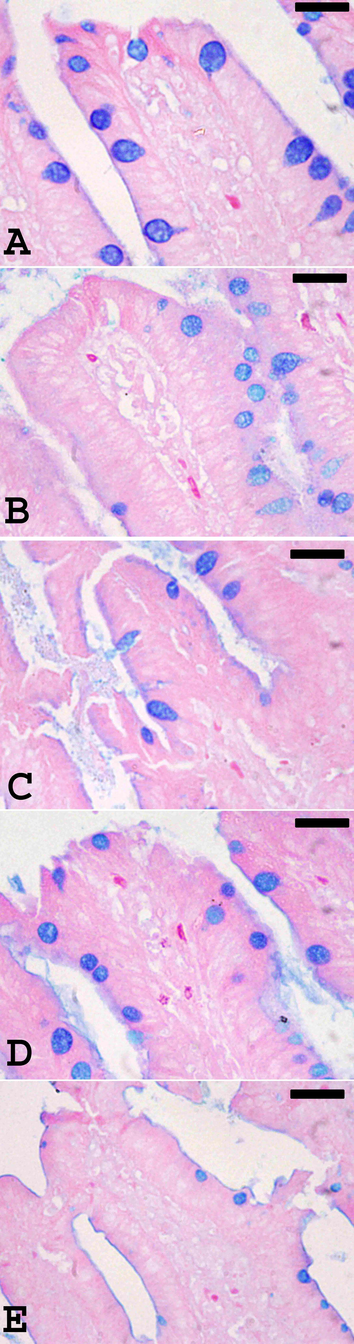
Alcian blue staining of caecum sections showing goblet cells in control chickens (A), RNE-treated chickens (B), E. tenella-infected chickens (C), and infected chickens treated with 200 mg/Kg RNE (D) and Amprolium (E). Scale bar = 50 μm.
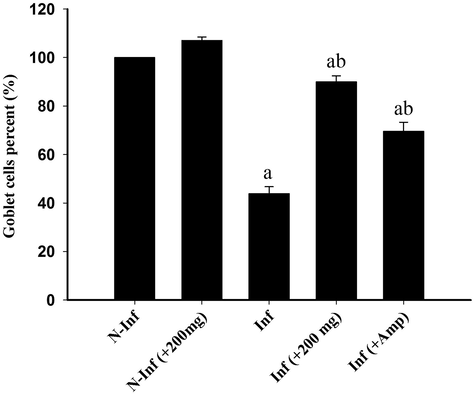
Changes in the number of caecal goblet cells in control chickens (A), RNE-treated chickens (B), E. tenella-infected chickens (C), and infected chickens treated with 200 mg/Kg RNE (D) and Amprolium (E). Values are means ± SEM. a: p < 0.05, significant change with respect to control group; b: p < 0.05, significant change with respect to infected group.
Fig. 5 showed that the mucin gene, Muc2 was downregulated by the E. tenella infection. This downregulation is about one fold compared to the control group. RNE could upregulate the expression of Muc2 in the infected-treated group nearly similar to that induced by the reference drug, Amprolium (Fig. 5).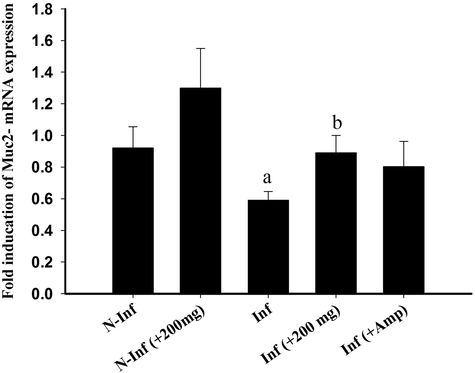
Effect of RNE on the mRNA expression of MUC2 in the caecum samples from E. tenella-infected chickens. The expression values obtained by RT-PCR analysis were normalized to the GAPDH mRNA level and are shown as fold induction (in log 2 scale) relative to the mRNA level in the control. a: P < 0.01, significant change with respect to the control group; b: P < 0.01, significant change with respect to the infected group.
The infection caused upregulation in the inflammatory cytokines IL1β, IL 6, INF-γ and LiTAF (Fig. 6). This increase in mRNA expression of IL1β, IL 6, INF-γ and LiTAF was about 5, 5.6, 4 and 5.8 fold, respectively when compared to the non-infected control animals (Fig. 5). RNE could significantly downregulate the expression of these genes nearly similar to Amprolium (Fig. 6).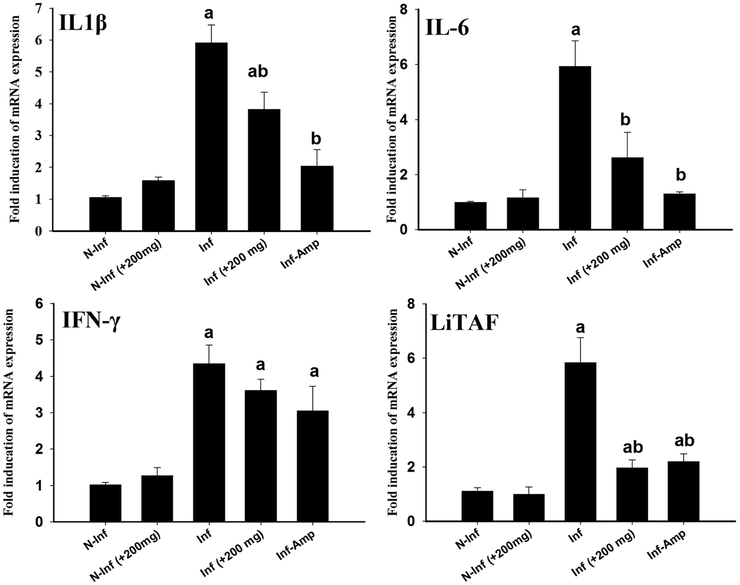
Effect of RNE on the mRNA expression of IL1β, IL 6, INF-γ and LiTAF in the caecum samples from E. tenella-infected chickens. The expression values obtained by RT-PCR analysis were normalized to the GAPDH mRNA level and are shown as fold induction (in log 2 scale) relative to the mRNA level in the control. a: P < 0.01, significant change with respect to the control group; b: P < 0.01, significant change with respect to the infected group.
5 Discussion
The infection of bird with E. tenella began with the oral uptake of the sporulated oocysts that invade the intestinal caecum resulting in destruction of the caecal epithelium due to the multiplication of E. tenella stages and finally the developed oocysts were released in faeces (Mehlhorn, 2014).
Researchers are seeking to find multiple prevention methods to monitor the effects of eimeriosis, this is to reduce the millions of dollars paid to overcome the induced damage in poultry industry (Ott et al., 2018). The consequences of damage vary from local, degeneration of the intestinal tissue to death in more severe cases (Vermeulen et al., 2001).
To ovoid adverse effects on animal performance, there's a need to develop new agents with low cost and reduced side effects against eimeriosis. Through this study, RNE was able to control the increased oocysts output in chicken’s faeces. This may be due to the presence of some active components in RNE acting against eimeriosis. Previous studies reported the effective role of another herbal extracts like Curcuma longa (Abbas et al., 2010), Azadirachta indica, Nicotiana tabacum, Calotropis procera and Trachyspermum ammi (Zaman et al., 2012), Piper sarmentosum (Wang et al., 2016) against coccidiosis.
RNE contained some active phytochemical compounds. One of these compounds is isophorone that reported to possess biological activity against a variety of microorganisms, and also had anti-oxidant properties (Kiran et al., 2013). Also, the phenolic compound, eugenol present in RNE was found to have antioxidant and antiapoptotic properties (Pandey and Mishra, 2004). Moreover, Sutili et al. (2014) and El-kady et al. (2019) confirmed the antiparasitic activity of eugenol. Interestingly, the anticoccidial activity of eugenol was reported by Remmal et al. (2013). The phytochemical compound, 2,5-Cyclohexadiene-1,4-dione, 2,6-bis(1,1-dimethylethyl)- present in RNE is related to Benzoquinones that possess potent pharmacological properties against inflammatory and microbial diseases (Pangal et al., 2013). Retinal is also called 9-cis-Vitamin A aldehyde, this compound could regulate the intestinal mucosal immune response (Sun et al., 2007).
Animal infected with Eimeria show a significant weight loss, due to the poor nutrient absorption and decreased immune response resulting in intestinal tissue damage (Chapman et al., 2010).
In this study, the caecal villi were damaged and also the goblet cells affected. The decrease in goblet cells number due to infection was previously studied not only in infected chicken with E. tenella (Yunus et al., 2007) but also in rodents infected with Eimeria. Dkhil et al. (2013) reported that the reason of the decreased goblet cells on mice jejunum infected with E. papillata is that the parasite destroys the stem cell producing goblet cells leading to the reduced number in the intestinal villi (Cheng, 1974).
Due to eimeriosis there was a disturbance in the population of some immune cell like lymphocytes, mast cells, goblet cells and macrophages (Huntley et al., 1985; Vervelde et al., 1996).
E. tenella infection induces tissue inflammation due to the invasion of the parasite to the caecal epithelium. These inflammations increase the expression of the inflammatory cytokines (Laurent et al., 2001; Hong et al., 2006). These cytokines were produced by the leucocytes as a regulatory response (Hong et al., 2006).
Chickens infected with E. tenella develop strong immune response through T-lymphocytes (Hong et al., 2006). Cytokines are considered to be one of the major molecules controlling the host response to poultry infection with Eimeria (Wang et al., 2016). Hong et al. (2006) reported that upregulation of cytokine mRNA levels after E. tenella infection. In this study, IL 1β was upregulated after infection. It played a critical role in initiating inflammation (Laurent et al., 2001; Dalloul et al., 2006). Also, our data showed that there was upregulation of IFN-γ after E. tenella infection. This result was supported by the previous observations carried out by Laurent et al. (2001).
Similarly, for homeostasis the proinflammatory cytokine, IL-6 was upregulated during infection (Lynagh et al., 2000). Moreover, LiTAF mRNA expression was increased due to the induced eimeriosis by E. tenella. This lipopolysaccharide induced TNF- γ factor was also found to be more expressed during Eimeria infection (Hong et al., 2006).
Collectively, R. nervosus is a promising medicinal plant with anticoccidial, and anti-inflammatory properties and could be used for the treatment of eimeriosis as a food additive but further studies are required to understand the metabolic activity and the mechanism of action of each phytochemical component during the infection.
Funding
This study was supported by Research Supporting Project (RSP-2019/23), Riyadh, King Saud University.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Botanicals: an alternative approach for the control of avian coccidiosis. Worlds. Poult. Sci. J.. 2012;68(2):203-215.
- [Google Scholar]
- Anticoccidial drug resistance in fowl coccidia: the state of play revisited. Worlds. Poult. Sci. J.. 2011;67(2):337-350.
- [Google Scholar]
- Anticoccidial activity of Curcuma longa L. in broilers. Braz. Arch. Biol. Technol.. 2010;53(1):63-67.
- [Google Scholar]
- Phytochemicals and antimicrobial activities of Rumex nervosus natural populations grown in Sarawat mountains, Kingdom of Saudi Arabia. Arab. J. Sci. Eng.. 2018;43(7):3465-3476.
- [Google Scholar]
- The role of mucus in the protection of the gastroduodenal mucosa. Scand. J. Gastrol.. 1986;21(125):71-78.
- [Google Scholar]
- Anti-Inflammatory and analgesic effects of ethanol extract of Dracaena Cinnabari Balf, as endemic plant in Yemen. Int. J. Pharm. Bio Sci.. 2012;3(2):96-106.
- [Google Scholar]
- Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. BioMed. Res. Int. 2015
- [Google Scholar]
- In vitro antiprotozoal activity of five plant extracts from Albaha region. World J. Pharmacol. Res.. 2016;5(3):338-346.
- [Google Scholar]
- A review on use of medicinal plants to control parasites. Indian J. Nat. Prod. Res.. 2015;6(4):268-277.
- [Google Scholar]
- Securing poultry production from the ever-present Eimeria challenge. Trend. Parasitol.. 2014;30(1):12-19.
- [Google Scholar]
- Forty years of monensin for the control of coccidiosis in poultry. Poult. Sci.. 2010;89(9):1788-1801.
- [Google Scholar]
- Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine II. Mucous cells. Am. J. Anat.. 1974;141(4):481-501.
- [Google Scholar]
- Chikoto, H., Eloff, J.N., 2005. Antioxidant. Patent NR 2005/09681.
- Unique responses of the avian macrophage to different species of Eimeria. Mol. Immunol.. 2006;44(4):558-566.
- [Google Scholar]
- Eimeriosis in cattle: current understanding. J. Vet. Med. Ser. B. 2005;52(10):417-427.
- [Google Scholar]
- Goblet cells and mucin related gene expression in mice infected with Eimeria papillata. Sci. World J. 2013
- [Google Scholar]
- eugenol, a potential schistosomicidal agent with anti-inflammatory and antifibrotic effects against Schistosoma mansoni, induced liver pathology. Infect. Drug. Resist.. 2019;12:709.
- [Google Scholar]
- European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections (EMEA), 2001. Committee for Veterinary Medicinal Products (Amprolium). /MRL/767/00-FINAL.
- Causes of mortality in laying hens in different housing systems in 2001 to 2004. Acta. Vet. Scand.. 2009;51(1):3.
- [Google Scholar]
- Analysis of chicken cytokine and chemokine gene expression following Eimeria acervulina and Eimeria tenella infections. Vet. Immunol. Immunopathol.. 2006;114(3–4):209-223.
- [Google Scholar]
- Systemic release of mucosal mast cell protease during infection with the intestinal protozoal parasite, Eimeria nieschulzi. Studies in normal and nude rats. Parasite Immunol.. 1985;7:489-501.
- [Google Scholar]
- Kiran, I., Özşen, Ö., Çelik, T., İlhan, S., Gürsu, B.Y., Demirci, F., 2013. Microbial transformations of isophorone by Alternaria alternata and Neurospora crassa. Nat. Prod. Commun. 8(1), 1934578X1300800114.
- Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun.. 2001;69(4):2527-2534.
- [Google Scholar]
- Anticoccidial effects of Galla rhois extract on Eimeria tenella-infected chicken. Lab. Anim. Res.. 2012;28(3):193-197.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402-408.
- [Google Scholar]
- Coccidiosis of Man and Domestic Animals. Florida: CRC Press, Boca Raton; 1990. p. :356.
- Interleukin-6 is produced during both murine and avian Eimeria infections. Vet. Immunol. Immunopathol.. 2000;76:89-102.
- [Google Scholar]
- Mehlhorn, H. (ed.), 2014. Encyclopedic Reference of Parasitology, 4th edn., Springer, Berlin.
- Variation of mucin distribution on the rat intestine, caecum and colon: effect of the bacterial flora. Comp. Biochem. Physiol. A. 1999;123:35-239.
- [Google Scholar]
- Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. Evid Based Complement. Alternat. Med.. 2016;2016:1-19.
- [Google Scholar]
- The impact of β-glucans on performance and response of broiler chickens during a coccidiosis challenge. Poult. Sci.. 2018;97(8):2713-2721.
- [Google Scholar]
- Modification of thymocytes membrane radiooxidative damage and apoptosis by eugenol. J. Environ. Pathol. Toxicol. Oncol.. 2004;23(2):117-122.
- [Google Scholar]
- Synthesis, characterization and study of antimicrobial activity of 2, 6-ditertiary butyl-1, 4-benzoquinone hydrazones. Int. Res. J. Pharm.. 2013;4(8):172-176.
- [Google Scholar]
- Control of avian coccidiosis: future and present natural alternatives. BioMed. Res. Int. 2015
- [Google Scholar]
- Oocysticidal effect of essential oil components against chicken Eimeria oocysts. Int. J. Vet. Med.. 2013;2013:599816
- [Google Scholar]
- Morphometric analysis of intestinal mucins under different dietary conditions and gut flora in rats. Dig. Dis. Sci.. 1995;40:2532-2539.
- [Google Scholar]
- Diareena is the best alternative of chemical drug salinomycin to cure and prevention from coccidiosis. World J. Pharm. Pharmacy. Sci.. 2015;5(1):943-949.
- [Google Scholar]
- Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med.. 2007;204(8):1775-1785.
- [Google Scholar]
- Anthelmintic activity of the phytochemical eugenol against the fish parasite Gyrodactylus sp. and acute toxicity in Daphnia pulex. Pan-Amer. J. Aquat. Sci.. 2014;9(3):223-227.
- [Google Scholar]
- In vivo anticoccidial activity of Salvadora persica root extracts. Pakistan J. Zool.. 2017;49(1):53-57.
- [Google Scholar]
- Diagnosing Helminthiasis Through Coprological Examination. Beerse, Belgium: Janssen Research Foundation; 1979. p. :34-36.
- Control of coccidiosis in chickens by vaccination. Vet. Parasitol.. 2001;100(1–2):13-20.
- [Google Scholar]
- In situ characterization of leucocyte subpopulations after infection with Eimeria tenella in chickens. Parasite Immunol.. 1996;18:247-256.
- [Google Scholar]
- Anticoccidial effect of Piper sarmentosum extracts in experimental coccidiosis in broiler chickens. Trop. Anim. Health. Prod.. 2016;48(5):1071-1078.
- [Google Scholar]
- Towards identifying novel anti-Eimeria agents: trace elements, vitamins, and plant-based natural products. Parasitol. Res.. 2014;113:3547-3556.
- [Google Scholar]
- Yunus, M., Suwanti, L.T., Mufasirin, 2007. Cecal goblet cell response on intracellular development of Eimeria tenella in susceptible and infected chickens, Med. Kedo. Hew. 23 (3), 132–138.
- Anticoccidial activity of herbal complex in broiler chickens challenged with Eimeria tenella. Parasitology. 2012;139(2):237-243.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.01.024.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







