Translate this page into:
Reduction in phytotoxicity of a textile wastewater against Vigna radiata using Citrobacter sp. M41 in a bioaugmented packed bed column bioreactor
⁎Corresponding author. sabir.hussain@gcuf.edu.pk (Sabir Hussain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The current research was carried out to devise a biological strategy to remediate dyes and metal ions from textile wastewater. For this purpose, Citrobacter sp. M41 was isolated from a textile wastewater sample. This strain efficiently decolorized > 90 % of the added reactive black-5 (RB5) dye using yeast extract as a C source at 8.5 pH and 30 °C in mineral salt medium containing a mixture of metals (Pb2+, Ni2+ and Cd2+). Moreover, M41 showed > 90 % decolorization of RB5 beside > 80 % concurrent removal of hexavalent Chromium [Cr(VI)]. Furthermore, the granulated corncob and its biochar bioaugmented with M41 and filled within packed bed column based bioreactors efficiently (>90 %) removed the RB5 and Cr(VI) from a textile wastewater. The M41 bioaugmented corncob biochar resulted into a 60.8 %, 57.8 % and 68.7 % decrease in chemical oxygen demand (COD), electrical conductivity (EC) and total dissolved solids (TDS) of the wastewater, respectively. A phytotoxicity study conducted by using mung bean (Vigna radiata) as a test crop indicated that the treated synthetic wastewater was relatively less toxic as compared with the untreated wastewater. Therefore, it can be concluded that Citrobater sp. M41 is an important bio-resource for treatment of textile effluent polluted with azo dyes and Cr(VI).
Keywords
Citrobacter sp. M41
Wastewater Treatment
Bioaugmented Packed Bed Column Bioreactors
Hexavalent Chromium
Azo Dyes
1 Introduction
Textile sector is reported to be a major producer of colored wastewaters because a significant amount of the applied dyes (5–50 %) remains unsuccessful to attach with the substrate at the time of dying process (Robinson et al., 2001). Apart from dyes, immense amount of some heavy metals like Cr+2, Pb+2, Ni+2, Cd+2, Mn+2, Cu+2, Co+2 are also used in dyeing processes and can be found in textile wastewater (Imran et al., 2015). Among various heavy metals, [Cr(VI)] is excessively utilized in dyeing processes. It is being used as a part of complexed dyes, oxidant in sulphur and vat dyeing and a mordant to fix dye color on fabrics during chrome dyeing process (Desai et al., 2009; Maqbool et al., 2016).
Releasing wastewater containing various azo-dyes and heavy-metals into surface water resources causes various issues including aesthetic problems, restriction in light absorption, hindrance in movement of oxygen and increase oxygen demands which poses serious threats for aquatic organisms (Li et al., 2012; Imran et al., 2015, Imran et al., 2019). Literature shows that several azo dyes and their metabolites as well as Cr(VI) have negative effects on biotic components of the environment (Mittal et al., 2005; Imran et al., 2019). Azo-dyes and Cr(VI) are found to be stable in soil and capable of affecting microbial processes and enzymatic activities (Imran et al., 2015). Hence, it is very important to design some suitable approaches to remove dyes and hexavalent chromium from textile wastewaters.
Recently, various scientists conducted different experiments to show the significance of microorganisms like bacteria, fungi, actinomycetes and algae for biological treatment of textile wastewater (Khalid et al., 2012; Hussain et al., 2017; Baig et al., 2019; Imran et al., 2019). Despite the fact that various fungal species have shown effectiveness in remediation of azo dyes and metal ions, bacteria are preferred due to rapid removal rate and shorter life span (Elisangela et al., 2009). Various strains of bacteria from several genera isolated from the wastewaters have shown potential to decolorize and detoxify various dyes (Hafeez et al., 2018; Imran et al., 2019; Hussain et al., 2020) and detoxify different metal ions including Cr(VI) (Maqbool et al., 2015). However, few recent studied showed the concurrent elimination of dyes and heavy metal like Cr(VI) by some of the bacteria isolated from different locations (Anwar et al., 2014; Maqbool et al., 2016; Hussain et al., 2020; Bilal et al., 2022).
Although, few bacterial isolates are already used for concurrent azo dyes decolorization and Cr(VI) removal, it is much needed to isolate and characterize novel more potential bacterial isolates for such simultaneous removal and test the possibility of their application for treatment of the wastewaters. In this regard, the current research work was carried out for isolation of a novel bacterial strain which might be harboring the capability of contemporaneous removal of reactive black 5 (RB5) dyes and Cr(VI) in the medium containing the metal ions. The current research is exclusive because this novel strain has the capability of concurrent removal of dyes and Cr(VI) in the medium containing other metals. Moreover, this strain was tested for its application for concurrent decolorization of RB5 and Cr(VI) removal in packed bed column based bioreactors using the granulated corncob and granulated corncob biochar as support materials for bioaugmentation with this strain. The untreated and treated textile wastewaters were also tested for their phytotoxicity using mung bean (Vigna radiata) as a test crop.
2 Materials and methods
2.1 Dyes, chemicals and culture media
The major characteristics of the dyes used in this research have been presented in Table S1. A mineral salt (MS) medium having the composition already reported in Hussain et al. (2013) was used for isolation of bacteria. However, for providing the metal stress, this medium was added with different metals (Cd2+, Pb2+ & Ni2+ @ 10 mg/L each) in the form of CdCl2·2H2O, Pb (NO3)2 and NiCl2. Wherever required, the agar (16 g/L) was added to medium. The composition of any other medium has been presented wherever used in this study.
2.2 Collection and characterization of textile wastewater samples
The textile wastewater samples were collected from six different industries of Faisalabad (Pakistan) in pre-sterilized plastic bottles and analyzed for different physicochemical properties following the standard procedures. The sources and physicochemical properties of effluent samples have been presented in Table 1. A component of the original samples was preserved (4 °C under dark) which were later on processed to isolate metal tolerant dye decolorizing microbial strains.
Sampling point
pH
E.C
(dS m−1)TSS
(mg/L)TDS
(mg/L)Na
(meq L-1)
Paharang Drain
(Sargodha Road Faisalabad)7.55
4.28
48
3875
41.54
Moti Drain
(Samundri Road Faisalabad)7.6
9.71
104
5194
34
Chenab Textiles
(Lahore Road Faisalabad)7.15
6.1
72
4461
58.6
Chanab Textiles 2
(Lahore Road Faisalabad)7.30
6.0
70
4052
59.02
Hina Sana Textiles
(Millat Road Faisalabad)8.05
4.63
50
3276
42.48
Pahari Nala
(Samundri Road Faisalabad)8.25
7.0
84
4956
42.48
2.3 Isolation of the potential reactive black 5 (RB5) decolorizing bacterium
The bacteria with potential to decolorize RB5 while tolerating the metal ions were isolated following an enrichment procedure followed by a dilution plating technique as already reported by Hussain et al. (2013). Among the purified bacterial colonies, 19 fast growing bacterial isolates were subjected to screening to check their ability for RB5 (200 mg/L) decolorization in MS containing multi-metal mixture (Cd2+, Pb2+, Ni2+). The isolate M41 was selected for use in further experiments because it efficiently decolorized RB5.
2.4 Identification of the isolate M41
Identification of M41 was done by amplifying and sequencing its 16S rRNA. Amplification of the 16S rDNA gene of M41 was done through PCR using 27f and 1492r primers in accordance with program and reaction mixture previously explained by Maqbool et al. (2016). Purification and sequencing of 16S ribosomal RNA was done by Macrogen (Seoul, Korea). BlastN analysis was carried out for comparison of 16S gene sequence of M41 with known sequences in the NCBI GenBank. Several alignments of sequence of M41 were carried out using clustalX software and a phylogenetic tree was constructed through neighbor joining method (Thompson et al., 1997; Maqbool et al., 2016).
2.5 Characterization of Citrobacter sp. M41 for its decolorizing potentials
The decolorizing capability of Citrobacter sp. M41 for selected different azo dyes was tested in MS media under stress of the mixture of metal ions (Cd2+, Pb2+ and Ni2+). The broth medium added with respective dye (200 mg/L) was inoculated with bacterial culture of strain M41 to develop a bacterial density (OD600) of 0.2. These samples and the un-inoculated controls of each dye were kept at 30 °C in static environment. Aliquots of each incubated sample were taken at different times, centrifuged (7000 rpm for 10 min) and analyzed for estimation of decolorization using UV–visible spectrophometer at their specific λmax (Table S1).
Reactive black 5 decolorization by Citrobacter sp. M41 was also tested under shaking as well as static incubations under given conditions. For this purpose, two set of MS broth media containing RB5 (@ 200 mg/L) were inoculated with strain M41 [optical density (OD600) of 0.2]. All of inoculated tubes and their un-inoculated controls were sealed tightly and one set was incubated in shaking (150 rpm) conditions and the other was incubated in static environment at 30˚C. The aliquots were separated and put under centrifugation (7000 rpm for 10 min). In order to estimate RB5 decolorization, supernatants were assessed using UV–visible spectrophotometer.
Glucose, maltose, D-mannitol and yeast extract carbon co-substrates were evaluated to see the impact on RB5 decolorizing potential of M41. For this, the carbon co-substrates were added in media separately along with a control without any co-substrate. After inoculation with M41 (OD600 of 0.2), all the vials as well as their counter un-inoculated controls were kept in static at 30˚C. During incubation, the samples were taken at different time intervals, centrifuged (7000 rpm for 10 min) and used to estimate decolorization (%) of RB5.
Strain M41 was also tested to decolorize RB5 at various pH levels ranging from 5.5 to 9.5 using MS broth media under stress due to the mixture of metal ions. The adjustment of pH of the media was done using standard solutions of HCl and NaOH. After inoculation with M41 (OD600 of 0.2), all the vials along with un-inoculated controls in triplicate were kept in static conditions at 30◦C. Over regular time periods, aliquots were collected, centrifuged (7000 rpm for 10 min) and examined with UV–Visible spectrophotometer for RB5 decolorization.
The efficiency of M41 for decolorization of RB5 dye at its varying initial concentrations was also tested. For this purpose, various concentrations of RB5 (from 25 to 1000 mg/L) were maintained and inoculated with M41 (OD600 of 0.2) in the media, separately. All of the inoculated samples and their un-inoculated controls in triplicates were incubated in static environment at 30˚C. Over regular time periods, aliquots of each incubated vial were taken and centrifuged for 10 min at 7000 rpm. The supernatants were analyzed to check decolorization of RB5.
The strain M41 was also tested to decolorize RB5 in the media containing different levels of metal ions. MS medium was edited with four separate tertiary combinations (0, 10, 50 and 100 mg/L of each selected metal ion) of the metal ions (Cd2+, Pb2+ and Ni2+). Addition of RB5 was done @ 200 mg/L. Optical density of 0.2 (OD600) was obtained after inoculating the strain M41 and incubated in triplicates at 30 °C in static incubator. Aliquots were collected, centrifuged (7000 rpm for 10 min) and analyzed for RB5 decolorization.
2.6 Concurrent RB5 decolorization and Cr(VI) removal by Citrobacter sp. M41
The strain M41 was also evaluated for concurrent RB5 decolorization and Cr(VI) removal. For this, 18 mL of the medium was added with of Cr(VI) (25 mg/L) using K2Cr2O7 and RB5 (200 mg/L). The bacterial culture of M41 was inoculated (OD600 equal to 0.2) and incubated statically in dark at 30 °C. All the experiment was carried in triplicates. Aliquots were taken at specific time intervals. Than it was subjected to centrifugation at 7000 rpm for 10 min. Supernatants were taken and divided in to two portions. One portion was used to measure Cr(VI) reduction by following diphenylecarbazide (DPC) method as previously explained by Anwar et al. (2014). However, second portion was used for estimation of RB5 decolorization.
2.7 Application of the strain M41 for removal of RB5 and Cr(VI) from a textile wastewater in packed bed columns based bioreactors
2.7.1 Use of corncob and biochar as biosorbent for concurrent removal of RB5 and Cr(VI)
For this study, corncob biomass obtained from an agricultural field was divided into small pieces and dried at 60 ◦C for overnight. After drying, corncob biomass was subjected to grinding using electrical grinder and sieved through ASTM round stainless sieve of mesh size (5 mm). Granulated biochar was prepared at experimental area of University of Agriculture Faisalabad using granulated corncob by method described by Shen et al. (2019). For this purpose, the ground corncob material was heated with a rate of 10 °C/min in a furnace with a limited supply of oxygen to a maximum temperature of 600 °C which was maintained for one hour.
After preparation of granulated corncob and biochar, 5 % of both biosorbent were added in separated flasks containing distill water. These flasks were amended with either RB5 (200 mg/L) or 25 mg/L of Cr (VI) or the combination of both RB5 and Cr(VI). These flasks were given a shaking incubation (150 rpm) at 30◦C in a shaking incubator. All the experiment was conducted in triplicates including their un-inoculated controls. Aliquots from every sample were collected followed by centrifugation at 7000 rpm for 10 min. The supernatants than used to analyze simultaneous treatment of RB5 and Cr (VI) as previously described in above sections.
2.7.2 Use of Granulated Corncob and Biochar Bio-augmented With Citrobacter sp. M41 For Simultaneous Removal of RB5 and Cr(VI) From A Textile Wastewater in Packed Bed Columns based bioreactors
This study also demonstrated the practical use of Citrobacter sp. M41 to treat colored textile wastewater having RB5 dye (200 mg/L) and Cr(VI) (25 mg/L) using the packed bed columns based bioreactors. The textile wastewater collected from an industrial area of Faisalabad was colorless and had the pH 7.9 and EC equal to 8.3 dS m−1. The centrifuged (10000 rpm for 5 min) wastewater was spiked with RB5 and Cr(VI) up to 200 mg/L and 25 mg/L, respectively. For this experiment, the granulated corncob and corncob biochar materials were first bioaugmented by immobilizing the cells of the strain M41 on the surface of these materials by using method of Lou et al. (2019). To achieve the objective, vertical and cylindrical glass columns (1 feet height and 2.5 cm in diameter) were prepared and separately filled with four different types of materials viz. granulated corncob, granulated biochar, granulated bioaugmented corncob and granulated bioaugmented biochar. Low pressure pumps were attached for passing the wastewater having RB5 (200 mg/L) and Cr(VI) (25 mg/L). After every pass of synthetic wastewater from the sorbent regions, aliquots were taken, centrifuged (7000 rpm for 10 min) and analyzed for simultaneous removal of RB5 and Cr(VI) as explained in above sections. The supernatants after the final pass were also analyzed for pH, electrical conductivity, total dissolved solids (TDS) and chemical oxygen demand (COD) using the standard methods already described in Maqbool et al. (2016).
2.8 Phytotoxicity evaluation of the treated wastewaters
In order to assess the toxicity, the centrifuged (10000 rpm for 5 min) untreated as well as treated wastewaters were separately applied for the germination of mung bean (Vigna radiata, cv. NM-2006) seeds obtained from Ayub Agricultural Research Institute, Faisalabad, Pakistan. Before germination, surface sterilization of the seeds was carried out for ten minutes with 3 % hydrogen peroxide and then seeds were rinsed with deionized water. The triplicate sets of 10 mung bean seeds in sand in petri plates were irrigated with 10 mL of untreated as well as treated wastewaters separately. The control was irrigated with equal amount of distilled water. After one week, the data regarding the germination (%) and length of plumule and radical were noted and statistically analyzed by analysis of variance followed by Fisher LSD test.
3 Results
3.1 Isolation and Identification of the strain M41
The results exhibited that the tested 19 bacterial isolates exhibited varying potential for decolorization of RB5 (Fig. S1). Among these 19 isolates, the isolate M41 exhibited maximum (>90 %) decolorization potential against RB5. Therefore, the isolate M41 was selected for further experiments. The BlastN analysis of the 16S rDNA gene of M41 indicated that this strain had the maximum (∼99 %) resemblance with bacterial isolates of genus Citrobacter. Additionally, the phylogenetic tree built by using neighbor joining method also confirmed the position of strain M41 in bacterial strains cluster of genus Citrobacter (Fig. 1). Hence, this isolate was named as Citrobacter sp. M41 and the sequence of its 16SrDNA gene was submitted in NCBI GenBank database under accession No. MT730590. This strain was found to carry out maximum decolorization of RB5 (92.5 ± 2.4 %) followed by RY2 (91.4 ± 3.0 %) in MS medium added with metals, but, the decolorization of RR120 and RO16 was found negligible even at last stage of the incubation (72 h) (Fig. S2).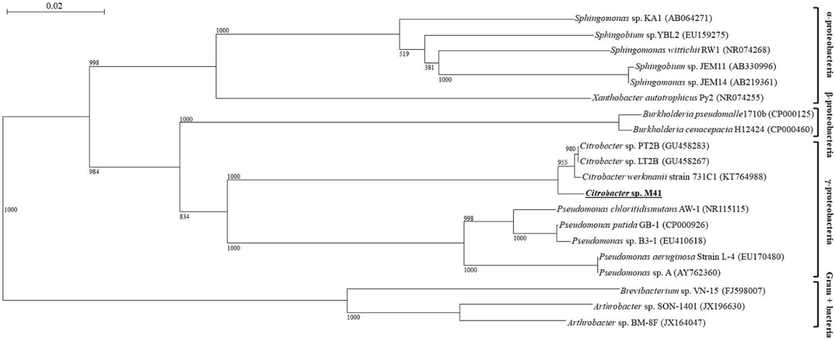
Phylogenetic tree of 16S rRNA of Citrobacter sp. M41 with those of the other bacteria in GenBank database.
3.2 Characterization of RB5 decolorizing capabilities of Citrobacter sp. M41
While studying the effect of shaking and static incubation, Citrobacter sp. M41 exhibited higher RB5 decolorization in static state of incubation in comparison with shaking incubation (Fig. 2a). According to results, 31.3 % and 18.1 % decolorization of added RB5 was observed under static and shaking conditions, respectively, within 24 h of incubation. After incubation of 72 h, the maximum RB5 decolorization was recorded under static incubation (91.4 %) followed by shaking incubation (56.7 %).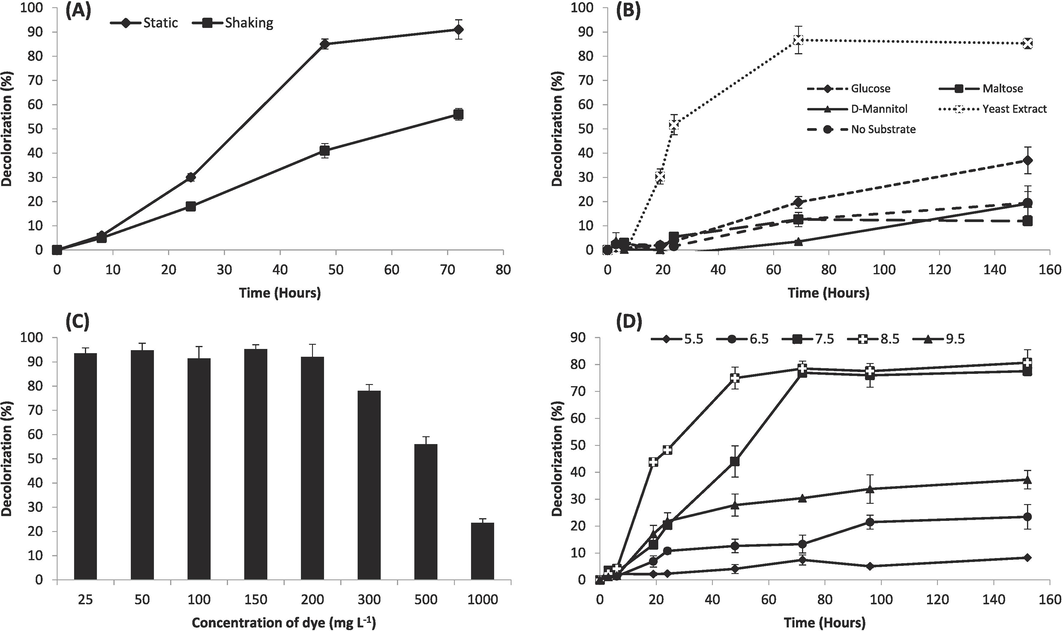
Characterization of RB5 decolorizing capabilities of Citrobacter sp. M41 in mineral salt medium under stress due to a mixture of Cd (10 mg/L), Pb (10 mg/L) and Ni (10 mg/L). (a) RB5 decolorization under static and shaking incubation, (b) RB5 decolorization in the media containing different carbon co-substrates, (c) RB5 decolorization at its varying initial concentrations, (d) RB5 decolorization in the media having different pH.
It was observed that addition of carbon co-substrates considerably enhanced the RB5 decolorization by M41 (Fig. 2b). After 24 h, 51.8 %, 5.4 %, 3.6 % and 2.1 % of the RB5 was decolorized while adding yeast extract, maltose, glucose and D-Mannitol, respectively. At the last stage of the incubation, the maximum RB5 removal was again recorded by adding yeast extract (85.3 %) followed by glucose (37.0 %), D-mannitol (19.2 %) and maltose (11.9 %).
Results regarding the effect of varying initial concentrations of RB5 on its decolorization in the presence of metal ions are shown in Fig. 2c. Results depicted that lower concentrations of RB5 (25–200 mg/L) showed non-significant impact on RB5 decolorization, however, the higher initial RB5 concentrations (300–1000 mg/L) exhibited negative effect on RB5 decolorization (Fig. 2c). Over the incubation period, > 90 % of RB5 was decolorized within the media having 25, 50, 100, 150 and 200 mg/L of initial concentrations of RB5. However, 78.3 %, 56.7 % and 23.5 % of RB5 was decolorized in the medium containing 300, 500 and 1000 mg/L of initial concentration of RB5 in the MS media containing the metal ions.
Results showed that optimum decolorization of RB5 was achieved at pH 8.5 and 7.5 (Fig. 2d). The maximum decolorization of RB5 over 24 h incubation was recorded at pH 8.5 (48.3 ± 1.5 %) followed by at pH 7.5 (21.8 ± 3.8 %) and pH 9.5 (20.3 ± 0.3 %). The maximum decolorization was at alkaline pH 8.5 (80.7 ± 4.8 %) followed by at 7.5 (77.6 ± 1.0 %), 9.5 (37.2 ± 3.5 %), 6.5 (23.5 ± 4.6 %) and 5.5 (8.2 ± 0.2 %) at the end of the incubation.
While studying the impact of the varying levels of multi-metal mixture (Cd2+, Pb2+ and Ni2+) on decolorization of RB5 by the strain M41, it was observed that the presence of the multi-metal mixtures did not inhibit the RB5 decolorization (Table 2). However, they affected the rate of decolorization of RB5 by the strain M41. Over 72 h incubation, 94.6 %, 92.8 %, 73.4 % and 38.4 % decolorization of RB5 was observed in the media containing no metals, lower concentration of the multi-metal mixtures [Cd2+ (10 mg/L) + Pb2+ (10 mg/L) + Ni2+ (10 mg/L)], medium concentration of the multi-metal mixtures [Cd2+ (50 mg/L) + Pb2+ (50 mg/L) + Ni2+ (50 mg/L)] and higher concentration of the multi-metal mixtures [Cd2+ (100 mg/L) + Pb2+ (100 mg/L) + Ni2+ (100 mg/L)], respectively.
Concentration of the mixture of metal ions (mg/L)
Decolorization (%)
14 Hours
38 Hours
72 Hours
No metal ions
31.5 ± 3.1
95.3 ± 2.5
94.6 ± 5.2
Cd: 10; Pb: 10; Ni: 10
18.2 ± 1.8
72.9 ± 2.3
92.8 ± 4.0
Cd: 50; Pb: 50; Ni: 50
16.9 ± 2.4
41.6 ± 3.5
73.4 ± 2.6
Cd: 100; Pb: 100; Ni: 100
10.5 ± 1.5
23.7 ± 2.8
38.4 ± 2.1
3.3 Concurrent RB5 and Cr(VI) removal by Citrobacter sp. M41
The strain M41 exhibited > 90 % removal of added Cr(VI) just in 48 h (Fig. 3a). Based on these results, Citrobacter sp. M41 was also subjected to see the concurrent removal of RB5 and Cr(VI) and we have found captivated response as shown in Fig. 3b. Throughout the experiment, Citrobacter sp. M41 decolorize faster RB5 dye than Cr(VI). Over 48 h incubation, Citrobacter sp. M41 exhibited > 80 % removal of RB5 and Cr(VI) in MS broth containing mixture of metal ions.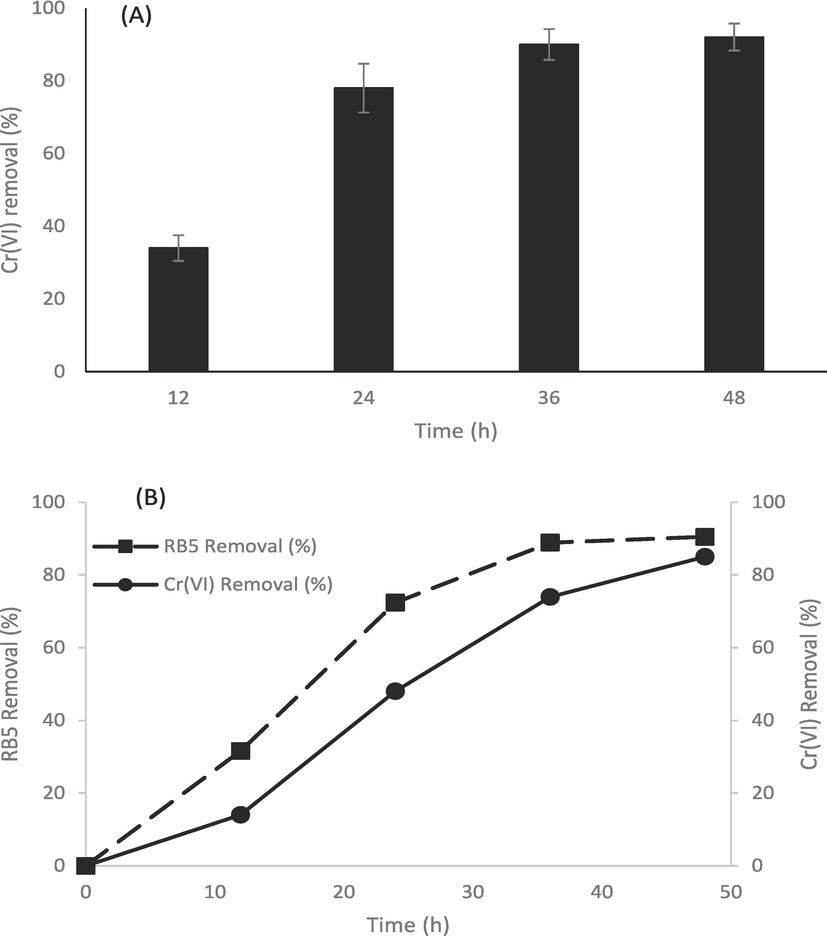
Simultaneous RB5 decolorization and Cr(VI) removal by Citrobacter sp. M41 in MS media amended with 10 mg/L of Pb, 10 mg/L of Cd and 10 mg/L of Ni (a) Cr(VI) removal (b) simultaneous RB5 decolorization and Cr(VI) removal.
3.4 Application of Citrobacter sp. M41 for treatment of wastewater using bio-augmented packed bed columns based bioreactors
Both the granulated corncob and its biochar exhibited considerable potential for removal of RB5 as both of the materials showed > 80 % decolorization of initially added RB5 dye. Although maximum removal of RB5 was achieved with biochar (93.4 ± 2.0 %) followed by corncob (83.3 ± 3.4 %) during 72 h (Fig. 4a). But in case of Cr(VI) removal, it was surprising to notice that corncob exhibited > 85 % removal of initially added Cr(VI) and biochar exhibited < 15 % removal of Cr(VI) during 72 h (Fig. 4b). As long as concurrent removal of RB5 and Cr(VI) is concerned, granulated corncob showed more considerable results in comparison to granulated corncob-biochar (Fig. 4c). It was noticed that granulated corncob exhibited 59.6 % RB5 removal along with 63.9 % simultaneous removal of Cr(VI). However, granulated corncob-biochar showed > 90 % removal of RB5 dye with < 10 % concurrent removal of Cr(VI) during 72 h incubation.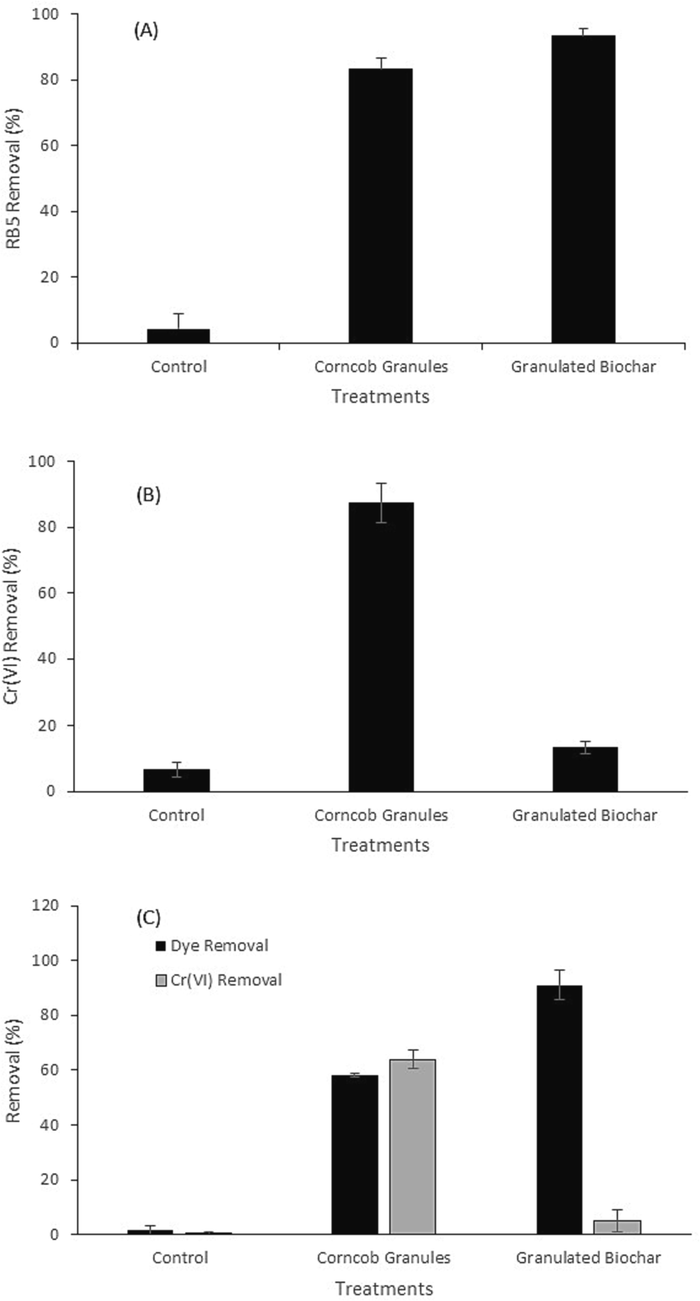
Potential of granulated corncob and granulated biochar as biosorbent for treatment of synthetic textile wastewater containing RB5 and Cr(VI) over 72 h of incubation (a) RB5 removal, (b) Cr(VI) removal, (c) simultaneous RB5 decolorization and Cr(VI) removal.
In bioaugmented packed bed columns based bioreactors, it was observed that removal of both RB5 and Cr(VI) was gradually increased as times of passing of wastewater from these column was increased (Fig. 5). After single time passing of the textile wastewater, 24.5 %, 43.2 %, 35.4 % and 48.1 % of RB5 was removed in the columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively (Fig. 5a). However, after passing the textile wastewater from the columns for five times, 82.1, 87.6 %, 91.3 % and 95.7 % of the initially added RB5 was removed in the columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively (Fig. 5a). There was no significant increase in RB5 decolorization after further passing of the wastewaters through the columns. As for as Cr(VI) removal is concerned, it was noticed that after single time passing of the textile wastewater, 18.2 %, 3.4 %, 25.5 % and 9.7 % of the initially added Cr(VI) was removed in the columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively (Fig. 5b). However, after passing the textile wastewater from the columns for five times, 81.9 %, 5.1 %, 89.4 % and 41.6 % of Cr(VI) was removed in the columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively (Fig. 5b). After 10th passing, 84.1 %, 4.6 %, 91.4 % and 80.7 % of Cr(VI) was removed in the in the wastewater samples treated through columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively (Fig. 5b).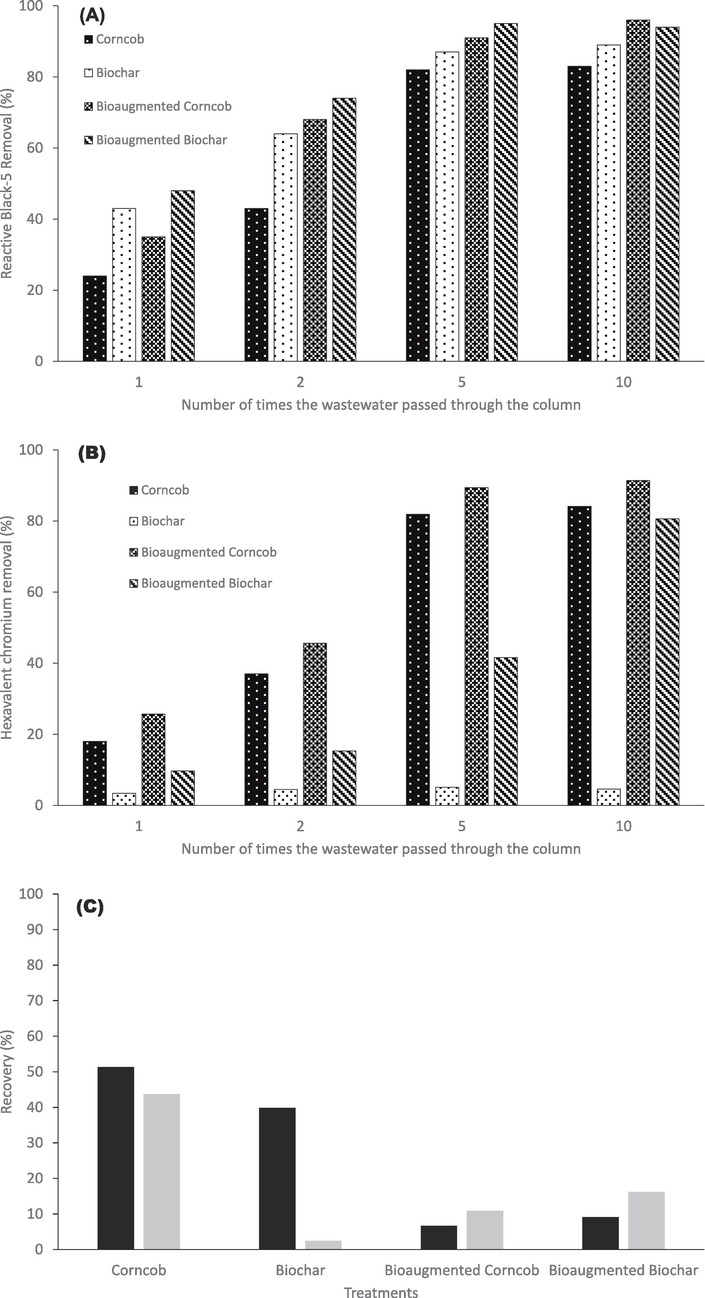
Simultaneous RB5 decolorization (a) and Cr(VI) removal (b) from synthetic wastewater in bio-augmented column bioreactors (c) recovery of RB5 and Cr(VI) from the columns at the end of the experiment.
At the end of the study, when RB5 and CR(VI) were recovered and extracted from the materials in the columns, it was found that 51.3 %, 39.8 %, 6.7 % and 9.1 % of RB5 was recovered from the materials in columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively (Fig. 5c). Similarly, 43.7 %, 2.4 %, 10.9 % and 16.2 % of the initially added Cr(VI) was recovered from the materials columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively.
It is also noteworthy that, at the end of the experiment, a significant decrease in the values of COD, EC and TDS was observed in the textile wastewater samples treated in different packed bed columns based bioreactors (Table 3). In this study, 41.9 % (±3.6), 53.6 % (±4.3), 63.5 % (±2.5) and 60.8 % (±5.1) of COD was observed to be removed in the samples treated through the packed bed columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively. The EC values of 3.9, 6.2, 4.1 and 3.5 dS m−1 were observed in the wastewater samples treated through the packed bed columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively. However, the EC of the untreated wastewater was dS m−1. Similarly, the TDS values of 513, 981, 532 and 426 mg/L were observed in in the wastewater samples treated through the packed bed columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively. However, the TDS of the untreated wastewater was 1365 mg/L.
Treatments
Parameters
COD Removal (%)
pH
EC
(dS m−1)
TDS
(mg/L)
Untreated wastewater
6.6 ± 1.5
7.9
8.3
1365
Wastewater treated in columns containing corncob
41.9 ± 3.6
7.6
3.9
513
Wastewater treated in columns containing corncob biochar
53.6 ± 4.3
7.2
6.2
891
Wastewater treated in columns containing M41 bioaugmented corncob
63.5 ± 2.5
6.9
4.1
532
Wastewater treated in columns containing M41 bioaugmented biochar corncob
60.8 ± 5.1
7.4
3.5
426
3.5 Phytotoxicity of the treated wastewaters against Vigna radiata
The impacts of untreated wastewater and wastewater treated in different packed bed columns based bioreactors on different parameters of mung bean has been presented in Table 4. It was observed that the mung bean seeds showed 93.3 % (±2.9) and 45.0 % (±5.0) germination when irrigated with distilled water and untreated wastewater, respectively. However, the germination values of 75.0 % (±5.0), 58.3 (±7.6), 91.7 % (±5.8) and 88.3 % (±7.6) were observed in the mung bean seeds irrigated with the wastewater samples treated through the packed bed columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively. The plumule lengths of the mung bean seedlings irrigated with distilled water and untreated wastewater were found to be 12.4(±0.50) cm and 5.7(±0.80) cm, respectively. However, the plumule lengths of 9.8(±0.44) cm, 7.9(±0.35) cm, 12.9(±1.1) cm and 12.6(±1.21) cm were observed in the mung bean seeds irrigated with the wastewater samples treated through the packed bed columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively. Similarly, the radical lengths of the mung bean seedlings irrigated with distilled water and untreated wastewater were found to be 6.43(±0.72) cm and 2.77(±0.31) cm, respectively. However, the plumule lengths of 5.80(±0.70) cm, 4.07(±0.57) cm, 6.90(±0.40) cm and 7.13(±0.31) cm were observed in the mung bean seeds irrigated with the wastewater samples treated through the packed bed columns filled with granulated corncob, granulated corncob biochar, bioaugmented granulated corncob and bioaugmented granulated corncob biochar, respectively.
Treatments
Parameters
Germination (%)
Plumule
(cm)
Radical
(cm)
Distilled Water
93.3 ± 2.9 a
12.4 ± 0.50 a
6.43 ± 0.72 ab
Untreated wastewater
45.0 ± 5.0 d
5.7 ± 0.80 d
2.77 ± 0.31 d
Wastewater treated in columns containing corncob
75.0 ± 5.0b
9.8 ± 0.44b
5.80 ± 0.70b
Wastewater treated in columns containing corncob biochar
58.3 ± 7.6c
7.9 ± 0.35c
4.07 ± 0.57c
Wastewater treated in columns containing M41 bioaugmented corncob
91.7 ± 5.8 a
12.9 ± 1.01 a
6.90 ± 0.40 a
Wastewater treated in columns containing M41 bioaugmented biochar corncob
88.3 ± 7.6 a
12.6 ± 1.21 a
7.13 ± 0.31 a
4 Discussion
In this study, total 19 bacterial isolates were observed to have varying capability for RB5 decolorization under stress due to a mixture of metal ions including Pb2+, Ni2+ and Cd2+. Variation in decolonization can be related to variant adaptation of these isolates for removal of dyes as already mentioned in previous studies (Maqbool et al., 2016; Baig et al., 2019) or due to a varying resistance to the metal ions in the medium (Abbas et al., 2016). Citrobacter sp. M41 was observed to harbor the highest potential to decolorize RB5 in mixture of metal ions and was selected for further studies. Despite that few bacterial strains belonging to genus Citrobacter are previously found having potential for decolorization of azo dyes (Sunkar et al., 2015; Schmidt et al., 2019), however, the strain M41 is unique because it has the potential not only to decolorize RB5 but also other related dyes i.e. RY2, RR120 and RO16 even under stress due to metal ions. Moreover, the strain M41 is also unique because it also harbors the potential for concurrent removal of RB5 and Cr(VI) while tolerating Pb2+, Ni2+ and Cd2+ in the medium.
The decolorizing ability of Citrobacter sp. M41 was optimized under various incubation conditions. Results revealed that Citrobacter sp. M41 carried out maximum decolorization in static conditions rather than in shaking conditions. Previous studies have also reported the same (Prasad and Aikat, 2014; Baig et al., 2019; Bilal et al., 2022). Relatively higher decolorization under static condition might be due to the fact that it provides partially anoxic environment which more suitably favors dye decolorization because the transfer of electrons to the azo bond and the activity of dyes reducing enzymes including azoreductase is assisted by anoxic environment (Tripathi and Srivastava, 2011; Imran et al., 2015; Imran et al., 2019). Presence of carbon co-substrates also increased the efficiency of Citrobacter sp. M41 to decolorize RB5 in the medium added with mixture of metal ions. It was found that yeast extract increased the decolorization of RB5 more as compared with other carbon co-substrates. Our results were in line with many other studies who also found efficient decolorization of different dyes including reactive black-5, reactive red-120 and reactive yellow-2 in the presence of yeast extract as co-substrate (Imran et al., 2016; Baig et al., 2019; Hussain et al., 2020; Bilal et al., 2022). Higher decolorization of RB5 in the presence of yeast extract might be related to yeast extract being not only carbon and nitrogen source for the growth of the microorganisms but also as redox mediator due to the presence of riboflavin in it which enhances the activity of the decolorizing enzymes including azoreductase (Imran et al., 2016). Our results showed that optimum decolorization of RB5 by Citrobacter sp. M41 was obtained at pH 8.5 followed by 7.5. Moreover, a decrease in decolorization was recorded as the pH moved away from these values. Higher decolorization between 7.5 and 8.5 pH might be attributed to the fact that the growth of the strain as well as the activity of its decolorizing enzymes including azoreductase is favored within this range of pH (Johansson et al., 2011). However, it is needed to have a detailed study about pH effects on dye decolorization by studying the microbial growth as well as the genes and enzymes which participate in dye removal in the presence of metal ions. The strain M41 was found to considerably decolorize even the higher concentration up to 1000 mg/L of RB5 but rate was decreased at higher concentrations. This reduction might be due to microbial toxicity of the dyes or their metabolites at their higher concentration (Das and Mishra, 2016). It is also possible that the active sights of the key enzyme i.e., azoreductase might be blocked by azo dyes with multiple structures, resulting in reduction of decolorization process.
Results showed that at different levels of the mixture of metal ions, M41 strain has a good potential to decolorize RB5 at lower concentrations, however, decolorization was decreased at higher concentrations. Previous studies reported the impacts of varying mixtures of metal ions on decolorization of different dyes (Hussain et al., 2013; Abbas et al., 2016). Interestingly a good potential of RB5 decolorization by M41 was observed even at higher concentrations of the mixture of metal ions. However, low decolonization of RB5 at higher concentration of the metal ions can be related to their inhibitory effects, supression of enzymatic activities as already reported by (Chen, 2011).
The results indicated that Citrobacter sp. M41 showed good potential not only for reduction of Cr(VI) but also for concurrent removal of RB5 dye. Few recent studies have reported the same findings (Mahmood et al., 2013; Anwar et al., 2014; Maqbool et al., 2016), however, in this study simultaneous removal was studied under stress due to the mixture of other metal ions (Pb2+, Ni2+ and Cd2+). Although mechanism of Cr(VI) removal by this strain has not been explored, however, few previous studies described that chromate play important role as electron acceptor in order to gain energy for Cr(VI) removal (Maqbool et al., 2015). Nevertheless, more investigations and research should be carried out to reveal a precise mechanism for Cr(VI) reduction, but, it is noteworthy that Citrobacter sp. M41 exhibited a good potential for concurrent RB5 decolorization and Cr(VI) removal which make it worthwhile bio-resource for its possible application in biotreatment of textile wastewater.
In the present study, practical application of Citrobacter sp. M41 was also carried out in packed bed columns based bireactors for treatment of a textile wastewater containing RB5 (200 mg/L) and Cr(VI) (25 mg/L). Relatively low sorption of the Cr(VI) on the corncob biochar might be due to the fact that the surface of this biochar mostly contains the negative charges due to the presence of a high magnitude of hydroxyl groups at alkaline pH (Tan et al., 2020). This study indicated that the corncob granules might serve as good materials for biosorption of Cr(VI) as well as RB5 from the solutions concurrently. While studying the treatment of textile wastewater containing RB5 (200 mg/L) and Cr(VI) (25 mg/L) in packed bed columns based bioreactors, it was observed that both RB5 and Cr(VI) were significantly removed in the columns containing granulated corncob biosorbent as well its biochar (Fig. 5). However, significant amount of RB5 was removed in the columns containing corncob biochar but Cr(VI) was not removed in these columns. However, it is noteworthy that a significantly higher removal of Cr(VI) was observed in case of the column filled with bioaugmented biochar. Similar type of removal of RB5 and Cr(VI) was also observed in a biochar packed bioreactor in which the biochar was bioaugmented with a dyes decolorizing strain Pseudomonas putida (Shahid et al., 2015). It is quite possible that the immobilization of M41 on the corncob biochar might have changed some surface properties of the biochar leading towards sorption and removal of the Cr(VI). However, there is need to get a deep insight into this process by studying the surface properties of the biochar. Further investigations revealed that bio-augmentation of corncob and corncob biochar with Citrobacter sp. M41 improved RB5 and Cr(VI) removal. The corncob and/or its biochar bioaugmented with immobilized functional microbial strains serve as a suitable combination for remediation of the wastewaters starting from the sorption on the solid surfaces followed by the decolorization of the dye molecules and reduction of the Cr(VI) by the functional bacteria on the surface interface of the solid material. The decolorization of RB5 and reduction of Cr(VI) by the immobilized strain M41 was supported by the fact that a relatively lower amount of either RB5 or Cr(VI) was recovered from the bioaugmented corncob and its biochar materials as compared to the corncob and its biochar without any microbial immobilization (Fig. 5C). Such sorption and then degradation/reduction has to be found in some previous studies focused on removal of different pollutants including dyes and metals from the wastewater using the functional microbial cultures immobilized on biochar materials (Shahid et al., 2015; Abu Talha et al., 2017; Bharti et al., 2019; Ayed et al., 2021). In this study, COD, EC and TDS were also significantly decreased in the wastewaters treated in the packed bed column bioreactors containing the bioaugmented corncob or its biochar. It further supports the treatment of the textile wastewaters in the bioreactors. While studying the treatments of a textile wastewater, Ceretta et al. (2017) reported that COD of the wastewater was significantly decreased due to a bacterial consortium in the presence of carbon co-substrates.
The phytotoxicity study conducted with Vigna radiata (mung bean) seeds while considering the germination rate and length of the plumule and radical in the mung bean seedlings indicated that the toxicity of all the treated textile wastewaters was significantly decreased as compared with the untreated textile wastewater (Table 4). Although several studies have been performed for the treatment of textile wastewaters, however, there are relatively few studies which also focus on studying the phytotoxicity of the treated wastewaters (Najme et al., 2015; Ceretta et al. 2017; Ayed et al., 2021). Our finding is in accordance with these few studies who reported the reduction in phytotoxicity of the dye solutions or dyes loaded textile wastewaters after treatment by exploiting the bacterial strains (Najme et al., 2015; Ceretta et al. 2017). For example, Ceretta et al. (2017) observed that the toxicity of a textile wastewater taken in terms of impacts on germination and radical length of Lactuca sativa seeds was significantly decreased after its treatment by a bacterial consortium. Similarly, Najme et al. (2015) reported that the impacts of a reactive yellow-2 loaded synthetic wastewater on germination, plumule length and radical length of mung bean were significantly reduced when it was treated with a bacterial strain Serratia sp. RN34. Recently, Ayed et al. (2021) reported that the phtotoxicity of a textile wastewater against the Triticum durum L. and Cucurbita pepo L. seeds was significantly reduced after its treatment through a consortium composed of bacterial and micro-algal strains. The reduction in toxicity of the textile wastewater might be not only due to reduction in load of Cr(VI) and RB5 in the treated textile wastewater due to biosorption in the columns but also due to biotransformation of these contaminants into relatively less toxic forms. For example, Cr(VI) has been reported to be reduced into less toxic form by several dyes decolorizing bacterial strains including Pseudomonas putida, Serratia proteamaculans, Pseudomonas aeruginosa and Acinetobacter junii in a number of previous studies (Mahmood et al., 2013; Anwar et al., 2014; Maqbool et al., 2016; Hussain et al., 2020). Hence this study shows that the application of the dye decolorizing strain Citrobacter sp. M41 as a bioresources in bioaugmented biochar based packed bed column bioreactors might lead towards the treatment of textile wastewaters along with reduction in their phytotoxic effects.
Conclusions and Future Perspectives.
It is concluded that Citrobacter sp. M41 isolated from textile wastewater possesses good potential for concurrent removal of RB5 and Cr(VI) even in the medium under stress due to a mixture of metal ions. Moreover, enhanced concurrent removal of RB5 and Cr(VI) in corncob packed bed column bioreactor supported with bio-augmentation of Citrobacter sp. M41 make it an important bio-resource to devise technologies for simultaneous RB5 decolorization and Cr(VI) removal using corncob as sorbent material in column bioreactor. However, there is still need to further have a thorough understanding of processes involved in concurrent removal of azo dyes and Cr(VI) in bench scale bioaugmented column bioreactors not only by studying the surface properties of the materials but also by targeting the metabolites of the dyes.
Acknowledgments
The authors are thankful to the researchers supporting project number (RSP2023R190), King Saud University, Riyadh, Saudi Arabia and the Higher Education Commission of Pakistan for providing funding under the project No. 8206/Punjab/NRPU/R&D/HEC/2017.
Declarations.
Funding.
The funds for the present research work were provided by Higher Education Commission (HEC) of Pakistan under the project No. 8206/Punjab/NRPU/R&D/HEC/2017 and the project number (RSP2023R190), King Saud University, Riyadh, Saudi Arabia.
Conflicts of interest/Competing interests.
All authors declare that there is no conflict of interest/competing interests in this original article.
Ethics approval.
This article does not contain studies with human participants or vertebrates performed by any of the authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Characterization of a salt resistant bacterial strain Proteus sp. NA6 capable of decolorizing reactive dyes in presence of multi-metal stress. World. J. Microbiol. Biotechnol.. 2016;32:1-12.
- [CrossRef] [Google Scholar]
- Characterization of reactive red-120 decolorizing bacterial strain Acinetobacter junii FA10 capable of simultaneous removal of azo dyes and hexavalent chromium. Water Air Soil Pollut.. 2014;225:1-16.
- [CrossRef] [Google Scholar]
- Decolorization and phytotoxicity reduction of reactive blue 40 dye in real textile wastewater by active consortium: Anaerobic/aerobic algal-bacterial-probiotic bioreactor. J. Microbiol. Methods.. 2021;181:106129-106139.
- [CrossRef] [Google Scholar]
- Characterization of a reactive yellow-2 decolorizing zinc tolerant bacterial strain Pseudomonas sp. LT10 isolated from textile industry wastewater. Asian J. Agric. Biol.. 2019;7(3):482-490.
- [Google Scholar]
- Biodegradation of methylene blue dye in a batch and continuous mode using biochar as packing media. Environ. Res.. 2019;171:356-364.
- [CrossRef] [Google Scholar]
- Characterization of Rhizospheric Bacillus Strains SG36 and SG42 for Decolorization of Reactive Yellow 2 Dye and Vigna radiata Growth Promotion in Dye Contaminated Soil. Int. J. Agri. Biol.. 2022;27:8-18.
- [Google Scholar]
- Biodegradation of textile wastewater: enhancement of biodegradability via the addition of co-substrates followed by phytotoxicity analysis of the effluent. Water Sci. Technol.. 2018;2017(2):516-526.
- [CrossRef] [Google Scholar]
- A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresource Technol.. 2011;102(2):716-723.
- [CrossRef] [Google Scholar]
- Decolorization of different textile azo dyes using an isolated bacterium Enterococcus durans GM13. Int. J. Curr. Microbiol. Appl. Sci.. 2016;5(7):675-686.
- [Google Scholar]
- Efficacy of bacterial consortium-AIE2 for contemporaneous Cr (VI) and azo dye bioremediation in batch and continuous bioreactor systems, monitoring steady-state bacterial dynamics using qPCR assays. Biodegradation. 2009;20:813-826.
- [CrossRef] [Google Scholar]
- Biodegradation of textile azo dyes by a facultative Staphylococcus arlettae strain VN-11 using a sequential microaerophilic/aerobic process. Int. Biodeterior. Biodegrad.. 2009;63(3):280-288.
- [CrossRef] [Google Scholar]
- Isolation and characterization of a lead (Pb) tolerant Pseudomonas aeruginosa strain HF5 for decolorization of reactive red-120 and other azo dyes. Ann. Microbiol.. 2018;68(12):943-952.
- [CrossRef] [Google Scholar]
- Biodecolorization of reactive black-5 by a metal and salt tolerant bacterial strain Pseudomonas sp. RA20 isolated from Paharang drain effluents in Pakistan. Ecotoxicol. Environ. Saf.. 2013;98:331-338.
- [CrossRef] [Google Scholar]
- Simultaneous removal of malachite green and hexavalent chromium by Cunninghamella elegans biofilm in a semi-continuous system. Int. Biodeterior. Biodegrad.. 2017;125:142-149.
- [CrossRef] [Google Scholar]
- Simultaneous removal of reactive dyes and hexavalent chromium by a metal tolerant Pseudomonas sp. WS-D/183 harboring plant growth promoting traits. Int. J. Agric. Biol.. 2020;23:241-252.
- [Google Scholar]
- Microbial biotechnology for decolorization of textile wastewaters. Rev. Environ. Sci. Biotechnol.. 2015;14(1):73-92.
- [CrossRef] [Google Scholar]
- Microbial biotechnology for detoxification of azo-dye loaded textile effluents: a critical review. Int. J. Agri. Biol.. 2019;22(5):1138-1154.
- [Google Scholar]
- BTI1, an azoreductase with pH-dependent substrate specificity. Appl. Environ. Microbiol.. 2011;77(12):4223-4225.
- [CrossRef] [Google Scholar]
- Accelerated decolorization of reactive azo dyes under saline conditions by bacteria isolated from Arabian seawater sediment. Appl. Microbiol. Biotechnol.. 2012;96:1599-1606.
- [CrossRef] [Google Scholar]
- Toxicity assessment on three direct dyes (D-BLL, D-GLN, D-3RNL) using oxidative stress bioassay and quantum parameter calculation. Ecotoxicol. Environ. Saf.. 2012;86:132-140.
- [CrossRef] [Google Scholar]
- Adsorption and degradation in the removal of nonylphenol from water by cells immobilized on biochar. Chemosphere. 2019;228:676-684.
- [CrossRef] [Google Scholar]
- Potential of newly isolated bacterial strains for simultaneous removal of hexavalent chromium and reactive black-5 azo dye from tannery effluent. J. Chem. Technol. Biotechnol.. 2013;88(8):1506-1513.
- [CrossRef] [Google Scholar]
- Isolating, screening and applying chromium reducing bacteria to promote growth and yield of okra (Hibiscus esculentus L.) in chromium contaminated soils. Ecotoxicol. Environ. Saf.. 2015;114:343-349.
- [CrossRef] [Google Scholar]
- Use of RSM modeling for optimizing decolorization of simulated textile wastewater by Pseudomonas aeruginosa strain ZM130 capable of simultaneous removal of reactive dyes and hexavalent chromium. Environ. Sci. Pollut. Res.. 2016;23:11224-11239.
- [CrossRef] [Google Scholar]
- Use of waste materials bottom ash and de-oiled soya, as potential adsorbents for the removal of amaranth from aqueous solutions. J. Hazard. Mater.. 2005;117:171-178.
- [CrossRef] [Google Scholar]
- Biodecolorization of Reactive Yellow-2 by Serratia sp. RN34 Isolated from textile wastewater. Water Environ. Res.. 2015;87(12):2065-2075.
- [CrossRef] [Google Scholar]
- Study of bio-degradation and bio-decolourization of azo dyeby Enterobacter sp. SXCR. Environ. Technol.. 2014;35(8):956-965.
- [CrossRef] [Google Scholar]
- Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol.. 2001;77(3):247-255.
- [CrossRef] [Google Scholar]
- Biodegradation potential of Citrobacter cultures for the removal of amaranth and congo red azo dyes. Int. J. Environ. Sci. Technol.. 2019;16(11):6863-6872.
- [CrossRef] [Google Scholar]
- Synthesis of MgO coated corncob biochar and its application in lead stabilization in a soil washing residue. Environ. Int.. 2019;122:357-362.
- [CrossRef] [Google Scholar]
- Citrobacter freundii mediated degradation of textile dye Mordant Black 17. J. Water Process Eng.. 2015;8:28-34.
- [CrossRef] [Google Scholar]
- Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Bioresour. Technol.. 2018;252:37-43.
- [CrossRef] [Google Scholar]
- Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd. J. Hazard. Mater.. 2020;384:121370-121380.
- [CrossRef] [Google Scholar]
- The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res.. 1997;25(24):4876-4882.
- [CrossRef] [Google Scholar]
- Ecofriendly treatment of azo dyes: biodecolorization using bacterial strains. Int. J. Biosci. Biochem. Bioinform.. 2011;1(1):37.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.103030.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







