Translate this page into:
Rapid naked eye detection of arginine by pomegranate peel extract stabilized gold nanoparticles
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Biosynthesized gold nanoparticles have shown remarkable size dependent colorimetric detection towards arginine among the twenty-three amino acids.
Abstract
Biosynthesized gold nanoparticles (AuNPs) of different size and shape was utilized for the visible detection of amino acids. The nanoparticles have shown sensitive and selective detection towards arginine among twenty-three different amino acids. The effect of pH on the detection of arginine is investigated. The AuNPs are able to sense 10−6 M of arginine in aqueous solution.
Abstract
The present work has been proposed for a simple colorimetric detection of arginine by the use of four different types of biogenic gold nanoparticles (AuNPs), stabilized by aqueous pomegranate (Punica granatum) peel extract. The detection is based on the shift in the surface plasmon resonance peak (SPR), which is attributed to the aggregation of the biosynthesized NPs in presence of arginine. The detection limits of arginine is 10−6 M. We have also examined the effect of pH, sensitivity and selectivity of the AuNPs towards the analyte. The present system is simple, cost effective, eco-friendly, and avoids tedious procedure for surface modification of nanoparticles to detect the analytes from aqueous solution.
Keywords
Gold nanoparticles
Arginine
Pomegranate peel
Biosynthesis
Colorimetric detection
Amino acids
1 Introduction
Gold nanoparticles (AuNPs) attracted much attention since they have been exploited to detect anions (Wei et al., 2012), metal ions (Kaviya and Prasad, 2014), explosives (Dasary et al., 2009), glucose (Radhakumary and Sreenivasan, 2011), amino acids (Sudeep et al., 2005), proteins (Xia et al., 2010), DNA (Xia et al., 2010) and bacterial spores (Cheng et al., 2012) through analyzing alterations in the unique surface plasmon resonance (SPR) absorption properties, which lie in the visible to near IR region (Kaviya and Prasad, 2015). Detection and measurement of amino acids in body fluids and tissue are essential for finding a disease and their therapy (Murakami et al., 1989). In the literature, there have been attempts to detect several amino acids using AuNPs via aggregation (Zakaria et al., 2013; Bhamore et al., 2015) and disaggregation mechanism (Li et al., 2011; Yuan et al., 2014). Exclusively, aggregation induced color changes of AuNPs in presence of the analyte is widely studied compared to the disaggregation mechanism (Zakaria et al., 2013; Bhamore et al., 2015; Li et al., 2011; Yuan et al., 2014; Zhou et al., 2012; Zhang et al., 2002, 2016; Bagci et al., 2015; Pu et al., 2013; Rawat and Kailasa, 2014). For example, selective detection of cysteine using photo chemically synthesized Au nano rods has been reported by Thomas and co-workers (Sudeep et al., 2005). Sodium citrate stabilized AuNPs have been utilized for detecting lysine (Zhou et al., 2012). Graphene oxide and gold nanoparticles based nanocomposite was used for the visible detection of glutamate, aspartate, and cysteine (Zhang et al., 2016). Apple juice stabilized gold NPs were used for the colorimetric detection of cysteine (Bagci et al., 2015). Naked eye detection of thiol holding amino acids such as homo-cysteine and cysteine was achieved using sodium citrate stabilized AuNPs (Zhang et al., 2002). Colorimetric detection of amino acids such as arginine, histidine, lysine and homocysteine was achieved by a citrate stabilized AuNPs and quercetin-functionalized gold nanoparticles (Pu et al., 2013; Rawat and Kailasa, 2014; Lim et al., 2007). Kailasa and his research group have studied the detection of arginine, histidine, methionine and tryptophan in biological samples by 4-amino nicotinic acid stabilised gold nanoparticles (Rawat and Kailasa, 2016). In another work, they have carried out the visual detection of cysteine and lysozyme in biofluids using dicoumaro decorated gold nanoparticles (Kasibabu et al., 2015).
In the present study, we have initiated the naked eye detection of arginine (Arg), the most efficacious amino acid for the stimulation of insulin and glucagon secretion (Floyd et al., 1966); in aqueous solution using biosynthesized gold nanoparticles at micro molar concentration of the analyte. Biosynthesis of nanoparticles using extract of plant (Kaviya and Prasad, 2016; Lee et al., 2016)/microorganism (Shi et al., 2015) having the advantages of simple, cost effective and biocompatibility over other physical (Polte et al., 2010) and chemical method (Mafune et al., 2002) of synthesis. Moreover, the surface modification of AuNPs (Son et al., 2012) has not been carried out for the detection of Arg. We also examined the effect of size, shape of the nanoparticles and pH of the medium on the detection of arginine.
2 Experimental
2.1 Synthesis of gold nanoparticles
Polydispersed, spherical, flower and urchin shaped gold nanoparticles were synthesized by a method already reported by our research group (Kaviya and Prasad, 2015). In brief, the aqueous solution of HAuCl4 (10 mL) was made in diverse concentration series from 10−2 M to 10−5 M. 200 µL of aqueous pomegranate peel extract was mixed to individual precursor solution. After the incorporation of extract, the solution was mixed by simple shaking. The as-prepared AuNPs are represented as: AuNP1 (10−2 M), AuNP2 (10−3 M), AuNP3 (10−4 M) and AuNP4 (10−5 M). The synthesized NPs were used for characterization. The detailed characterization has already been published elsewhere (Kaviya and Prasad, 2015). The reproduced results are given in Fig. S1.
2.2 Colorimetric analyse
Colorimetric finding experiments in presence of several amino acids such as L, β and dl-alanine (Ala), aspartic acid (Asp), arginine (Arg), asparagine (Asn), cysteine (Cys), Glutamic acid (Glu), phelylalanine (Phe), glycine (Gly), glutamine (Gln), histidine (His), isoleucine (Ile), lysine (Lys), leucine (Leu), methionine (Met), proline (Pro), serine (Ser), threonine (Thr), valine (Val), tryptophan (Trp), tyrosine (Tyr) and Ornithine was accomplished in aqueous solution using biosynthesized AuNPs (1–4) at room temperature. The concentration of arginine was changed from 10−1 M to 10−6 M. 100 µL of analyte was mixed to 200 µL of AuNPs, together with the addition of ∼1 mL of doubly distilled water. The performance of the analytes were observed through naked eye color change of the NPs and UV–vis absorption spectroscopy to examine the variations in the SPR peak of gold nanoparticles.
2.3 Characterization
UV-3100 Hitachi spectrometer is used for UV–visible spectroscopic measurements. The IR spectra have been recorded by Perkin-Elmer FT-IR spectrometer. Malvern- Zeta sizer is used for the Zeta potential and particle size analysis. The morphology of AuNP was scanned using transmission electron microscopy (TEM) (Philips Tecnai 12). The picture was taken with digital camera (Canon-A3200 IS).
3 Results and discussion
3.1 Detection of arginine by AuNPs
We have used the as-synthesized AuNPs for the finding of amino acids in aqueos solution. The aqueous pomegranet peel extract contains phytochemicals such as as punicalagin and punicalin (Cam and Hisil, 2010; Ismail et al., 2012). These polophenols are responsible for the reduction and the stabilization of AuNPs in solution (Kaviya and Prasad, 2015). We hypothesis that, the function group in the extract attached on the surface of AuNPs (1–4) can also intract with the amino acid through non-covalent interaction which can lead to nanoparticle aggregration and such aggregration leads inter plasmonic oscillation and concomitant color changes in the solution (Kaviya and Prasad, 2014).
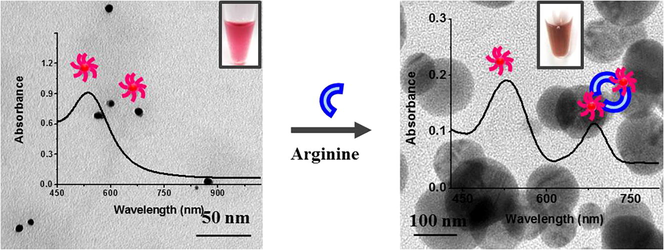
Initially, we have treated the four different AuNP systems with twenty-three amino acids in aqueous solution (Figs. S2 and S3). Among them, all the four nanoparticles show immediate color change upon addition of arginine and the color change was visible by naked eye (violet (AuNP1), red (AuNP2), blue (AuNP3) and ash (AuNP4)) changed into brown for AuNP1 and AuNP2; black for AuNP3 and AuNP4) (Inset in Fig. 1). This result indicates the rapid detection of arginine by biosynthesized AuNPs (1–4). We have taken the UV–vis absorption spectra of the AuNPs with the addition of arginine in order to find out the change in the SPR peak of the NPs (Figs. 1, S2 and S3). The intensity of SPR peek of AuNP1 and AuNP2 is declined as the concentration of arginine is increased, along with the formation of new peaks at ∼760 nm and 650 nm, respectively (Fig. 1a and b). Similarly, the intensity of SPR of AuNP3 and AuNP4 were declined and additional peaks appeared at ∼807 and 870 nm, respectively (Fig. 1c and d). Fig. S4 depicts the absorption ratio of AuNP2 between 650 nm and 527 nm was linearly related to the concentration of Arg with the linear range from 1 to 6 µM. The visible finding of arginine was more viable in AuNP2 and AuNP3, compared to AuNP1 and AuNP4, due to the enhanced color contrast.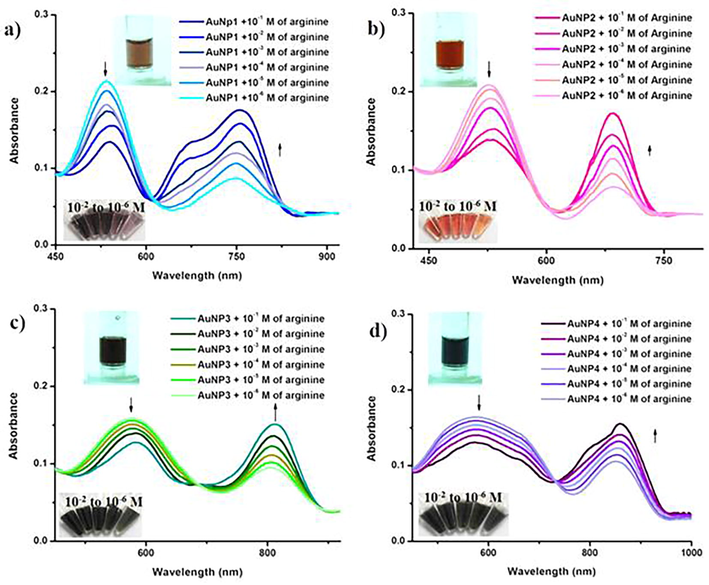
UV-vis absorption spectra of AuNPs (1–4) with different concentration of arginine (10−1 M–10−6 M). The inset shows the photos indicating the corresponding color changes of the NPs upon addition of arginine. As the concentration of arginine increases, the surface plasmon peak of AuNPs undergoes a shift in the peak, leading to a visible color change.
Fig. 2 shows the TEM images of AuNPs (1–4) in presence of arginine. The TEM images clearly indicate that, the distance between the two adjacent NP (Fig. S1b–e) is decreased with the addition of arginine. As a result, merging/overlapping of the particle was occurred which effectively shows the aggregation of AuNPs. We have examined the zeta potential of AuNPs in presence and absence (Kaviya and Prasad, 2015) of amino acids (Table S1). The results clearly indicate that, the surface charge on AuNPs is decreased only with the addition of arginine which eventually causes the aggregation of the particles (Kaviya and Prasad, 2014). We measured the variation in the size of the AuNPs with the incorporation of Arg (10−1–10−6 M) using dynamic light scattering method (Fig. S5). The result shows that the size of the particle is increased with the inclusion of arginine. These results are consistent with the results from TEM analysis (Fig. 2).![TEM images of a) AuNP1, b) AuNP2, c) AuNP3 and d) AuNP4 with the addition of arginine [10−4 M]. The inset is the higher magnification of corresponding AuNP and AuNP aggregates before and after the addition of arginine.](/content/185/2019/31/4/img/10.1016_j.jksus.2017.12.001-fig3.png)
TEM images of a) AuNP1, b) AuNP2, c) AuNP3 and d) AuNP4 with the addition of arginine [10−4 M]. The inset is the higher magnification of corresponding AuNP and AuNP aggregates before and after the addition of arginine.
3.2 Mechanism for the detection of arginine by AuNPs
Among the amino acids, arginine alone contains two primary and secondary amino groups (Johannessen et al., 2005). Hence, it is likely that arginine can have enhanced interaction with the capping biomolecules (punicalagin and punicalin) (Kaviya and Prasad, 2015). This could reduce the distance between the adjacent AuNPs, causing aggregation as well as SPR band shift (Guan et al., 2008) (Scheme 1). The interaction between the functional groups from the pomegranate peel extract which is anchored on the surface of AuNPs and with arginine was studied by FT-IR spectroscopy (Fig. S6). Fig. S6a shows the FT-IR spectrum of aqueous pomegranate peel extract where spectral bands observed at 3342, 2926, 2315, 2138, 1644, 1450, 1211, 1047 and 763 cm−1. The bands are correspond to the stretching vibration of O–H (3342 cm−1), C–H (2926 and 763 cm−1), O—C—O (2315 and 2138 cm−1), C⚌C (1644 and1450 cm−1), C—O (1211 cm−1) and C—OH (1047 cm−1). These are the functional groups responsible for the reduction and formation of AuNPs (Kaviya and Prasad, 2015). FT-IR spectrum of arginine is given in Fig. S6b. A bands appeared at 3151 and 2928 cm−1 are because of the stretching vibrations of NH and CH2 group, respectively. The peaks at 1680, 1575, 1470, 896 and 796 cm−1 are resemble to bending vibrations of NH2 (1680 and 896 cm−1), stretching vibrations of CO group (1575 cm−1), bending vibrations of CH2 group (1470 cm−1) and stretching of CNH group (796 cm−1) (Kumar and Rai, 2010). FT-IR spectrum of pomegranate peel extract capped AuNPs + arginine (Fig. S6c) showed the peaks at 3342, 2932, 2315, 2120, 1646, 1450, 1211, 896 and 763 cm−1 which are the characteristic functional groups of pomegranate peel extract and arginine. The intensity of peaks at 3151, 1575 and 796 cm−1 were decreased which indicates that arginine bound on the surface of biogenic AuNPs either through amine or carboxylic groups (Pu et al., 2013). Further, the presence of gold and nitrogen distribution in the AuNP2 + arginine system is confirmed by EDS analysis and mapping (Fig. S7).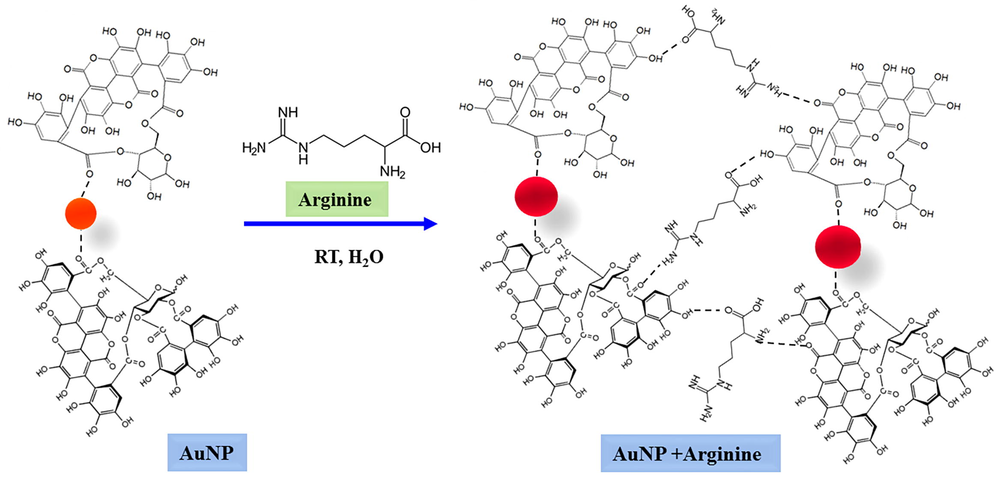
Schematic representation for the detection of arginine by pomegranate peel stabilized AuNPs.
3.3 Effect of pH on the detection of arginine
We have examined the effect of pH on the detection of arginine by AuNP2 (as a model nano system) was carried out at a pH range from 5 to 10. The different media pH was achieved by adjusting different concentration ratios of acids and base. The experimental results showed that pH variation affects the binding affinity between the AuNPs and Arg (Fig. 3a). Lower pH (pH 5 and 6) leads the protonation of nitrogen group in the arginine (Chen et al., 2010) as well as affects the stability of the nanoparticles (Yuan et al., 2014). At higher pH (pH 8 and 9) value cause more deprotonation of the functional group (OH and COOH) on the surface of AuNPs and arginine (Kaviya et al., 2017). As a result, the electrostatic replusion among the AuNPs and arginine (Chen et al., 2010). Self aggregration of nanoparticles were observed when the pH was at 10 due to the fact that shielding effect of ions (Pu et al., 2013). Hence, we choose a neutral pH condition for the ananlysis.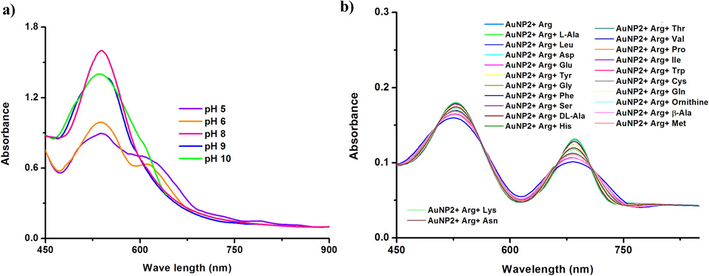
a) UV–vis absorption spectra of AuNP2 with the addition of arginine at different pH condition and b) interference study of AuNP2 + arginine in the presence of other amino acid (10−2 M).
3.4 Selectivity of AuNPs
Interference studies were carried out in presence of other amino acids. The outcomes indicate that, there is no effect in the SPR absorption of AuNP-arginine system with the addition of other amino acids (Figs. 3b and S8). The UV–vis absorption studies of the peel extract and arginine indicate no color change in the absence of AuNP (Fig. S9). The detection limit for arginine by the biologically synthesized AuNPs falls in the range of 10−6 M, which is comparable with the existing chemically synthesized AuNPs based sensors (Pu et al., 2013; Rawat and Kailasa, 2014). Table S2 gives the outline assessment of our work with the reported colorimetric detection of Arg by gold nanoparticles. Hence, the biosynthesized nanoparticles can be a suitable candidate for the detection of Arg due to its simple procedure.
4 Conclusion
The present method to detect arginine using AuNPs is simple and eco-friendly. Unlike reported, the detection performed by AuNPs is achieved without surface functionalization of NPs and the naked eye detection ability are notable qualities of the system. The present study opens up new possibilities of utilizing bio-synthesized NPs for the detection of amino acid (arginine).
Acknowledgments
Author thank Dr. Edamana Prasad, Associate Professor, Department of Chemistry, Indian Institute of Technology Madras for his constant support, the DST-INSPIRE, Govt. of India for INSPIRE fellowship and SAIF, IITM for FT-IR and SEM.
References
- A simple and green route for room-temperature synthesis of gold nanoparticles and selective colorimetric detection of cysteine. J. Food Sci.. 2015;80(80):N2071-N2078.
- [Google Scholar]
- Influence of molecular assembly and NaCl concentration on gold nanoparticles for colorimetric detection of cysteine and glutathione. Sens. Actuators B: Chem.. 2015;212:526-535.
- [Google Scholar]
- Pressurised water extraction of polyphenols from pomegranate peels. Food Chem.. 2010;123:878-885.
- [Google Scholar]
- Sensitive and selective detection of glutathione based on resonance light scattering using sensitive gold nanoparticles as colorimetric probes. Analyst. 2010;137:3132-3137.
- [Google Scholar]
- Nanoparticle-based substrates for surface-enhanced Raman scattering detection of bacterial spores. Analyst. 2012;137:3601-3608.
- [Google Scholar]
- Gold nanoparticle based lable-free SERS probe for ultrasensitive and selective detection of trinitrotoluene. J. Am. Chem. Soc.. 2009;131:13806-13812.
- [Google Scholar]
- Stimulation of insulin secretion by amino acids. J. Clin. Invest.. 1966;45:1487-1502.
- [Google Scholar]
- pH-dependent aggregation of Histidine-functionalized Au nanoparticles induced by Fe3+ ions. J. Phys. Chem. C. 2008;112:3267-3271.
- [Google Scholar]
- Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol.. 2012;143:397-405.
- [Google Scholar]
- Historical trends in mercury sedimentation and mixing in the strait of Georgia, Canada. Environ. Sci. Technol.. 2005;39:4361-4368.
- [Google Scholar]
- Dicoumarol assisted synthesis of water dispersible gold nanoparticles for colorimetric sensing of cysteine and lysozyme in biofluids. RSC Adv.. 2015;5:39182-39191.
- [Google Scholar]
- Sunlight induced synthesis of reversible and reusable biocapped nanoparticles for metal ion detection and SERS studies. ACS Sustain. Chem. Eng.. 2014;2:699-705.
- [Google Scholar]
- Sequential detection of Fe3+ and As3+ ions by naked eye through aggregation and disaggregation of biogenic gold nanoparticles. Anal. Methods. 2015;7:168-174.
- [Google Scholar]
- Eco-friendly synthesis of ZnO nano pencils in aqueous medium: a study of photocatalytic degradation of methylene blue under direct sunlight. RSC Adv.. 2016;6:33821-33827.
- [Google Scholar]
- Room temperature biosynthesis of greatly stable fluorescent ZnO quantum dots for the selective detection of Cr3+ ions. Mater. Res. Bull.. 2017;95:163-168.
- [Google Scholar]
- Spectroscopic studies of L-arginine molecule. Indian J. Pure Appl. Phys.. 2010;48:251-255.
- [Google Scholar]
- Biosynthesis of gold nanoparticles using Ocimum sanctum extracts by solvents with different polarity. ACS Sustain. Chem. Eng.. 2016;4:2651-2659.
- [Google Scholar]
- Anti-aggregation of gold nanoparticle-based colorimetric sensor for glutathione with excellent selectivity and sensitivity. Analyst. 2011;136:196-200.
- [Google Scholar]
- S. Zhou, Homocysteine-mediated reactivity and assembly of gold nanoparticles. Langmuir. 2007;23:826-833.
- [Google Scholar]
- Full physical preparation of size-selected gold nanoparticles in solution: Laser ablation and laser-induced size control. J. Phys. Chem. B. 2002;106:7575-7577.
- [Google Scholar]
- Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism. 1989;38:753-758.
- [Google Scholar]
- Mechanism of gold nanoparticle formation in the classical citrate synthesis method derived from coupled in situ XANES and SAXS evaluation. J. Am. Chem. Soc.. 2010;132:1296-1301.
- [Google Scholar]
- Visual detection of arginine based on the unique guanidino group-induced aggregation of gold nanoparticles. Anal. Chim. Acta. 2013;764:78-83.
- [Google Scholar]
- Naked eye detection of glucose in urine using glucose oxidase immobilized gold nanoparticles. Anal. Chem.. 2011;83:2829-2833.
- [Google Scholar]
- Visual detection of arginine, histidine and lysine using quercetin-functionalized gold nanoparticles. Microchim. Acta. 2014;181:1917-1929.
- [Google Scholar]
- 4-Amino nicotinic acid mediated synthesis of gold nanoparticles for visual detection of arginine, histidine, methionine and tryptophan. Sens. Actuators B: Chem.. 2016;222:780-789.
- [Google Scholar]
- Biosynthesis of gold nanoparticles assisted by the intracellular protein extract of Pycnoporus sanguineus and its catalysis in degradation of 4-nitroaniline. Nanoscale Res. Lett.. 2015;10(147):1-8.
- [Google Scholar]
- A BODIPY-functionalized bimetallic probe for sensitive and selective color-fluorometric chemosensing of Hg2+. Analyst. 2012;137:3914-3916.
- [Google Scholar]
- Selective detection of cysteine and glutathione using gold nanorods. J. Am. Chem. Soc.. 2005;127:6516-6517.
- [Google Scholar]
- Selective detection of iodide and cyanide anions using gold-nanoparticle-based fluorescent probes. ACS Appl. Mater. Interface. 2012;4:2652-2658S.
- [Google Scholar]
- Colorimetric detection of DNA, small molecules, proteins and ions using unmodified gold. PNAS. 2010;107:10837-10841.
- [Google Scholar]
- Colorimetric detection of D-amino acids based on anti-aggregation of gold nanoparticles. Chin. Chem. Lett.. 2014;25:995-1000.
- [Google Scholar]
- Small molecule- and amino acid-induced aggregation of gold nanoparticles. Langmuir. 2013;29:7661-7673.
- [Google Scholar]
- Colorimetric detection of thiol-containing amino acids using gold nanoparticles. Analyst. 2002;127:462-465.
- [Google Scholar]
- Label-free amino acid detection based on nanocomposites of graphene oxide hybridized with gold nanoparticles. Biosens. Bioelectron.. 2016;77:963-970.
- [Google Scholar]
- Colorimetric detection of lysine using gold nanoparticles aggregation. Anal. Methods. 2012;4:2711-2714.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jksus.2017.12.001.
Appendix A
Supplementary data







