Translate this page into:
Polymorphisms of the matrix metalloproteinase 9 gene are associated with duodenal ulcer in a Caucasian population of Central Russia
⁎Corresponding author at: Department of Medical Biological Disciplines, Belgorod State University, 85 Pobedy St., Belgorod, 308015, Russia. churnosov@bsu.edu.ru (Mikhail Churnosov)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The aim of this study was to analyze functionally significant polymorphisms of matrix metalloproteinases genes (MMP-1, -2, -3, -8, -9) for their association with duodenal ulcer (DU) in the Caucasian population from Central Russia.
Methods
The study sample included 364 DU patients (208 had H. pylori and 156 were uninfected) and 347 controls (H. pylori-negative). Ten polymorphisms of the MMP-1, -2, -3, -8, -9 genes were examined for association with DU by the logistic regression analysis (used the three main genetic models). The polymorphisms of the MMP-9 gene associated with DU and 59 proxy variants (r2 ≥ 0.80) were studied in silico for their functionality.
Results
Allele G of rs17576 and haplotype GG [rs17576-rs3787268] of the MMP-9 gene may increase risk for DU (adjOR = 1.46–2.09, pperm ≤ 0.006 and adjOR = 1.60, pperm = 0.016 respectively). Five SNPs of the MMP-9 gene may increase risk for H. pylori-positive DU: alleles T of rs3918242 (adjOR = 1.95, pperm = 0.007), G of rs17576 (adjOR = 1.68–2.81, pperm ≤ 0.002), and A of rs17577 (adjOR = 1.96, pperm = 0.008), haplotypes GG [rs17576-rs3787268] (adjOR = 1.95, pperm = 0.006) and GGC [rs17576-rs3787268-rs2250889] (adjOR = 1.96, pperm = 0.006). These loci and 59 proxy SNPs may have functionally significant epigenetic effects, amino acid replacements in the MMP9, and correlate with the expression and alternative splicing of 17 and 6 genes respectively.
Conclusions
Polymorphisms rs17576 and rs3787268 of the MMP-9 were associated with DU and five MMP-9 gene SNPs were associated with H. pylori-related DU in Caucasians of Central Russia.
Keywords
Association
Duodenal ulcer
H. pylori
Matrix metalloproteinases
SNP
1 Introduction
Duodenal ulcer (DU) is a peptic ulcer disease affecting the duodenum. In the general population, the peptic ulcer prevalence is estimated at approximately 5–10% (Lanas and Chan, 2017). DU may be caused by various factors, e.g., Helicobacter pylori infection, some medications, e.g., aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), alcohol and tobacco consumption, dietary factors, stress, etc. (Kuna et al., 2019; Lanas and Chan, 2017). Both NSAIDs and H. pylori infection induce secretion of gastric acid and damage the mucus barrier that allow gastric acid to cause inflammation and ulceration of the duodenal lining (Zhang et al., 2013). On the other hand, far not all people using NSAIDs or infected by H. pylori develop DU. This assumes that individual susceptibility is important for the mucosal damage to begin (Kuna et al., 2019). DU is a multi-factorial disease and thus determined by a complex of environmental and genetic factors (Datta De and Roychoudhury, 2015).
Matrix metalloproteinases (MMPs) play a key role in extracellular matrix (ECM) degradation, one of the causes of gastrointestinal ulcer (Shahin et al., 2001; Tarnawski, 2005). MMPs comprise a family of neutral endopeptidases capable to degrade extracellular matrix proteins and remodel connective tissue (Cui et al., 2017). In addition to this, MMPs activate some chemokines, thus contributing to the recruitment of white blood cells into inflamed tissues (Kruidenier et al., 2006). MMPs are overexpressed in H. pylori-associated gastrointestinal ulcers (Hellmig et al., 2006; Pender and MacDonald, 2004). The MMP proteins can be expressed by many cell types in response to various signals from soluble factors or cell-matrix interactions (Parks et al., 2004). In H. pylori-infected mucosa, epithelial cells are apparently one of the major producers of MMPs (Mori et al., 2003). In addition to H. pylori infection (Bebb et al., 2003), other major stimuli for MMP production are cytokines produced by mucosal T cells and macrophages (Pender and MacDonald, 2004).
Despite the apparent significance of MMP for the development of DU, the association of these genes with the disease has been studied poorly: we were able to find only three articles on this topic (Shaymardanova et al., 2016; Shan et al., 2010; Yeh et al., 2010). Shaymardanova et al. (2016) analyzed seven loci from five MMP genes (rs1799750 and rs494379 MMP-1, rs2285052 MMP-2, rs3025058 MMP-3, rs3918242 and rs17576 MMP-9, rs2276109 MMP-12) in DU patients from the Republic of Bashkortostan (Russia) and showed the association of two polymorphisms, rs494379 MMP-1 and rs17576 MMP-9, with the disease. Yeh et al. (2010) studied loci of three MMP genes (MMP-3-1612 6A/5A, MMP-7-181 A/G, and MMP-9 exon 6 A/G) for their association with DU in H. pylori-infected Taiwanese patients and found that the MMP-3 promoter polymorphism (-1612 6A/5A) might contribute to DU susceptibility after H. pylori infection in Taiwanese females (a 2.4-fold increased risk of DU for the 6A6A genotype as compared to the 5A allele carriers). On the other hand, no association with the disease was detected for rs3918242 MMP–9 in Chinese children (Shan et al., 2010).
The available literature data, despite being contradictory, suggests that MMP genes may contribute to DU. This study analyzed the association of ten loci of the MMP-1, -2, -3, -8, -9 genes with DU in Caucasians from Central Russia.
2 Materials and methods
2.1 Study participants
The study sample included 364 DU patients and 347 controls. To be recruited for the study, the participants should meet the following inclusion criteria: Russian ethnicity (self-reported) and birthplace in Central Russia (Reshetnikov et al., 2015; Litovkina et al., 2014).
DU was diagnosed by clinical/endoscopic examination. The group of control included otherwise healthy participants without any symptoms of gastrointestinal disease (Minyaylo, 2020). Endoscopy was not performed in the participants from the control group because, in addition to ethical reasons, the chance to find an active ulcer in asymptomatic individuals is very low (García-González et al., 2003). Those individuals who were on long-term treatment with NSAIDs, corticosteroids, and aspirin were excluded.
The infection of H. pylori in DU participants was diagnosed by the histologic examination of endoscopic biopsies. Among 364 patients with DU, 208 had H. pylori and 156 were uninfected. Serology testing in the controls was used to detect H. pylori-specific IgG (H. pylori-positive controls were excluded from the further analysis. All participants were evaluated for the possible presence of psycho-social stressful factors such as numerous home/work stressful situations, the unsatisfactory (or absence) of social support, stressful family situations (single/separated/widowed), poor social or/and economic participants status (Moskalenko et al., 2019a).
The study protocol was approved by the Regional Ethics Committee at the medical institute of Belgorod State National Research University. All participants were asked to sign an informed consent prior to entering the study. All medical examinations of the participants were conducted at the Gastroenterology Division of the St. Joasaph Belgorod Regional Hospital.
2.2 DNA isolation, SNPs selection, and genotyping
About 4–5 ml of blood from each participant was drawn to a vacutainer tube (Vacutainer®) by a certified nurse. Total DNA was isolated from peripheral blood leucocytes using the phenol–chloroform protocol (Tikunova et al., 2017).
The selection of SNPs of the MMP genes was based on their previously reported associations with disorders of the digestive system (duodenal and gastric ulcer, cancer of gastric and other organs of digestive systems), predicted functional characteristics, and minor allele frequency >0.05 in Europeans (Ponomarenko et al., 2020a). The ten common SNPs of the five MMP genes were selected for the study: MMP-1 (rs1799750), MMP-2 (rs243865), MMP-3 (rs679620), MMP-8 (rs1940475), MMP-9 (rs2250889, rs17577, rs3918249, rs17576, rs3787268, rs3918242) (Moskalenko et al., 2019b; Starikova et al., 2021). The SNPs functionality was assessed in silico by the available online at the HaploReg tool (Ward and Kellis, 2016). All 10 loci MMP genes included in this study had potential functional significance (Supplementary Table 1). According to previously published association studies, eight loci were associated with various diseases of the digestive system (duodenal and gastric ulcer, gastritis, esophageal and gastric cancer, other digestive cancers) (among them with peptic ulcer were associated 2 loci, rs1799750 MMP3 and rs17576 MMP9) (Supplementary Table 2).
The selected loci were genotyped by the MassARRAY 4 system. About five percent of the samples were regenotyped at random (Golovchenko et al., 2020) and showed complete (100%) reproducibility.
2.3 Data analysis
The Hardy-Weinberg equilibrium of the observed genotype (allele) frequencies was assessed by the χ2-test. The associations of the candidate SNPs with DU were examined using the logistic regression analysis (according to the recessive, dominant, and additive models) (Ponomarenko et al., 2020b). The following covariates we used for the regression analysis: BMI as continuous variable, whereas stress, tobacco and alcohol consumption, positive peptic ulcer family history as categorical variables (data are presented in Table 1). The «Solid Spine» procedure of the linkage disequilibrium (at D' > 0.80) executed in the HaploView program (Barrett et al., 2005) was used to determine haplotype blocks. The association analysis (OR, 95% CI) and the permutation test (multiple comparisons adjustment) were carried out using PLINK v. 2.050 (Purcell et al., 2007). The value of Pperm ≤ 0.017 was applied as the significance level assuming the Bonferroni correction (n = 3) based on the number of pairwise comparisons (control vs DU, control vs H. pylori-infected patients, and control vs H. pylori-uninfected patients). P values < 0.05 are shown in bold.
Parameters
Control
mean ± SD, % (n)DU patients
mean ± SD, % (n)p
N
347
364
–
Age, years
(min–max)48.47 ± 13.69
(22–79)48.00 ± 14.54
(20–78)0.71
Gender ratio, f/m
66.28/33.72
(230/117)65.93/34.07 (240/124)
0.98
BMI, kg/m2
26.83 ± 5.09
25.75 ± 5.39
0.004
Age of developing peptic ulcer, years
–
36.12 ± 13.67
–
Family history of peptic ulcer
4.32 (15)
19.78 (72)
0.0005
Current smoking
14.99 (52)
42.31 (154)
0.0005
Alcohol consumption
32.28 (112)
53.30 (194)
0.0005
Stress
37.17 (129)
74.73 (272)
0.0005
Positivity H. pylori test (endoscopic biopsy and histological identification)
–
57.14 (208)
–
Somatic pathologies
Cardiovascular pathology
26.80 (93)
33.52 (122)
0.06
Endocrine pathology
3.17 (11)
2.20 (8)
0.57
Kidney pathology
2.59 (9)
2.75 (10)
0.99
Respiratory system pathology
4.32 (15)
6.04 (22)
0.40
Nervous system pathology
7.78 (27)
9.34 (34)
0.54
Musculoskeletal system pathology
6.91 (24)
7.69 (28)
0.80
2.4 SNPs functionality effects
The DU-associated genetic variants and their proxies were further studied in silico for their functional significance (Ponomarenko et al., 2021). The SIFT online tool (Kumar et al., 2009) was used to analyze missense SNPs. The regulatory potential was analyzed using HaploReg (Ward and Kellis, 2016) and RegulomeDB (Dong and Boyle, 2019). The RegulomeDB database values of the rank and the score were applied to estimate SNP regulatory potential: the higher RegulomeDB score (from 0 to 1) and lower RegulomeDB rank (from 1 to 7) indicate a higher regulatory potential (Dong and Boyle, 2019). The data from the GTExportal browser (The GTEx Consortium, 2017) was used to estimate the effect of the DU candidate loci on expression QTLs and splicing QTLs. Likewise, regulatory potential, eQTL, and sQTL parameters of the SNPs in LD with the DU-associated polymorphisms were assessed (Moskalenko et al., 2021). The polymorphisms linked (r2 ≥ 0.80) to the DU-associated ones were identified by HaploReg (Ward and Kellis, 2016).
3 Results
A summary of the phenotypic characteristics (demographic and clinical) of the enrolled subjects is provided in Table 1 and Table 2. The DU-affected participants had lower BMI, higher incidence of peptic ulcer in family history, stress, tobacco and alcohol consumption, as compared to the control subjects (p = 0.004–0.0005) (data are presented in Table 1). Therefore, these variables were applied as covariates (continuous (BMI) and categorical (all other aforementioned demographic parameters) variables) in the association data analyses. The prevalence of frequent somatic pathologies such as essential hypertension (24.18% DU and 19.02% controls, p = 0.11), heart ischemia (15.11% DU and 12.97% controls, p = 0.47), heart atherosclerosis (10.98% DU and 8.93% controls, p = 0.43), spine osteochondrosis (9.07% DU and 7.20% controls, p = 0.44), osteoartrosis (6.87% DU and 5.19% controls, p = 0.43), chronic bronchitis (4.67% DU and 4.03% controls, p = 0.81), diabetes (2.20% DU and 3.17% controls, p = 0.57), chronic glomerulonephritis/pyelonephritis (2.75% DU and 2.59% controls, p = 0.99) among DU patients and controls were not significantly different (p > 0.05).
Parameters
DU
mean ± SD, % (n)
Anatomical characteristics of the ulcer
Location: Bulb
100.00 (364)
Ulcer diameter, cm
0.74 ± 0.42
Ulcer diameter by categories:Small
(<0.5 cm)Medium
(0.5–1.0 cm)Large
(>1.0 cm)23.07
(84)64.29
(234)12.64
(46)
Associated complications
Bleeding
6.04 (22)
Perforation
10.99 (40)
Stenosis
11.54 (42)
Malignancy
1.10 (4)
3.1 Association analysis
The distribution of genotypes/alleles of the studied SNPs in the case and control groups is shown in Supplementary Table 3. All polymorphisms were in the HWE (p > 0.005, pbonf > 0.05). The risk value for DU had allele G rs17576 (recessive model, the parameter of OR adjusted for covariates adjOR = 2.09, pperm = 0.004, power – 96.86%; additive model, adjOR = 1.46, pperm = 0.006, power – 93.66%) (Table 3). All results were obtained after adjustment for covariates. OR, odds ratio; 95%CI, 95% confidence interval.
SNP
Gene
MAF
n
Additive model
Dominant model
Recessive model
OR
95%CI
P
OR
95%CI
P
OR
95%CI
P
L95
U95
L95
U95
L95
U95
rs1940475
MMP-8
T
704
1.02
0.78
1.32
0.912
0.96
0.63
1.47
0.860
1.08
0.70
1.67
0.714
rs1799750
MMP-1
2G
695
0.90
0.68
1.17
0.422
0.93
0.61
1.42
0.740
0.79
0.49
1.25
0.312
rs679620
MMP-3
T
705
0.96
0.74
1.25
0.760
0.86
0.56
1.33
0.499
1.04
0.67
1.62
0.856
rs243865
MMP-2
T
701
0.92
0.68
1.26
0.606
0.91
0.62
1.34
0.631
0.88
0.41
1.88
0.742
rs3918242
MMP-9
T
703
1.36
0.95
1.94
0.093
1.56
1.04
2.33
0.032
0.61
0.16
2.30
0.464
rs3918249
MMP-9
C
703
1.20
0.92
1.58
0.184
1.41
0.95
2.11
0.087
1.07
0.63
1.82
0.794
rs17576
MMP-9
G
710
1.46
1.11
1.91
0.006
1.43
0.97
2.13
0.074
2.09
1.27
3.45
0.004
rs3787268
MMP-9
A
703
0.99
0.71
1.38
0.971
0.96
0.64
1.43
0.836
1.19
0.48
2.94
0.706
rs2250889
MMP-9
G
700
0.65
0.41
1.02
0.062
0.62
0.37
1.04
0.068
0.46
0.09
2.26
0.340
rs17577
MMP-9
A
692
1.34
0.95
1.90
0.096
1.56
1.04
2.35
0.031
0.68
0.21
2.02
0.518
Three SNPs of the MMP-9 were associated with H. pylori-related DU: allele T of rs3918242 was associated according to the dominant model (adjOR = 1.95, pperm = 0.007, power – 95.98%), allele G of rs17576 – according to the additive (adjOR = 1.68, pperm = 0.002, power – 98.49%) and recessive (adjOR = 2.81, pperm = 0.001, power – 99.73%) models, allele A of rs17577 – according to the dominant model (adjOR = 1.96, pperm = 0.008, power – 96.20%) (Table 4). All results were obtained after adjustment for covariates. OR, odds ratio; 95%CI, 95% confidence interval.
SNP
Gene
MAF
n
Additive model
Dominant model
Recessive model
OR
95%CI
P
OR
95%CI
P
OR
95%CI
P
L95
U95
L95
U95
L95
U95
H. pylori-positive DU
rs1940475
MMP-8
T
550
1.04
0.75
1.43
0.823
1.04
0.62
1.74
0.892
1.07
0.63
1.82
0.814
rs1799750
MMP-1
2G
541
0.95
0.68
1.31
0.734
0.88
0.53
1.48
0.635
0.98
0.56
1.70
0.940
rs679620
MMP-3
T
551
0.93
0.67
1.29
0.668
0.78
0.46
1.30
0.337
1.07
0.63
1.82
0.798
rs243865
MMP-2
T
545
1.02
0.70
1.47
0.938
0.94
0.59
1.51
0.810
1.31
0.57
3.04
0.526
rs3918242
MMP-9
T
549
1.58
1.03
2.41
0.036
1.95
1.20
3.18
0.007
0.33
0.04
2.71
0.304
rs3918249
MMP-9
C
549
1.41
1.01
1.97
0.045
1.66
1.01
2.72
0.045
1.45
0.78
2.73
0.243
rs17576
MMP-9
G
554
1.68
1.21
2.34
0.002
1.60
0.98
2.60
0.060
2.81
1.56
5.03
0.001
rs3787268
MMP-9
A
551
0.95
0.63
1.43
0.800
0.89
0.54
1.45
0.636
1.26
0.42
3.81
0.683
rs2250889
MMP-9
G
546
0.56
0.30
1.03
0.062
0.58
0.30
1.12
0.107
0.00
0.00
inf
0.998
rs17577
MMP-9
A
542
1.56
1.03
2.35
0.035
1.96
1.20
3.18
0.007
0.56
0.12
2.63
0.461
H. pylori-negative DU
rs1940475
MMP-8
T
502
1.02
0.72
1.44
0.931
0.92
0.53
1.61
0.770
1.14
0.64
2.02
0.656
rs1799750
MMP-1
2G
493
0.87
0.61
1.25
0.464
1.03
0.58
1.83
0.915
0.63
0.33
1.23
0.177
rs679620
MMP-3
T
499
0.79
0.55
1.14
0.205
0.72
0.41
1.25
0.241
0.75
0.40
1.41
0.370
rs243865
MMP-2
T
499
0.80
0.52
1.23
0.317
0.84
0.50
1.42
0.524
0.47
0.13
1.65
0.237
rs3918242
MMP-9
T
497
1.07
0.66
1.72
0.787
1.10
0.63
1.91
0.731
0.93
0.20
4.43
0.930
rs3918249
MMP-9
C
499
1.01
0.70
1.46
0.953
1.18
0.70
1.98
0.546
0.75
0.35
1.63
0.468
rs17576
MMP-9
G
502
1.22
0.85
1.76
0.283
1.25
0.74
2.11
0.406
1.41
0.70
2.83
0.337
rs3787268
MMP-9
A
497
1.09
0.70
1.69
0.697
1.10
0.65
1.86
0.731
1.19
0.36
3.87
0.775
rs2250889
MMP-9
G
496
0.77
0.43
1.38
0.389
0.69
0.35
1.37
0.290
1.08
0.22
5.32
0.922
rs17577
MMP-9
A
490
1.07
0.67
1.72
0.774
1.13
0.65
1.97
0.666
0.82
0.18
3.83
0.804
Haplotype GG [rs17576-rs3787268] of MMP-9 was associated with DU (adjOR = 1.60, p = 0.006, pperm = 0.016). Also, MMP-9 haplotypes GG [rs17576-rs3787268] and GGC [rs17576 – rs3787268 – rs2250889] were associated with H. pylori-related DU (adjOR = 1.95, p = 0.0009, pperm = 0.006 and adjOR = 1.96, p = 0.001, pperm = 0.006 respectively) (Fig. 1).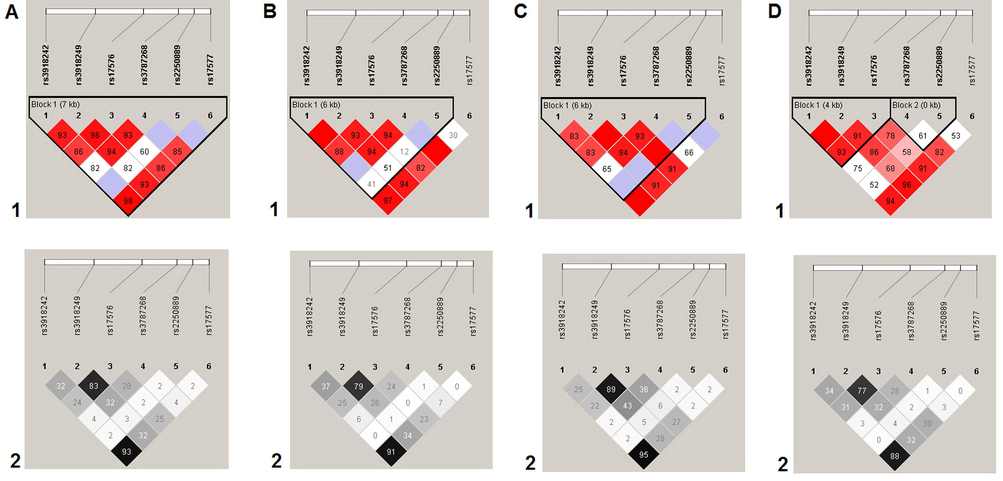
Linkage disequilibrium (LD) between SNPs rs3918242, rs3918249, rs17576, rs3787268, rs2250889, and rs17577 of the MMP9 gene in DU patients. A, summary; B, H. pylori-positive DU patients; C, H. pylori-negative DU patients; D, control group. LD values are given as Lewontin’s standardized coefficient D′ (Figure sections 1) and the square of the Pearson's correlation coefficient (r2) (Figure sections 2) between SNPs.
3.2 Functional SNPs
Non-synonymous SNPs. Among the five DU- and H. pylori-positive DU-associated polymorphisms of the MMP-9 gene, three SNPs (rs17576, rs17577, rs2250889) cause amino acid replacements in the encoded protein (Gln279Arg, SIFT predictor value “tolerated”; Arg668Gln, SIFT predictor value “deleterious”; Arg574Pro, SIFT predictor value “tolerated” respectively).
Regulatory effects. According to the RegulomeDB, five MMP9 loci associated with DU and H. pylori-positive DU are characterized by significant epigenetic potential. Variant rs17576 MMP9, individually associated with both DU and H. pylori-positive DU, had RegulomeDB rank 4 (transcription factor (TF) binding + DNAse-1 hypersensitivity peak) and RegulomeDB score 0.61. Another polymorphism, rs17577, had RegulomeDB rank = 2b (TF binding + any motif + DNase Footprint + DNase peak) and RegulomeDB rank score equal 0.79. The other H. pylori-positive DU-associated SNPs MMP9 gene had RegulomeDB rank and RegulomeDB score equals 4–5 and 0.59–0.61 respectively. Data of the HaploReg showed that all five polymorphisms were located in the DNAse hypersensitivity region, four loci had a DNA position in the histone modification region corresponding to enhancer (H3K4me1 and H3K27ac) and promoter (H3K9ac and H3K4me3) elements in several tissues/organs and the twelve motifs site to the factors of transcription (TFs); two SNPs – in the protein-bound region (Supplementary Table 1). Herewith, alleles of the five SNPs of the MMP-9 gene, which are risk variants for H. pylori-positive DU (Table 4), decrease affinity to the ten TFs and increase affinity to two TFs (Supplementary Table 4).
In addition to the five H. pylori-positive DU-associated SNPs, 59 proxy SNPs were analyzed for their functionality (Supplementary Table 5). Seven SNPs (including four missense mutations) were mapped to MMP9 exons, 24 and 28 loci were in introns and intergenic regions, respectively. All 59 proxy SNPs possessed a significant regulatory potential; several of them had pronounced epigenetic effects (Supplementary Table 5). For example, rs10432735 (linked to rs3918242, r2 = 0.89) is located in the DNAase I hypersensitive region (21 tissues), in the several protein-bound regions (POL2, ZNF143, YY1, CTCF, ELF1, FOSL1, HMGN3) and a motif DNA regions (Pax-4, ZNF219, Zfp281). Importantly, both the H. pylori-positive DU-associated SNPs and their proxies manifested significant epigenetic effects in the target organs of DU in an adult (gastric, small intestine, duodenum mucosa and smooth muscle, mucosa and smooth muscle of stomach) and fetus (small intestine and stomach).
Expression and splicing QTLs. According to the GTExportal database, five DU- and H. pylori-positive DU-associated MMP9 loci and 50 proxy SNPs affected the mRNA transcript level of seventeen genes (e.g., ZNF335, CD40, SLC12A5, MMP9, etc.) (Supplementary Table 6 and Supplementary Table 7) and might affect alternative splicing of six genes (e.g., CD40, SLC12A5, PLTP, etc.) (Supplementary Table 8 and Supplementary Table 9) in more than 20 various tissues/organs.
4 Discussion
The present study reports the association of several MMP-9 polymorphic variants with DU in Caucasians from the central region of Russia: variant allele G rs17576 and haplotype GG[rs17576-rs3787268] of the MMP-9 gene increased risk for DU (adjOR = 1.46–2.09 and adjOR = 1.60 respectively). Also, five MMP-9 SNPs increased risk for H. pylori-related DU: alleles T of rs3918242 (adjOR = 1.95), G of rs17576 (adjOR = 1.68–2.81), and A of rs17577 (adjOR = 1.96), haplotypes GG[rs17576-rs3787268] (adjOR = 1.95) and GGC[rs17576-rs3787268-rs2250889] (adjOR = 1.96).
MMP-9 or gelatinase-B is a type IV collagenase located on chromosome 20q11.2-q13.1. MMP-9 is expressed by a variety of cells including epithelial cells, macrophages, T-cells, etc. (Cui et al., 2017). MMP-9 contributes to the degradation of large substrates such as elastin, various collagen types, fibronectin, laminin, proteoglycan, etc., and may cause ECM breakdown and loss of tissue integrity (Cui et al., 2017). Previous studies demonstrated an important role of ECM degradation in gastrointestinal ulceration (Shahin et al., 2001; Tarnawski, 2005). In addition, DU is associated with infiltration of the duodenal mucosa by monocytes, lymphocytes, plasma cells, neutrophils, which release proinflammatory cytokines (IL-6, IL-1, IL-8, TNF-α) (Lanas and Chan, 2017; Zhang et al., 2013). There is evidence that cytokines released by mucosal macrophages and T cells are also major inducers of MMP production (Pender and MacDonald, 2004).
The literature data about the association of MMP-9 gene polymorphisms with DU is scarce and contradictory. Shaymardanova et al. (2016) reported the significant association of the AG rs17576 genotype (OR = 1.57) in a multiethnic cohort from the Republic of Bashkortostan (Russia). Our results are in general agreement with these findings: allele G of rs17576 MMP-9 was shown to elevate the risk of DU in the Caucasian population from Central Russia. On the contrary, Yeh et al. (2010) did not find a significant association of rs17576 with H. pylori-positive DU in Taiwanese. Similarly, no association with DU was reported for rs3918242 in Chinese (Shan et al., 2010) and in the multiethnic sample from the Republic of Bashkortostan (Shaymardanova et al., 2016), which contradicts the results of the present study. On the other hand, our results are in agreement with those of Shaymardanova et al. (2016) who did not find an association of rs1799750 MMP-1 with DU. In summary, our study is the first to report the association of the MMP-9 polymorphic variants with H. pylori-infected DU.
According to our results, MMP-9 SNPs were associated with H. pylori-related DU but not with H. pylori-negative DU. There is evidence that in H. pylori-infected mucosa, epithelial cells are among the major producers of the MMP proteins (Mori et al., 2003). Furthermore, H. pylori by itself can stimulate gastric epithelial cells to release MMPs (Bebb et al., 2003). H. pylori infections apparently induce the release of MMP-9 by activating nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) (Mori et al., 2003). Antral gastric mucosa of H. pylori-infected patients with gastritis manifested a ten-fold increase of the MMP-9 gene expression and 19-fold higher MMP-9 activity versus the uninfected patients (Bergin et al., 2004). The MMP-9 serum levels were higher in individuals with H. pylori-positive gastritis than that in uninfected controls (Rautelin et al., 2009). A significant proportion of H. pylori-infected individuals (80–85%) develop mild antrum and body gastritis characterized by hypergastrinemia with normal gastric acid levels (Gravina et al., 2018), while about 10–15% of H. pylori-infected patients develop prevalent antrum gastritis characterized by hypergastrinemia and elevated gastric mucosa secretion resulting in duodenal ulceration (Censini et al., 2001).
The associations of the MMP-9 polymorphisms determined in the present study may be backed by various functional effects of these loci and their proxies as suggested by the results of the in silico analysis. The predicted effects include amino acid replacements in the MMP-9 protein, alteration of expression and alternative splicing of several genes (e.g., CD40, SLC12A5, MMP9, ACOT8, SNX21, PLTP), etc. Importantly, these effects were predicted in both the target organs of DU (e.g., small intestine, duodenal mucosa and smooth muscle, and the others) and those involved in the pathophysiology of the disease (e.g., the frontal cortex of the brain, pituitary, blood, thyroid, adrenal gland, adipose (visceral and subcutaneous), etc.) (Lanas and Chan, 2017). The functional significance of the loci analyzed in the present study was also predicted for cardiovascular diseases (arterial hypertension) in the studied population of Central Russia (Moskalenko et al., 2019a; Moskalenko et al., 2019b; Moskalenko et al., 2021). Worth noting that some cardiovascular diseases (e.g, coronary artery disease) and body fat-related traits have a significant positive SNP-based genetic correlation with peptic ulcer disease (Wu et al., 2021), so that MMP-9 gene polymorphisms and their proxies may be among syntropic genes contributing to the pathophysiology of DU and cardiovascular diseases.
5 Conclusion
Allele G rs17576 and haplotype GG [rs17576-rs3787268] of the MMP-9 gene may increase risk for DU (adjOR = 1.46–2.09 and adjOR = 1.60 respectively). Also, five SNPs of the MMP-9 gene may increase risk for H. pylori-positive DU: alleles T of rs3918242 (adjOR = 1.95), G of rs17576 (adjOR = 1.68–2.81), and A of rs17577 (adjOR = 1.96), haplotypes GG[rs17576-rs3787268] (adjOR = 1.95) and GGC [rs17576-rs3787268-rs2250889] (adjOR = 1.96). These loci and 59 SNPs linked to them appear to have functionally significant epigenetic effects, amino acid replacements in MMP9, may affect the expression and alternative splicing of 17 and 6 genes respectively.
Disclosure of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263-265.
- [CrossRef] [Google Scholar]
- Helicobacter pylori upregulates matrilysin (MMP-7) in epithelial cells in vivo and in vitro in a Cag dependent manner. Gut. 2003;52:1408-1413.
- [Google Scholar]
- Increased production of matrix metalloproteinases in Helicobacter pylori-associated human gastritis. Helicobacter. 2004;9:201-210.
- [CrossRef] [Google Scholar]
- Cellular responses induced after contact with Helicobacter pylori. Curr. Opin. Microbiol.. 2001;4:41-46.
- [Google Scholar]
- Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci.. 2017;147:1-73.
- [CrossRef] [Google Scholar]
- To be or not to be: The host genetic factor and beyond in Helicobacter pylori mediated gastro-duodenal diseases. World J. Gastroenterol.. 2015;21:2883-2895.
- [CrossRef] [Google Scholar]
- Predicting functional variants in enhancer and promoter elements using RegulomeDB. Hum. Mutat.. 2019;40:1292-1298.
- [CrossRef] [Google Scholar]
- Association of interleukin 1 gene family polymorphisms with duodenal ulcer disease. Clin. Exp. Immunol.. 2003;134:525-531.
- [Google Scholar]
- Functionally significant polymorphisms of ESR1 and PGR and risk of intrauterine growth restriction in population of Central Russia. Eur. J. Obstet. Gynecol. Reprod. Biol.. 2020;253:52-57.
- [CrossRef] [Google Scholar]
- Helicobacter pylori and extragastric diseases: A review. World J. Gastroenterol.. 2018;24:3204-3221.
- [CrossRef] [Google Scholar]
- Genetic variants in matrix metalloproteinase genes are associated with development of gastric ulcer in H. pylori infection. Am. J. Gastroenterol.. 2006;101:29-35.
- [Google Scholar]
- Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant CXCL7 from intestinal epithelial cells. Gastroenterology. 2006;130:127-136.
- [Google Scholar]
- Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc.. 2009;7:1073-1081.
- [Google Scholar]
- Peptic ulcer disease: A brief review of conventional therapy and herbal treatment options. J. Clin. Med.. 2019;8:179.
- [CrossRef] [Google Scholar]
- Genes involved in the regulation of vascular homeostasis determine renal survival rate in patients with chronic glomerulonephritis. Gene. 2014;546:112-116.
- [CrossRef] [Google Scholar]
- Minyaylo, O.N., 2020. Allele distribution and haploblock structure of matrix metalloproteinase gene polymorphism in patients with H. pylori-negative gastric ulcer and duodenal ulcer. Res. Results Biomed. 6, 488-502. Russian. doi: 10.18413/2658-6533-2020-6-4-0-5.
- Helicobacter pylori induces matrix metalloproteinase-9 through activation of nuclear factor κB. Gastroenterology. 2003;124:983-992.
- [Google Scholar]
- Moskalenko, M.I., Ponomarenko, I.V., Polonikov, A.V., Zhernakova, N.I., Efremova, O.A., Churnosov, M.I. 2019. The role of stress factors and genetic predisposition in the development of stroke in patients with essential hypertension. Zh Nevrol Psikhiatr Im S S Korsakova. 2019a. 119(3. Vyp. 2), 11-17. Russian. doi: 10.17116/jnevro201911903211.
- Moskalenko, M.I., Milanova, S.N., Ponomarenko, I.V., Polonikov, A.V., Churnosov, M.I., 2019b. Study of associations of polymorphism of matrix metalloproteinases genes with the development of arterial hypertension in men. Kardiologiia. 59(7S), 31-39. Russian. doi: 10.18087/cardio.2598.
- Polymorphisms of the matrix metalloproteinase genes are associated with essential hypertension in a Caucasian population of Central Russia. Sci. Rep.. 2021;11:5224.
- [CrossRef] [Google Scholar]
- Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol.. 2004;4:617-629.
- [Google Scholar]
- Matrix metalloproteinases and the gut: new roles for old enzymes. Curr. Opin. Pharmacol.. 2004;4:546-550.
- [Google Scholar]
- Candidate genes for age at menarche are associated with endometriosis. Reprod. Biomed. Online. 2020;41:943-956.
- [CrossRef] [Google Scholar]
- Candidate genes for age at menarche are associated with endometrial hyperplasia. Gene. 2020;757:144933.
- [CrossRef] [Google Scholar]
- Candidate genes for age at menarche are associated with uterine leiomyoma. Front. Genet.. 2021;11:512940.
- [CrossRef] [Google Scholar]
- PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet.. 2007;81:559-575.
- [CrossRef] [Google Scholar]
- Enhanced systemic matrix metalloproteinase response in Helicobacter pylori gastritis. Ann. Med.. 2009;41:208-215.
- [Google Scholar]
- The insertion-deletion polymorphism of the ACE gene is associated with increased blood pressure in women at the end of pregnancy. J. Renin Angiotensin Aldosterone Syst.. 2015;16:623-632.
- [CrossRef] [Google Scholar]
- Remodeling of extracellular matrix in gastric ulceration. Microsc. Res. Technol.. 2001;53:396-408.
- [Google Scholar]
- Relationship between gene polymorphisms in MMP-9 and Helicobacter pylori-related upper gastrointestinal disease in children. Zhongguo Dang Dai Er Ke Za Zhi. 2010;12:262-266. Chinese
- [Google Scholar]
- Role of Allelic Genes of Matrix Metalloproteinases and Their Tissue Inhibitors in the Peptic Ulcer Disease Development. Russ. J. Genet.. 2016;52:320-330.
- [CrossRef] [Google Scholar]
- Novel data about association of the functionally significant polymorphisms of the MMP-9 gene with exfoliation glaucoma in the Caucasian population of Central Russia. Ophthalmic Res.. 2021;64(3):458-464.
- [CrossRef] [Google Scholar]
- Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig. Dis. Sci. Suppl. 2005;1:S24-S33.
- [CrossRef] [Google Scholar]
- Genes of tumor necrosis factors and their receptors and the primary open angle glaucoma in the population of Central Russia. Int J Ophthalmol.. 2017;10:1490-1494.
- [CrossRef] [Google Scholar]
- HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res.. 2016;D1:D877-D881.
- [Google Scholar]
- GWAS of peptic ulcer disease implicates Helicobacter pylori infection, other gastrointestinal disorders and depression. Nat. Commun.. 2021;12(1):1146.
- [CrossRef] [Google Scholar]
- Matrix metalloproteinase-3 promoter polymorphisms but not dupA-H. pylori correlate to duodenal ulcers in H. pylori-infected females. BMC Microbiol.. 2010;10:218.
- [CrossRef] [Google Scholar]
- Association between TNF α gene polymorphisms and the risk of duodenal ulcer: a meta-analysis. PLoS One. 2013;8:e57167.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102142.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







