Translate this page into:
Neuroprotective and antioxidant capability of RW20 peptide from histone acetyltransferases caused by oxidative stress-induced neurotoxicity in in vivo zebrafish larval model
⁎Corresponding author at: Department of Biotechnology, College of Science and Humanities, SRM Institute of Science and Technology, Kattankulathur 603 203, Chennai, Tamil Nadu, India. jesuaroa@srmist.edu.in (Jesu Arockiaraj)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Oxidative stress causes disruption of cell macromolecules and resulting neurodegeneration when the antioxidant defense mechanism is inhibited. Oxidative stress is emerging as a key therapeutic target for nerve agent toxicity. Therefore, antioxidant treatment methods are a novel and effective approach to attenuate oxidative stress and neurodegeneration. This study evaluated the neuroprotective and antioxidant ability of a short peptide, RW20 derived from histone acetyltransferases (HATs).
Using bioinformatics tools, the short amino acid sequence of RF13 peptide with antioxidant activity was predicted from HAT of Channa striatus transcriptome. The synthesized antioxidant peptide at various concentrations (10–50 μM) was analyzed in in vitro and in vivo antioxidant assay. The neuroprotective effect of RF13 peptide was evaluated in H2O2 stressed zebrafish larvae.
The results showed the neuroprotective effect by restoring antioxidant enzymes including SOD, CAT, GPx, GSH, and GST treated with RW20. In addition, the oxidative stress indicator of lipid peroxidation was gradually declined with an increase in the concentration of peptides. It was also shown to attenuate neuronal damage by inhibiting nitric oxide overproduction, thus increasing AChE activity. Further, to validate the neuroprotective effect of RW20, the intracellular ROS level and apoptosis in the zebrafish larvae were analyzed by incubating the larvae along with RW20, which decreased intracellular ROS level and reduced the cell death.
Overall, the study demonstrated that RW20 possessed prominent antioxidant activity. Hence, we conclude that RW20 can be developed as a potent therapeutic agent against oxidative stress-induced neurodegenerative diseases.
Keywords
Antioxidant peptide
Histone acetyltransferases
Oxidative stress
Neuroprotection
Neurotoxicity
1 Introduction
Central nervous system cells were considered more vulnerable to ROS due to their intrinsic higher oxidative metabolism and lower antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD) (Olmez and Ozyurt, 2012). Accumulated evidence that elevated ROS levels could cause serious cell damage due to oxidative stress has shown in most prevalent many neurodegenerative disorders such as Alzheimer's disease, multiple sclerosis, Parkinson's disease and Huntington's disease (Nabavi et al., 2013; Si et al., 2013). Hydrogen peroxide (H2O2) in the central nervous system is a ubiquitous neuronal signaling substance that affects transcription factors, phosphatases, kinases and ion channels (Kishida and Klann, 2007). In the presence of H2O2, divalent metals can produce ROS and pro-inflammatory mediators, resulting in oxidative stress, mitochondrial dysfunction, inflammation and apoptosis, which can promote neurodegeneration. Accumulation of H2O2 can also raise the risk of various neurodegenerative diseases significantly. Extensive studies of oxidative stress-induced neuronal death have been documented using H2O2 as a method to build in vitro neurodegeneration models to screen potential neuroprotective agents (Shalgum et al., 2019).
Bioactive peptides from natural bio-resources produce a large number of potent and unique bioactive molecules (Arasu et al., 2017). These peptides are residues of 3–20 amino acids, and their sequence composition demonstrates their ability (Elias et al., 2008). Histone acetyltransferases (HATs) are a diverse class of enzymes involved in acetylation where an acetyl group transfers acetyl-CoA to ε-amino group of a lysine residue. HATs play an essential role in the post-translational modification of histones (Cress and Seto, 2000). HATs complex can control gene expression by responding to external signals and altering the chromatin structure (Kim et al., 2018). Accordingly, in the present study, a full length HATs cDNA was obtained from the transcriptome dataset of stripped murrel, Channa striatus constructed previously (Arasu et al., 2013; Arockiaraj et al., 2014; Kumaresan et al., 2015) and was characterized by in silico analysis. The RW20 peptide amino acid sequence composition was found in the HATs protein of Channa striatus; the multiple sequence analysis validates that RW20 amino acid segment was also present in the histone acetyl transferase of the other species. This evidence suggests that RW20 peptide is a highly conserved region of HAT. The recognized peptide region was synthesized and investigated its potential in vitro, in vivo antioxidant and neuroprotection on H2O2 induced neurotoxicity in zebrafish larvae by analyzing the changes in the biomarkers of neurodegeneration.
2 Materials and methods
2.1 In silico analysis of HATs and identification of antioxidant peptide
HATs sequence was obtained from the previously constructed transcriptome library of a teleost Channa striatus (Arockiaraj et al., 2014). The Expasy translate tool was used to translate the cDNA of HATs into protein sequences (Arasu et al., 2013). The physicochemical parameters of HATs were determined by the Prot-Param tool (Arockiaraj et al., 2015). The BLAST program was used to identify the homologous sequence of HATs (Sathyamoorthi et al., 2017). Domains and motif analysis for HATs were performed by Prosite analysis (Arockiaraj et al., 2014). The multiple sequence alignment was run using Bioedit (ver 7.1.3.0) (Arasu et al., 2014). The 3D structure was recognized using the I-TASSER program and visualized over Pymol.
The RW20 peptide (1RPVKRKKGWPKGVKRGPPKW20) from HATs was identified using in silico analysis. The antioxidant property of RW20 was identified by its molecular weight, hydrophobicity and amino acid composition using the Expasy Prot-Param tool (https://web.expasy.org/protparam). The hydrophobic nature of the RW20 was studied using a peptide program (https://www.peptide2.com/). The helical wheel of RW20 was identified using an online server (http://www.bioinformatics.nl/cgi-bin/emboss/pepwheel). The primary structure of the peptide RW20 was predicted using pep draw (www.pepdraw.com). The predicted RW20 peptide was synthesized at Zhengzhou Peptides Pharmaceutical Technology Co. Ltd., China.
2.2 In vitro antioxidant activity of RW20
To determine the antioxidant activity of RW20, the assays such as DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), superoxide anion radical scavenging activity and H2O2 scavenging assay were performed using UV–Vis spectrophotometer (UV 1800, SHIMADZU, Kyoto, Japan) as reported in our earlier study (Velayutham et al., 2021). Trolox was used as a positive control for the assays.
2.3 Toxicity studies
2.3.1 Cytotoxicity assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay procedure was performed as mentioned earlier (Guru et al., 2021a; Kumar et al., 2020). The N9 murine microglial cell lines were seeded (5 × 105 cell/well) with different concentrations of RW20 peptide and incubated for 24 h. After incubation, 5 mg/mL MTT solution was added to the incubated cells, and it was further incubated in the dark for 4 h at 37 °C. The formazan crystal was formed during the addition of 100 µL of dimethyl sulfoxide (DMSO), and the resulting absorbance was measured at 570 nm with a microplate reader.
2.3.2 Hemolytic assay
The hemolytic effect of RW20 on human erythrocytes was performed as described earlier (Gopinath et al., 2021). The RW20 peptide was treated to human erythrocytes (Ethical Clearance No. 885/IEC/2015) with various concentrations (10–50 µM). The mixture was allowed to incubate at room temperature for 1 h. After incubation, the sample was centrifuged for nearly 5 min at 4000 rpm. Then, 100 µL of the supernatant was diluted with 1 mL of PBS, and the absorbance was measured at 560 nm.
2.4 Zebrafiah larvae maintenance and treatment
Male and female zebrafish were purchased from SB aquarium, Guduvanchery, Tamilnadu, India. After acclimating for one month, the fishes were used for breeding and the embryos were collected for the study (Guru et al., 2021c). The fertilized embryos are observed under the microscope and unfertilized embryos are discarded. For further assay, the fertilized zebrafish embryos are placed in a six well plate with embryo medium. The zebrafish embryo or larvae were randomly divided into seven groups (n = 30 each) as control group (I), in which zebrafish embryo or larvae are untreated and kept in embryo medium, H2O2 group (II), in which zebrafish embryo or larvae are exposed to H2O2 (1 mM), and RW20 peptide pre-treated group (III, IV, V, VI, and VII) with different concentration before H2O2 exposure. The overall exposure paradigm used in the current study is illustrated in E-Suppl. Fig. 3.
2.5 Developmental toxicity test on zebrafish embryos
In a 4 h post fertilized (hpf) zebrafish embryo, the toxicity test was assayed using six well plate containing RW20 peptide at different concentrations. H2O2 (1 mM) was used as a positive control, and the untreated embryos in the embryo medium were used as an experimental control. Around 15 embryos were allowed in each well containing embryo medium (3 mL). During the period between 4 hpf and 72 hpf, the embryo was examined carefully to monitor the mortality, hatching, heart rate, and morphological abnormalities (Ramachandran et al., 2017).
2.6 Assessment of protective effect on developmental toxicity
In a 48 hpf zebrafish embryo, the protective effect of RW20 in H2O2 induced developmental toxicity was analyzed. Initially, the RW20 was pre-treated to the zebrafish embryo (48 hpf) before being treated with the H2O2 (1 mM) and the larvae were exposed to H2O2 for a period lasting upto to 6 dpf. For image analysis of the protective effect on developmental toxicity at 6 dpf after H2O2 exposure, lateral view of larvae was imaged using a stereographic microscope (Na et al., 2009).
2.7 Behavioural test
The zebrafish larvae of 48 hpf are used for the behavioural test to analyze the neurocognitive function. The partition preference test and horizontal compartment test was performed as described previously by Dubey et al. (2015).
2.7.1 Partition preference test
At 48 hpf, the larvae were pretreated with RW20, and then larvae were exposed to H2O2 till 6 dpf. Once the larvae reached 6 dpf, the partition preference test was performed. In brief, in a glass chamber water level was maintained up to 3 cm; the chamber was divided into two equal parts with a glass plate which allowed for vertical operation to divide or undivide the chamber. The RW20 pretreated zebrafish larvae exposed to H2O2 (6 dpf) (n = 30) were allowed to the one side (right) of the chamber, which was considered as ‘home chamber’. In the home chamber, the larvae were left undisturbed for 45 min as acclimatization period. Then, the vertically partitioned glass plate was lifted to 1 cm, so that the larvae can move from the home chamber to the other side (left) of the tank, which was considered as ‘target chamber’. The assay was performed by counting the number of larval entries from home chamber to target chamber, thus cognitive function was examined. The partition preference test was estimated based on the number of larvae moved from home to target chamber, which was calculated and reported as “percentage of entry to the target chamber” (% ETC) and it was also calculated the time that larvae spent in the target chamber, that was expressed as sec in “Time spent in the target chamber” (TSTC).’
2.7.2 Horizontal compartment test
At 48 hpf, the larvae were pretreated with RW20 and then larvae were exposed to H2O2 till 6 dpf. Once the larvae reached 6 dpf, the horizontal compartment test was performed. In brief, a glass chamber was filled with water until a height of 21 cm. Then, the water height in the chamber was divided into three horizontal segments as lower, middle and upper with an equal height of 7 cm. For each group, 30 larvae (6 dpf) were allowed into the chamber and left undisturbed for a day as acclimatization, and they were freely swimming. For the assay, the “time spent in the upper segment” (TSUS) and “time spent in the lower segment” (TSLS) of the larvae was calculated based on their healthy and neurodegenerative status. Thus cognitive impairment of zebrafish larvae was examined.
2.8 Mobility test
Once the larvae reached 6 dpf, the mobility test was performed. In a 2 L tank filled with 1 L water system, the locomotor activity of RW20 pre-treated zebrafish larvae (6 dpf) was measured. The locomotor activity was calculated by taking the total number of lines crossed by the zebrafish, dividing it by the time, and expressing it as the number of crossed lines/5 min.
2.9 Enzyme assay
Random zebrafish larvae from each group were anesthetized with tricaine, and the head portion without eyes and yolk sac regions were homogenized with 0.1 M ice-cold phosphate buffer (pH 7.4). The homogenate was then centrifuged at 5000 rpm for 15 min. The supernatant was collected, and the Bradford method was used to determine the protein content in the sample (Braford, 1976). The antioxidant enzyme assays of SOD, CAT, lipid peroxidation, reduced glutathione (GSH), glutathione peroxidase (GPx), Glutathione S-transferase (GSH), acetylcholinesterase, and nitric oxide assay was performed as reported in our previous study (Issac et al., 2021a).
2.10 Quantification of ROS and apoptosis level in zebrafish larvae
DCFDA and acridine orange staining dye were used to study intracellular ROS and apoptosis (Issac et al., 2021b). The untreated control and RW20 pre-treated larvae (96 hpf) for 1 h were transferred to 6 well plates containing 3 mL of embryo medium and then incubated for 3 h with H2O2 (1 mM). The larvae were stained with 20 µg/mL of DCFDA and acridine orange (7 µg/ml) for 30 min. Then, the larvae were washed with PBS after incubation, and the intracellular ROS level and apoptosis were analyzed by the fluorescence intensity of different exposure groups. The fluorescence image was captured using Leica DM6 fluorescent microscope. Image J software was used to quantify the fluorescence intensity.
2.11 Statistical analysis
The values reported in this study are the average of three independent experiments, and the data were expressed as mean ± standard deviation. The significance of data is analyzed using One-way analysis of variance (ANOVA) and Tukey multiple range test using GraphPad Prism (ver. 5.0). Statistically significant was determined by p value less than 0.05.
3 Results
3.1 Bioinformatic characterization of HAT
RW20 was obtained from the cDNA sequence of the previously constructed transcriptome library of C. striatus (Cs). The recognized CsHAT sequence was submitted to The European Molecular Biology Laboratory (EMBL) nucleotide sequence database under the accession number LS999894. The CsHAT cDNA sequence length was 441 base pairs, which encodes a protein sequence of 200 amino acids with a molecular mass of 23225.54 Da and the theoretical isoelectric point (pI) 9.66. The putative protein sequence has 35 negatively charged residues (Asp + Glu) and 45 positively charged residues (Arg + Lys). The aliphatic index of the protein is 52.20, and the instability index is estimated to be 58.17. The multiple sequence alignment of HATs and other homologous sequences of fishes was highly conserved (E-Suppl. Fig. 1A). I-Tasser server predicted a 3D structure of HATs with arginine and glycine rich peptide region (E-Suppl. Fig. 1B).
3.2 Identification of peptide sequence
RW20 peptide identified from HAT cDNA sequence of C. striatus contained 20 amino acids (1RPVKRKKGWPKGVKRGPPKW20). The RW20 peptide had amino acids such as arginine, lysine, glycine, proline, valine and tryptophan, which naturally reveal a strong antioxidant character. The RW20 peptide includes a net charge of +9, a molecular weight of 1.4 kDa and the sequence composition is predicted based on the peptide property calculator (ver 3.1). The basic (45%) and neutral (20%) residues in the sequence are identified by the helical wheel prediction, which also predicted the peptide's polar and non-polar regions (E-Suppl. Fig. 1C).
3.3 Antioxidant activity of RW20
DPPH radical scavenging activity of RW20 increased in a concentration-dependent manner. RW20 showed significant (p < 0.05) radical scavenging activity at the minimum concentration of 10 μM (33.6%) and maximum radical scavenging activity at 40 μM (76.33%), whereas Trolox maximum activity 88.3% was observed at 50 μM (E-Suppl. Fig. 2A).
In the ABTS radical scavenging assay, the maximum radical scavenging activity of trolox was observed with 83% at 50 µM, and the minimum activity of 53% was found in 10 µM. The RW20 peptide exhibited 71% of maximum activity at 40 µM concentration, and the lower concentration (10 µM) showed 34% of radical scavenging activity. A dose-dependent increase in the concentration of RW20 intensified the activity significantly (p < 0.05) (E-Suppl. Fig. 2B).
The superoxide scavenging activity of RW20 increased remarkably with an increase in concentration. Thus, the inhibitory activity of RW20 on superoxide anion formation at lower concentration was 31.6% (10 μM), whereas maximum activity was observed in 40 µM of concentration, 69.6% compared to the corresponding Trolox activity, 76.3% (E-Suppl. Fig. 2C).
Endogenous ROS such as H2O2 has been recognized as destructive molecules. Our results showed a strong H2O2 scavenging activity at the concentration 40 µM (60%), and Trolox showed an increase in activity of 82% at 50 µM. The values were differed significantly (p < 0.05) between control and experimental (E-Suppl. Fig. 2D).
3.4 Cytotoxic and hemolytic effect of RW20
The cytotoxic effect of the RW20 peptide was determined in N9 murine microglial cells (Fig. 1A). RW20 at 50 µM showed a significant (p < 0.05) effect on cell viability after 24 h treatment. The study was also conducted on the human blood cells. The result indicates that RW20 had no hemolytic activity at concentrations between 10 µM and 40 µM (Fig. 1B).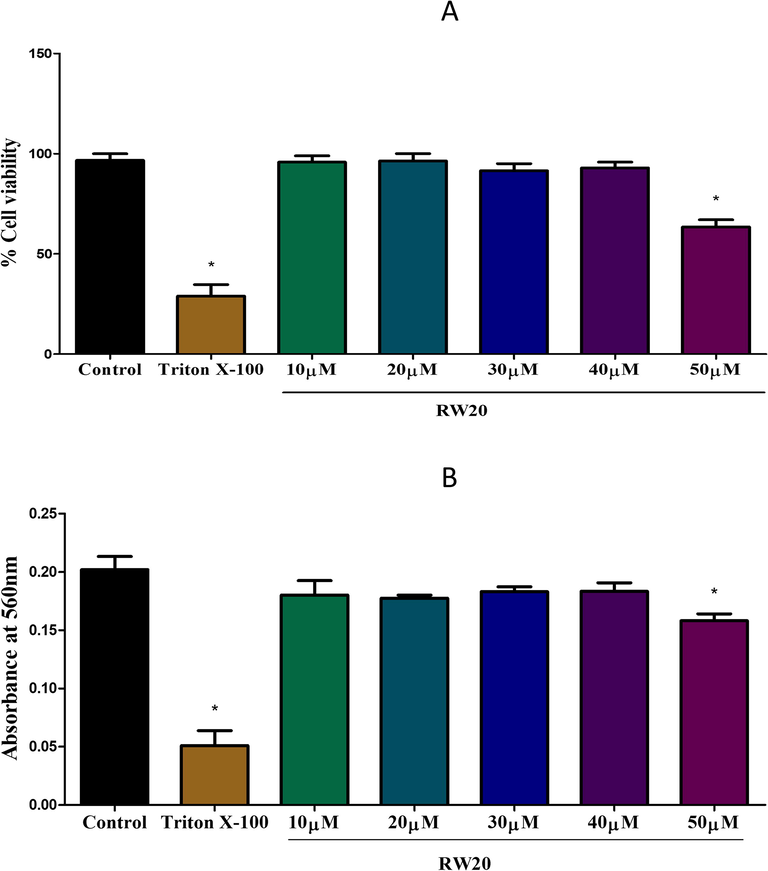
(A) Effect of RW20 peptide on the viability of N9 murine microglial cells, evaluated by MTT assay. Triton X-100 (0.01%) and PBS are used as positive control and control for the experiment, respectively. The peptide treatment for 24 h did not alter the cell viability, except higher concentration (50 µM). (B) Hemolytic activity of the RW20 peptide against human erythrocytes. Data were expressed as mean ± SD. * denotes p < 0.05 as compared to the control.
3.5 Toxicity assay in zebrafish larvae
3.5.1 Mortality, hatching, and heart rate
The larvae of zebrafish treated with RW20 peptide (10–40 μM) showed no significant mortality at 96 hpf compared to H2O2 treatment. With a peptide concentration of 50 μM, a moderate mortality rate was observed (E-Suppl. Fig. 4A). The hatching rate from 48 hpf to 72 hpf was estimated in the zebrafish embryo; our results showed that more than 90% of the embryos had hatched from 48 hpf to 72 hpf in the RW20 peptide treatment (10–40 μM). Even at a higher concentration of 50 μM, only a slight reduction in the hatching rate and heart rate was observed (E-Suppl. Fig. 4B & C).
3.5.2 Morphological analysis
E-Suppl. Fig. 4D shows the developmental toxicity of zebrafish larvae exposed to H2O2 from 4 hpf to 72 hpf. Many malformations, including bent spine (46%) and yolk sac edema (66%) were observed in the H2O2 treated group at 72 hpf. Additionally, we found that RW20 peptide treatment (10–40 µM) showed no malformation in larvae. However, the larvae treated with the higher concentration (50 µM) of RW20 developmental abnormalities of the bent spine (21%) became more apparent(Fig. 2).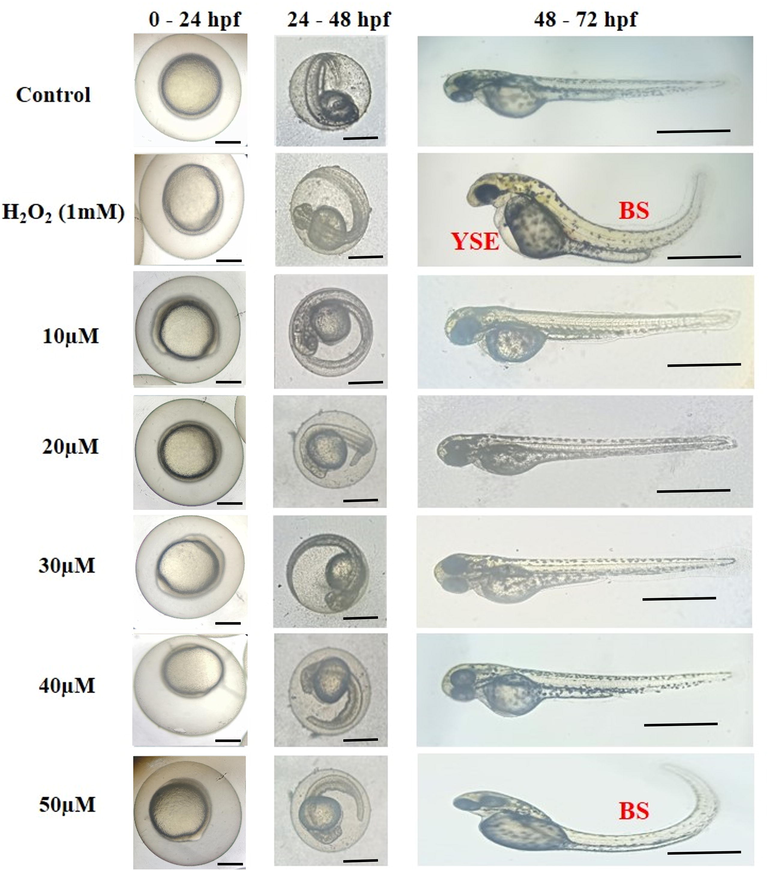
Representative photomicrographs of morphological malformation observed during the exposure period. Control with PBS treatment and RW20 peptide at different concentrations (10–50 µM). RW20 at 50 µM concentration showed a bent spine (BS), whereas H2O2 treatment group showed with malformations such as yolk sac edema (YSE) and BS. Scale bar = 500 µm.
3.6 Effect of RW20 in H2O2 induced developmental toxicity
The image analysis revealed that RW20 prevented the morphological abnormalities caused by H2O2 (Fig. 3). The size of the yolk sac region in the H2O2 treatment group was almost four times that of the control group. RW20, on the other hand, clearly protected zebrafish larvae from H2O2. The zebrafish larvae exposed to H2O2 showed a shortening of the body length due to a bent spine at 6 dpf, but no growth arrest occurred when the larvae were pre-treated with RW20.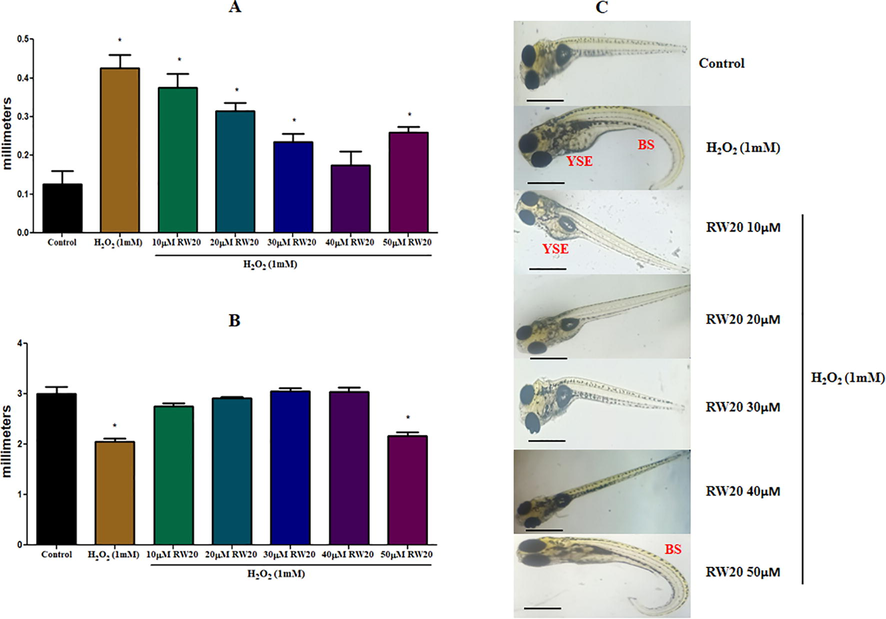
Effect of RW20 on the H2O2 induced malformation of increased in size of (A) YSE and (B) decrease in body length due to bent spine in zebrafish larvae. (C) Lateral view of zebrafish larvae was photographed, and data were quantitated by image J analysis. N = 30 zebrafish larvae per group. Data were expressed as mean ± SD. * denotes p < 0.05 as compared to the control. Scale bar = 500 µm.
3.7 Effect of RW20 in H2O2 induced cognitive impairment
In the partition preference and horizontal compartment test, exposure to H2O2 (1 mM) causes cognitive impairment, and it was detected based on the decrease in the percentage of ETC and TSTC values and a reduction in TSUS and increase in TSLS values compared to the control group (Figs. 4 and 5). Pre-treatment of RW20 has been shown to produce important (p < 0.05) dose-dependent neuroprotective activity against cognitive impairment caused by H2O2.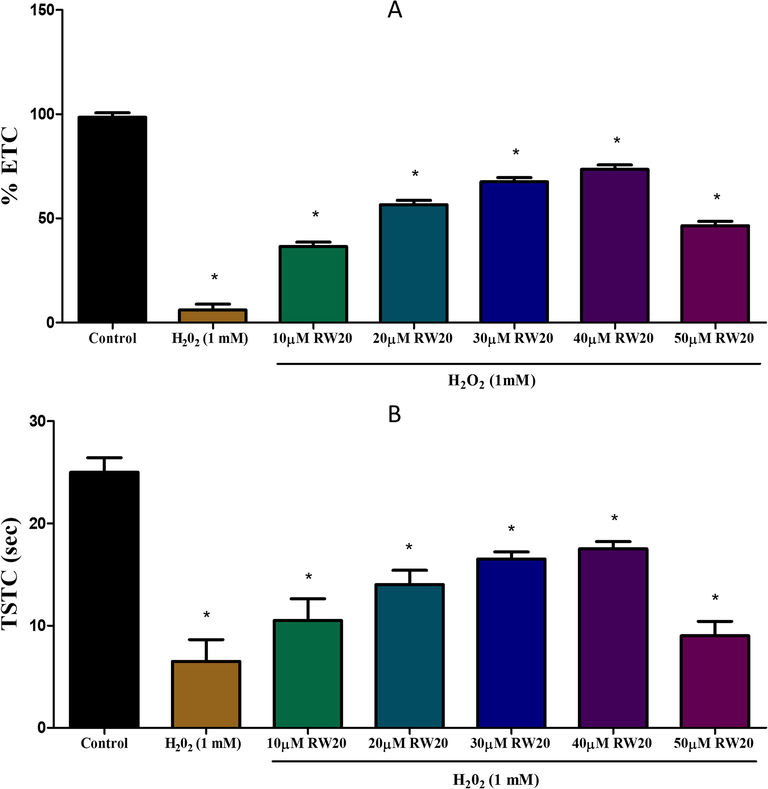
Effect of RW20 peptide in H2O2 induced cognitive impairment of partition test. N = 30 zebrafish larvae per group. Data were expressed as mean ± SD. * denotes p < 0.05 as compared to the control.
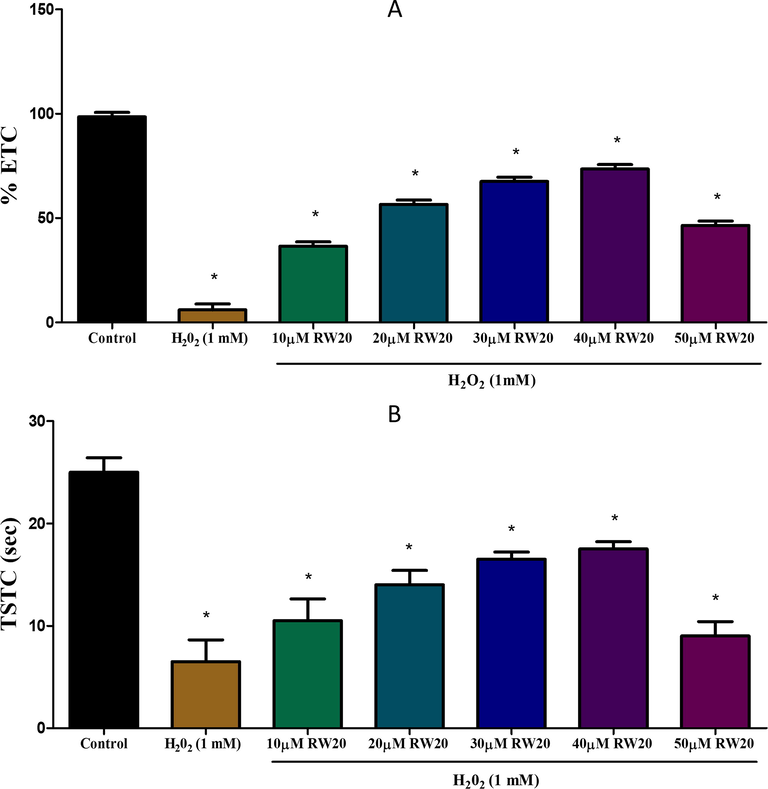
Effect of RW20 peptide in H2O2 induced cognitive impairment of partition test. N = 30 zebrafish larvae per group. Data were expressed as mean ± SD. * denotes p < 0.05 as compared to the control.
3.8 Effect of RW20 on zebrafish larvae mobility
To determine the effect of RW20 on locomotor activity, we evaluated zebrafish motility for 5 min. The pre-treatment of RW20 resulted in significant changes in the mobility of H2O2 exposed zebrafish larvae (Fig. 6). The results demonstrated that H2O2 reduced motility when compared to the control. Pre-treatment with RW20 administration significantly (p < 0.05) improved zebrafish motility in a dose-dependent manner.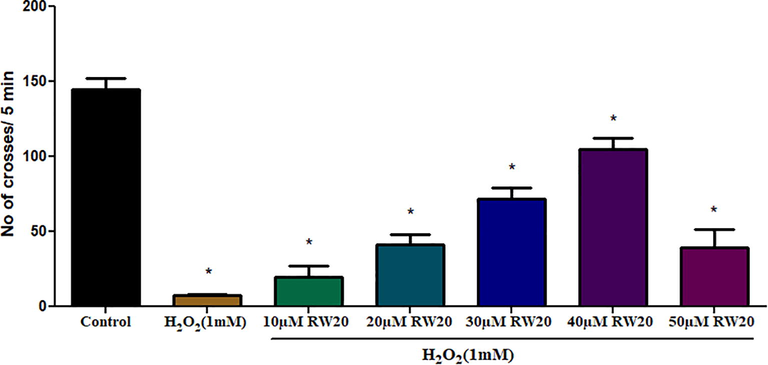
Effect of RW20 peptide on mobility of zebrafish larvae. Values are presented as mean ± SD (n = 30/group). * Symbol indicated the values are statistically significant at p < 0.05.
3.9 Antioxidant enzyme activity OF RW20
SOD catalyzes the dismutation of the superoxide radical into H2O2; and CAT catalyzes H2O2 to water and oxygen. The SOD (5 U/mg of protein) and CAT (4 µmol/mg of protein) activity were remarkably decreased in the H2O2 treated group. Meanwhile, the RW20 peptide at 40 μM showed a higher level of SOD (19 U/mg of protein) and CAT (18 µmol/mg of protein) activity compared to the control. The total SOD and CAT activity of RW20 against the H2O2 stressed zebrafish larvae were shown in E-Suppl. Fig. 5A & B.
The lipid peroxidation disrupts the cell membrane assembly and resulting in severe cellular dysfunction. The H2O2 (1 mM) treated group showed an increased level of lipid peroxidation levels (31 nmol/L) than the control group (E-Suppl. Fig. 5C). However, a dramatic reduction in lipid peroxidation level was observed in the RW20 peptide treated group. A higher level of lipid peroxidation inhibition was observed in 40 µM (13 nmol/L).
The RW20 peptide treatment showed maximum activity of GSH (6 nmol/mg of protein), GPx (15.2 U/mg of protein) and GST (6.3 U/mg of protein) at 40 µM concentration. There was a significant (p < 0.05) increase in enzyme activity compared with the control. This indicates that RW20 is a potent antioxidant peptide by inducing enzyme activity, which plays a crucial role in protecting cells from oxidative damage (E-Suppl. Fig. 6A, B & C).
3.10 Estimation of acetylcholinesterase and nitric oxide
The H2O2 induced zebrafish larvae showed decreased acetylcholinesterase activity of 2.3 μmoles/mL. Treatment with RW20 peptide resulted in significant (p < 0.05) restoration of acetylcholinesterase activity at dose-dependent concentrations. The maximum acetylcholinesterase activity was observed in 40 µM (6.3 μmoles/mL) compared to control (Fig. 7).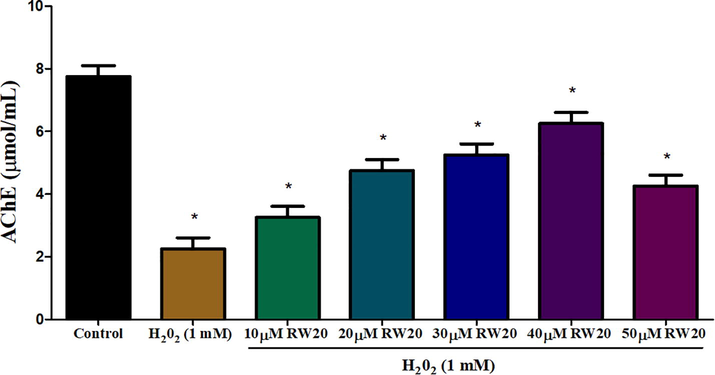
Estimation of AChE activity in oxidative stressed zebrafish larvae head portion at 96 hpf of control and RW20 peptide treated group. Values are presented as mean ± SD (n = 30/group). * Symbol indicated the values are statistically significant at p < 0.05.
NO is produced at a high concentration under pathological conditions such as aging and inflammation. The Griess reagent assay was used to determine the NO levels in the supernatants of zebrafish larvae. As shown in E-Suppl. Fig. 7, H2O2 increased NO production level compared to the normal NO level in a control group, whereas RW20 treatment was shown to suppress H2O2 induced NO production in a concentration dependent manner. In particular, treatment with 40 µM of RW20 substantially inhibited NO overproduction.
3.11 Potential of RW20 to protect H2O2 induced zebrafish larvae in ROS accumulation
The intracellular ROS scavenging activity of RW20 peptide against H2O2 induced zebrafish larvae was investigated by DCFDA and acridine orange. The results were observed based on fluorescence intensity. The H2O2 treated larvae showed an increase in fluorescence intensity in DCFDA (97%) and acridine orange (83%), indicating an increase in ROS and apoptosis (Figs. 8 and 9). In the RW20 peptide treated group (40 µM), there was a decrease in ROS (39%) and apoptosis (43%), which implies RW20 peptide exhibit as a protective antioxidant agent against neuroinflammation caused by oxidative stress.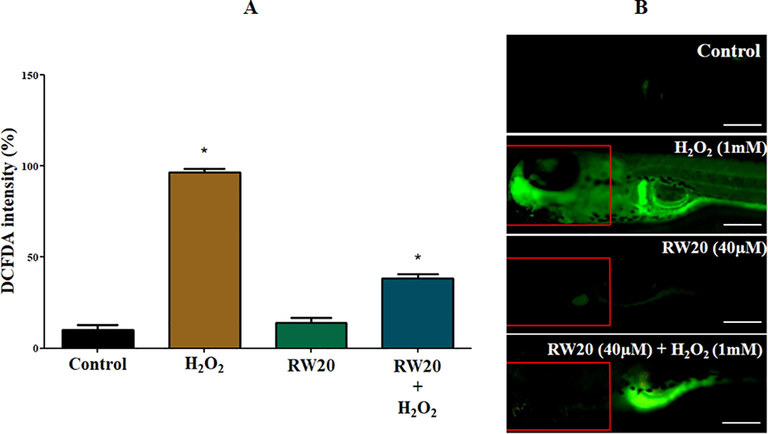
(A) Quantitative analysis of in vivo ROS generation in the head of zebrafish larvae. The fluorescence intensity was quantified using ImageJ. (B) Representative photomicrographs of 96 hpf zebrafish fish larvae by an oxidation-sensitive DCFDA fluorescent probe. The fluorescence image was captured using a fluorescence microscope. Experiments were performed in triplicate, and the data were expressed as mean ± S (n = 30/group). * represents the statistical significance at p < 0.05. Scale bar = 250 µm.
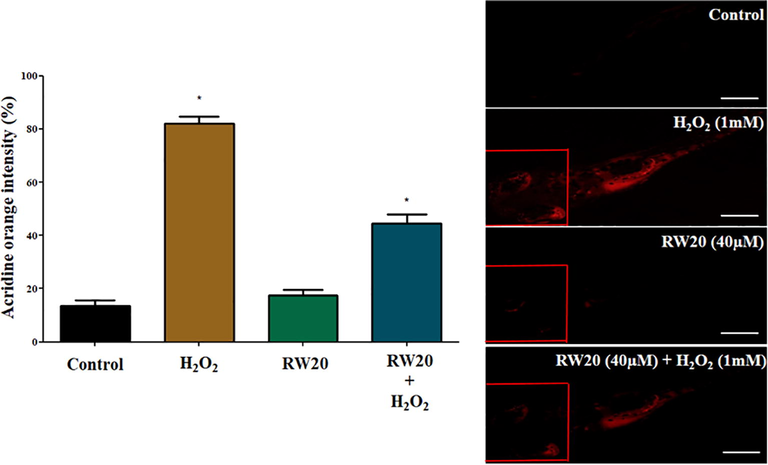
(A) Quantitative analysis of apoptosis activity in the head of zebrafish larvae. The fluorescence intensity was quantified using ImageJ. (B) Representative photomicrographs of 96 hpf zebrafish fish larvae stained with acridine orange. The fluorescence image was captured using a fluorescence microscope. Experiments were performed in triplicate, and the data were expressed as mean ± SD (n = 30/group). * represents the statistical significance at p < 0.05. Scale bar = 250 µm.
4 Discussion
The present study reported the neuroprotective effect of RW20 peptide from C. striatus in terms of oxidative stress markers and apoptosis in zebrafish larvae. We found that RW20 peptide prevents zebrafish larvae from neurotoxicity induced by H2O2, as indicated by the improved recovery of neurological function, decreases in oxidative stress, increases in antioxidant enzyme activities and reduction in apoptosis. RW20 peptide from HATs contains a high percentage of positively charged basic amino acids such as arginine and lysine and hydrophobic residues like glycine, valine and proline; altogether, the hydrophobic amino acids produced the potent radical scavenging and lipid peroxidation activity (Rajapakse et al., 2005). Accordingly, in our study, the synthesized peptide RW20 contains hydrophobic amino acids (glycine, proline, valine), basic amino acids (lysine, arginine) and aromatic amino acid (tryptophan), which are all exhibited a high level of scavenging efficacy (Wang et al., 2014).
In vitro antioxidant activity of RW20 exhibits radical scavenging activity in a dose-dependent manner. The hydrophobic, basic and aromatic amino acid sequence of the RW20 peptide strengthens its free radical scavenging activity with an increase in concentration from 10 µM to 40 µM when compared with Trolox. The hemolytic and cytotoxic effect of RW20 peptide was analyzed to confirm the non-toxic nature of the peptide towards the brain cell line (N9 murine microglial cells) and human blood cells. The result proved that RW20 had no cytotoxic effect against N9 murine microglial cells and was non-hemolytic to human blood cells until the concentration of 40 µM concentration. To act as a therapeutic agent, the peptide should be non-toxic (Comegna et al., 2010); thus RW20 peptide can act as a safe and efficient therapeutic agent.
The zebrafish is a versatile and easy to breed vertebrate model that provides major benefits for investigating in vivo drug screening and developmental neurotoxicity (Brenet et al., 2020). Zebrafish embryos and larvae were used to evaluate the effect of H2O2 and different concentrations of RW20 peptide. The results showed that RW20 peptide treated zebrafish larvae at various concentrations (10–40 µM) did not indicate any adverse effect in the morphology of the larvae, whereas the H2O2 (1 mM) and 50 µM of RW20 peptide treated group showed mortality, decreased hatching rate, reduced heart rate and malformation like yolk sac edema and bent spine. In zebrafish larvae, the RW20 has a protective effect against the morphological alterations caused by H2O2 exposure. The pre-treated larvae with RW20 showed the protective effect against morphological disruption, such as yolk sac edema and body axis shortening due to a bent spine. There are various basic and complex behavioural programs for zebrafish larvae, including spontaneous swimming, responses to multiple stimuli and cognitive behaviour (Nishimura et al., 2015) were observed. The treatment with H2O2 induced cognitive impairment in zebrafish larvae; different neurocognitive behavioural changes were expressed, such as decreased ETC and TSTC in the partition test, a decrease in TSUS and an increase in TSLS in the horizontal compartment test. In addition, pre-treatment with RW20 demonstrated a substantial ameliorative effect against cognitive dysfunction caused by H2O2. Interestingly, pre-treatment with RW20 resulted in marked improvement in the mobility of zebrafish larvae and the effect was dose-dependent suggesting neuroprotective activity.
Oxidative stress is considered a toxic condition for the normal functioning of the brain. The brain is vulnerable to oxidative stress since it uses chemically diverse reactive species for signal transmission (Lee et al., 2020). Enzymatic and non-enzymatic antioxidant systems play a crucial role in maintaining the balance between the pro-oxidant and antioxidant agents in the brain and minimizing oxidative stress (Guru et al., 2021b; Jiang et al., 2016). In this study, the SOD and CAT antioxidant activity after exposure to H2O2 (1 mM) declined considerably in the head of zebrafish larvae, but the pre-treatment of RW20 enhanced the SOD and CAT activity in a dose-dependent manner (10–40 µM). A high level of ROS interacts with polyunsaturated fatty acid and triggers the release of reactive aldehyde metabolites (MDA) and other toxic substances. RW20 peptide treatment reduced lipid peroxidation level in the H2O2 induced supernatant of zebrafish larvae head compared with the control group. GPx reduces H2O2 to hydroxyl group with GSH as substrate and detoxifies ROS by oxidizing GSH to GSSG (Borutova et al., 2008). The RW20 peptide proved that it is the potent activator of the GPx, GST, and GSH antioxidant enzyme and acts as an inducer in the antioxidant defense system.
AChE was found to play an important role in the conduction of nerve impulses at the neuromuscular junction. AChE inhibition was associated with exposure to toxins, resulting in a harmful effect on the body (Rodríguez-Fuentes et al., 2015). In our analysis, AChE activity was significantly reduced in the head of H2O2-treated zebrafish larvae. The AChE activity level was restored close to the control group when the larvae sample were treated with RW20 peptide. NO may inhibit key energy metabolism enzymes, damage DNA, deplete intracellular glutathione, and respond to the formation of peroxynitrite with superoxide. Under conditions of excessive production, NO appears to be a neurotoxin, suggesting that NO has a role in neurodegenerative diseases (Schulz et al., 1995). The present study demonstrates that exposure to H2O2 increased NO production, and the RW20 peptide reduced the NO overproduction in the head of zebrafish larvae. Based on the result, it shows that RW20 provides the neuroprotective activity against the H2O2 induced toxicity.
At a concentration of 40 μM, the RW20 peptide demonstrated better activity with no toxicity effect. The optimal concentration (40 μM) was therefore selected for further study. We further investigate the effect of RW20 peptide on H2O2 induced intracellular oxidative stress in the head of zebrafish larvae. In our results, the zebrafish larvae treated with RW20 prior to the H2O2 treatment showed a significant reduction in the intracellular ROS level in the head of zebrafish larvae, indicating the strong neuroprotective and antioxidant potential of RW20. ROS production is also well known to be closely associated with cell apoptosis in fish in response to contamination (Deng et al., 2009). In our results, the control larvae had intact cells, while H2O2 treatment caused cell death in the head of zebrafish larvae. However, a substantial reduction in cell death was observed when the larvae were treated with RW20 prior to H2O2 treatment. This study showed that RW20 peptides have a prominent neuroprotective effect and antioxidant activity against neuroinflammation and neuron apoptosis in the head of zebrafish larvae.
5 Conclusion
We have validated the evidence that RW20 can restore behavioural phenotypes by suppressing H2O2 induced oxidative stress and neurotoxicity in zebrafish larvae. Furthermore, the neuroprotective effect of RW20 in this study is mediated by reducing the generation of intracellular ROS, inhibiting cellular apoptosis and restoring the level of antioxidants in the head of zebrafish larvae. Further investigations are required to show the mechanism by which RW20 demonstrates its neuroprotection against the Alzheimer's disease model. Therefore, we conclude that RW20 derived from CsHAT protein might help treat the neurodegenerative disorder.
Acknowledgements
We acknowledge Dr. Kanchana K. Mala for providing necessary help in performing haemolytic assay and associated ethical clearance (885/IEC/2015). The authors extend their appreciation to the Researchers Supporting Project (Number: RSP-2021/393), King Saud University, Riyadh, Saudi Arabia.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bacterial membrane binding and pore formation abilities of carbohydrate recognition domain of fish lectin. Dev. Comp. Immunol.. 2017;67:202-212.
- [CrossRef] [Google Scholar]
- Molecular characterization of a novel proto-type antimicrobial protein galectin-1 from striped murrel. Microbiol. Res.. 2014;169(11):824-834.
- [CrossRef] [Google Scholar]
- Fish lily type lectin-1 contains β-prism architecture: Immunological characterization. Mol. Immunol.. 2013;56(4):497-506.
- [CrossRef] [Google Scholar]
- Fish chemokines 14, 20 and 25: A comparative statement on computational analysis and mRNA regulation upon pathogenic infection. Fish Shellfish Immunol.. 2015;47(1):221-230.
- [CrossRef] [Google Scholar]
- A murrel interferon regulatory factor-1: Molecular characterization, gene expression and cell protection activity. Mol. Biol. Rep.. 2014;41(8):5299-5309.
- [CrossRef] [Google Scholar]
- Effects of deoxynivalenol and zearalenone on oxidative stress and blood phagocytic activity in broilers. Arch. Anim. Nutr.. 2008;62(4):303-312.
- [CrossRef] [Google Scholar]
- A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [CrossRef] [Google Scholar]
- Organophosphorus diisopropylfluorophosphate (DFP) intoxication in zebrafish larvae causes behavioral defects, neuronal hyperexcitation and neuronal death. Sci. Rep.. 2020;10:1-13.
- [CrossRef] [Google Scholar]
- Design, synthesis and antimicrobial properties of non-hemolytic cationic α-cyclopeptoids. Bioorganic Med. Chem.. 2010;18(5):2010-2018.
- [CrossRef] [Google Scholar]
- Histone deacetylases, transcriptional control, and cancer. J. Cell. Physiol.. 2000;184:1-16.
- [CrossRef] [Google Scholar]
- Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat. Toxicol.. 2009;93(1):29-36.
- [CrossRef] [Google Scholar]
- Protective effect of rutin on impairment of cognitive functions of due to antiepileptic drugs on zebrafish model. Indian J. Pharmacol.. 2015;47:86-89.
- [CrossRef] [Google Scholar]
- Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr.. 2008;48(5):430-441.
- [CrossRef] [Google Scholar]
- 6-Gingerol and semisynthetic 6-Gingerdione counteract oxidative stress induced by ROS in zebrafish. Chem. Biodivers.. 2021;11:807-813.
- [CrossRef] [Google Scholar]
- Deteriorating insulin resistance due to WL15 peptide from cysteine and glycine-rich protein 2 in high glucose-induced rat skeletal muscle L6 cells. Cell Biol. Int.. 2021;45(8):1698-1709.
- [CrossRef] [Google Scholar]
- Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep.. 2021;48(1):743-761.
- [CrossRef] [Google Scholar]
- Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev. Comp. Immunol.. 2021;114:103863.
- [CrossRef] [Google Scholar]
- Oxidative stress induced antioxidant and neurotoxicity demonstrated in vivo zebrafish embryo or larval model and their normalization due to morin showing therapeutic implications. Life Sci.. 2021;283:119864.
- [CrossRef] [Google Scholar]
- Tryptophan-tagged peptide from serine threonine-protein kinase of Channa striatus improves antioxidant defence in L6 myotubes and attenuates caspase 3 – dependent apoptotic response in zebrafish larvae National Centre for Cell Science. Fish Physiol. Biochem.. 2021;47(2):293-311.
- [Google Scholar]
- Role of nitric oxide in neurodegenerative diseases. Curr. Opin. Neurol.. 1995;8(6):480-486.
- [Google Scholar]
- Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol.. 2016;147:1-19.
- [CrossRef] [Google Scholar]
- Epigenetic control of oxidative stresses by histone acetyltransferases in Candida albicans. J. Microbiol. Biotechnol.. 2018;28(2):181-189.
- [CrossRef] [Google Scholar]
- Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid. Redox Signal.. 2007;9(2):233-244.
- [CrossRef] [Google Scholar]
- Molecular process of glucose uptake and glycogen storage due to hamamelitannin via insulin signalling cascade in glucose metabolism. Mol. Biol. Rep.. 2020;47(9):6727-6740.
- [CrossRef] [Google Scholar]
- A novel antimicrobial peptide derived from fish goose type lysozyme disrupts the membrane of Salmonella enterica. Mol. Immunol.. 2015;68(2):421-433.
- [CrossRef] [Google Scholar]
- Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci.. 2020;21(19):7152.
- [CrossRef] [Google Scholar]
- Protective effects of vitamin E against 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) induced toxicity in zebrafish embryos. Ecotoxicol. Environ. Saf.. 2009;72(3):714-719.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of methyl-3-O-methyl gallate against sodium fluoride-induced oxidative stress in the brain of rats. Cell. Mol. Neurobiol.. 2013;33(2):261-267.
- [CrossRef] [Google Scholar]
- Zebrafish as a systems toxicology model for developmental neurotoxicity testing. Congenit. Anom. (Kyoto). 2015;55(1):1-16.
- [CrossRef] [Google Scholar]
- Reactive oxygen species and ischemic cerebrovascular disease. Neurochem. Int.. 2012;60(2):208-212.
- [CrossRef] [Google Scholar]
- Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem.. 2005;16(9):562-569.
- [CrossRef] [Google Scholar]
- Anticancer activity of biologically synthesized silver and gold nanoparticles on mouse myoblast cancer cells and their toxicity against embryonic zebrafish. Mater. Sci. Eng. C. 2017;73:674-683.
- [CrossRef] [Google Scholar]
- Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following Chlorpyrifos exposure. Comp. Biochem. Physiol. Part - C Toxicol. Pharmacol.. 2015;172–173:19-25.
- [CrossRef] [Google Scholar]
- Gene expression and in silico analysis of snakehead murrel interleukin 8 and antimicrobial activity of C-terminal derived peptide WS12. Vet. Immunol. Immunopathol.. 2017;190:1-9.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of Hibiscus Sabdariffa against hydrogen peroxide-induced toxicity. J. Herb. Med.. 2019;17-18:100253.
- [CrossRef] [Google Scholar]
- Antioxidant properties and neuroprotective effects of isocampneoside II on hydrogen peroxide-induced oxidative injury in PC12 cells. Food Chem. Toxicol.. 2013;59:145-152.
- [CrossRef] [Google Scholar]
- NV14 from serine O -acetyltransferase of cyanobacteria influences the antioxidant enzymes in vitro cells, gene expression against H 2 O 2 and other responses in vivo zebrafish larval model. Cell Biol. Int.. 2021;45(11):2331-2346.
- [CrossRef] [Google Scholar]
- Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J. Funct. Foods. 2014;6:176-185.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.101861.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







