Translate this page into:
Microwave-assisted regioselective synthesis of substituted-9-bromo-9,10-dihydro-9,10-ethanoanthracenes via Diels-Alder cycloaddition
⁎Corresponding authors. mujeeb.AA@just.ac (Mujeeb A. Sultan), ambarakat@ksu.edu.sa (Assem Barakat)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The substituted-9-bromo-9,10-dihydro-9,10-ethanoanthracenes ortho 8a-12a and meta 8b-13b have been synthesized via Diels-Alder reaction under microwave conditions. The cycloadduct isomers ortho 8a-11a and meta 8b-11b, with priority to ortho 8a-11a, were obtained from the reaction of 9-bromoanthracene 1 with acrylonitrile 2, 2-chloroacrylonitrile 3, methacryloyl chloride 4 and acrylic acid 5, while ortho 12a and meta 12b, with priority to meta 12b, was obtained from the reaction of 9-bromoanthracene 1 with 1-cynao vinyl acetate 6. Interestingly, the only isomer meta 13b was obtained from the reaction of 9-bromoanthracene 1 with phenyl vinyl sulfone 7. The results proved that the steric or/and electronic nature of the dienophile substituent is/are playing significant roles in the regioselectivity and isomers ratio.
Keywords
Diels-Alder cycloaddition
Regioselectivity
Microwave synthesis
Ethanoanthracene derivatives
- OLED
organic light emitting diodes
- NIR
near-infrared
- DA
Diels-Alder
- NMR
nuclear magnetic resonance
- EWG
Electron Withdrawing Group
- EDG
Electron Donating Group
- Cl
chlorine atom
- CN
nitrile group
- OAc
acetoxy group
- COOH
carboxyl group
Abbreviations
1 Introduction
Anthracene derivatives have been employed in versatile applications; for example in the organic electronics particularly organic light emitting diodes (OLED) as light-emitting materials (Varol et al., 2016; Peng et al., 2019; Chen et al., 2020) and in the chemotherapeutic field as antidepressants (Wilhelm and Schmidt, 1969; Wadler et al., 1986; Huang et al., 2002), anti-proliferative agents (Cloonan et al., 2010; Cloonan and Williams, 2011; McNamara et al., 2014), antimalarial agents (Millet et al., 2004; Henry et al., 2008) and as glucocorticoid receptor modulators (Yang et al., 2009). 9-Bromoanthracene, which serve as a precursor for the synthesis of anthracene carboxyimides, has been considered as a promising candidate for bioimaging applications (Xu et al., 2017). These compounds are soluble and stable near-infrared (NIR) dyes (Yao et al., 2009). Many compounds have been reported and synthesized starting from 9-bromoanthracene, for example a series of bidentate bis-(pyridine) anthracene isomers (2,3-PyAn, 3,3-PyAn, 2,2-PyAn) were designed as nanocrystal photosensitizer (Li et al., 2017). Additionally, 9-bromoanthracene was incorporated into deoxyadenosine to prepare fluorescent nucleotide for biological applications (Le et al., 2017) indeed, it was hybridized with fluorescein to produce chemodosimeters for detection of singlet oxygen within the live cell (Chercheja et al., 2019).
Diels-Alder (DA) cycloaddition is one of the most efficient approach for the C–C bond formation. The catalysis is one the most important variables that affect the regioselectivity of DA cycloaddition, in particular, gold catalyst creates new opportunities for the regioselective cycloaddition (Praveen, 2019). Microwave-assisted organic synthesis has been gained significant attention better than conventional method because it makes reactions faster, safer, greener, more selective and more economic (Lidström et al., 2001; Wathey et al., 2002; Praveen et al., 2013; Gawande et al., 2014). The effect of substituents at the 9- or 10-position of the anthracene on the regioselectivity and photophysical properties have been investigated (Yang and Doweyko, 2005; Kim et al., 2008; Adel and Farooqui, 2013; Mallesham et al., 2014; Zhang et al., 2017). Based on the above findings, the substituents effect of the dienophiles on the DA regioselectivity has been reported through investigation the microwave-assisted reactions of 9-bromoanthracene 1 with six dienophiles individually; acrylonitrile 2, 2-chloroacrylonitrile 3, methacryloyl chloride 4, acrylic acid 5, 1-cynao vinyl acetate 6 and phenyl vinyl sulfone 7.
2 Results and discussion
As a part of our ongoing interest in DA cycloaddition, in particular reaction of anthracene derivatives with many dienophiles (Sultan et al., 2016, 2017a, 2017b), our group has recently reported thei DA cycloaddition and tested the cycloadducts in in vitro as antidepressants (Karama et al., 2016a) and anticancer agents (Karama et al., 2016b). More recently, we reported the regioselective DA reactions between 10-allyl-1,8-dichloroanthracene and three substituted dienophiles (Sultan and Karama, 2016). Unfortunately we failed to obtain the cycloadducts in dichloromethane or toluene at room temperature. In fact, from our experiences, we can say; chlorinated anthracenes are not reactive enough for DA reactions at room temperature and even under conventional heating. In this line, the DA reaction of phenyl vinyl with sulfoxide 1,8-dichloroanthracene afford cycloadduct in 30% yield after 8 days (del Rosario Benites et al., 1999), in comparison, the reaction of phenyl vinyl sulfoxide with anthracene afford 83% of the cycloadduct (Paquette et al., 1978). So in this work, 9-bromoanthracene 1 was subjected to react with six dienophiles 2–7 separately under microwave conditions affording ortho/ meta cycloadducts with variable regioselectivity (Scheme 1, Table 1). EWG: Electron Withdrawing Group; EDG: Electron Donating Group.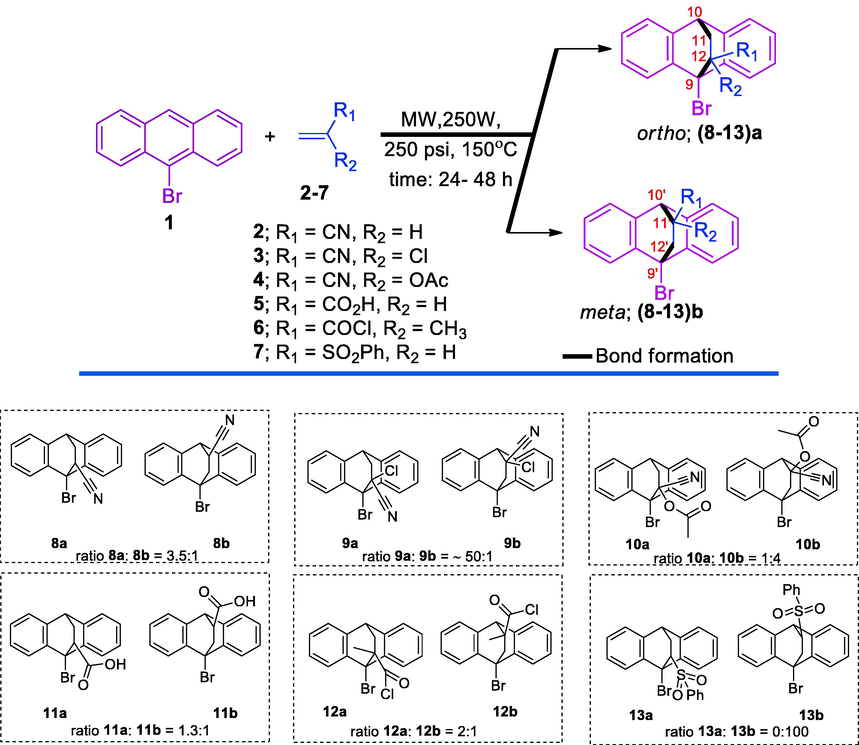
The DA cycloaddition of 9-bromoanthracene 1 with six dienophiles 2–7.
Entry
Dienophile
Substituent Nature
Cycloadducts Ratio
ortho (8–13)a: meta (8–13)b
1
, 2
EWG
8a: 8b = 3.5:1
2
, 3
EWG, EWG
9a: 9b = ∼50:1
3
, 4
EDG, EWG
10a: 10b = 1:4
4
, 5
EWG
11a: 11b = 1.3:1
5
, 6
EWG, EDG
12a: 12b = 2:1
6
, 7
EWG
13a: 13b = 0: 100
The DA reaction of 9-bromoanthracene 1 with acrylonitrile 2 was successfully carried out in xylene affording two isomers ortho 8a: meta 8b in a ratio 3.5:1 respectively as deducted from nuclear magnetic resonance spectrum (NMR) of the cycloadduct crude. It looks that nitrile group as a strong EWG on the dienophile 2 exerted an electronic effect and may a slightly steric effect also leading to the formation meta 8b as a minor isomer. Meek et al, reported the DA cycloaddition of various ethylene equivalents with 9-Nitroanthracene and 9-Anthramide affording either or both isomer, the authors indicated that the negativity of the 9-substituent on the anthracene as a diene may play a significant role in determining the isomer ratio, but the negativity of the substituent on the dienophile do not (Meek et al., 1960). In a communication study, bicyclic amino acids were obtained in highly regioselectivity from DA cycloaddition of 2-acetamidoacrylate with 9-substituted anthracenes (Yang and Doweyko, 2005). This study observed that the ortho isomer was not formed at high temperatures, on the other hand, the DA cycloaddition of 2-acetamidoacrylate with 9-chloroanthracene or 9-methylanthracene were not sufficiently regioselective (Yang and Doweyko, 2005). The configuration assignment and regioselectivity of Diels-Alder cycloadducts could be clear in help of understanding the nature of the substituent (Verma and Singh, 1976, 1977). The NMR spectra of the isomer ortho 8a showed the bridge-head proton H-10 at chemical shift (δ) 4.37 ppm as triplet with coupling constants (J) 2 Hz while the corresponding bridge-head proton H-10′ of the isomer meta 8b appeared at chemical shift (δ) 4.55 ppm as doublet with coupling constants (J)2 Hz. The slight downfield shifting of the H-10′ in the isomer meta 8b, in compare to isomer ortho 8a, could be attributed to the deshielding effect of the nitrile group (CN), since its position on C-11′ is close to /H-10 of the isomer meta 8b.
The reaction of 9-bromoanthracene 1 with 2-chloroacrylonitrile 3 afforded almost isomer ortho 9a with insignificant amount of the isomer meta 9b. The presence chlorine atom as EWG on this dienophile 3, compare to dienophile acrylonitrile 2, exhibited an electronic effect but not steric effect, since the ratio of the isomer ortho 9a is increased. Sultan and Karama reported that chlorine atom on 2-chloroacrylonitrile had no steric effect in the DA cycloaddition of 2-chloroacrylonitrile and 10-allyl-1,8-dichloroanthracene.
The bridge-head proton H-10 of isomer ortho 9a appeared at chemical shift (δ) 4.36 ppm as a triplet with coupling constant (J) 2.4 Hz. However the amount of the isomer meta 9b was insignificant, the assignment of this isomer was scientifically valuable; the bridge-head proton H-10′ of the isomer meta 9b appeared at chemical shift (δ) 4.72 ppm as a singlet signal. The shifting of H-10′ of the isomer meta 9b, in relative to H-10 of the isomer ortho 9a, could be attributed to the deshielding effect of the chlorine (Cl) atom and nitrile group (CN), since their positions on C-11′ are very close to H-10′.
In contrast, the isomer meta 10b was the major resulting from the reaction of 9-bromoanthracene 1 with 1-cynao vinyl acetate 4. NMR assignment was recruited to understand the regioselectivity and then identify the isomers and their ratio. It’s clear that acetate group on the dienophile exerted a significant steric or/and electronic effects leading to the formation isomer meta 10b as a major in a ratio 1:4 for ortho 10a: meta 10b respectively as deducted from NMR spectrum of the cycloadduct crude. For these cycloadducts; the bridge-head proton H-10′ of isomer meta 10b appeared at chemical shift (δ) 5.07 ppm as a singlet while the bridge-head proton H-10 of the isomer ortho 10a appeared at chemical shift (δ) 4.37 ppm as triplet with coupling constants (J) 2.8 Hz. The downfield shifting of the H-10′ in the isomer meta 10b, in compare to isomer ortho 10a, could be attributed to the deshielding effect of the nitrile (CN) and acetoxy (OAc) groups, since their positions on C-/11 are close to H-10′ of the isomer meta 10b.
The DA cycloaddition of 9-bromoanthracene 1 with acrylic acid 5 was carried out under the same condition to its reaction with acrylonitrile 2 and the ratio isomers ortho 11a: meta 11b was in the same direction where isomer ortho 11a is the major. But the amount of isomer ortho 11a is less and vice versa regarding amount of isomer meta 11b. This is could be referred to the steric effect exerted by carboxyl group (COOH) that is more than nitrile group (CN) do. The isomers ortho 11a: meta 11b ratio is 1.3:1 while it was 3.5:1 in case of acrylonitrile. The NMR spectra of the isomer ortho 11a exhibited the bridge-head proton H-10 at chemical shift (δ) 4.39 ppm as broad singlet and after enlarge the spectra it appears as triplet with coupling constants (J) 2.4 Hz while the bridge-head proton H-10′ of the isomer meta 11b appeared at chemical shift (δ) 4.74 ppm as doublet with coupling constants (J) 2 Hz. The slight downfield shifting of the H-10′ in the isomer meta 11b, in compare to isomer ortho 11a, is may due to the deshielding effect of the carboxyl group (COOH), since its position on C-11′ is close to H-10′ of the isomer meta 11b.
The effect of methyl group as an electron donating group (EDG) beside to an electron withdrawing group (EWG) on the dienophile, as in methacryloyl chloride 6, is studied. The DA cycloaddition of 9-bromoanthracene 1 with methacryloyl chloride 6 led to the formation isomers ortho 12a and meta 12b in a ratio 2:1 respectively. The 1H NMR spectrum was employed to distinguish between the isomers ortho 12a and meta 12b; the bridge-head proton H-10 of the isomer ortho 12a appeared as a triplet at chemical shift (δ) 4.29 ppm with coupling constants (J) 2.4 Hz whereas the corresponding bridge-head proton H-10′ of isomer meta 12b appeared as singlet signal at chemical shift (δ) 4.42 ppm.
Herein, we also report the DA cycloaddition, where the only isomer meta 13b was formed. The DA cycloaddition of 9-bromoanthracene 1 with phenyl vinyl sulfone 7 gave only isomer meta 13b. However, the phenyl sulfone group is EWG, the big significant volume of the phenyl sulfone group exhibited a significant steric effect leading to no formation of the isomer ortho 13a but only isomer meta 13b. The assignment of the isomer meta 13b by 1H NMR analysis was clear. The bridge-head proton H-10′ of the isomer meta 13b exhibited as a doublet signal and appeared at chemical shift (δ) 4.91 ppm with coupling constants (J) 1.6 Hz.
3 Conclusion
In conclusion, microwave-assisted DA cycloadditions of 9-Bromoanthracene 1 with six different-substituted dienophiles 2–7 have been reported. The ortho 8a-13a and meta 8b-13b cycloadducts–were obtained in different ratios. The dienophiles; acrylonitrile 2, 2-chloroacrylonitrile 3, methacryloyl chloride 4 and acrylic acid 5 were independently reacted with 9-bromoanthracene 1 affording ortho 8a-11a as major isomers while dienophile 1-cynao vinyl acetate 6 was reacted with 9-bromoanthracene 1 affording meta 12b as a major isomer. In contrast, the dienophile phenyl vinyl sulfone 7 was reacted with 9-bromoanthracene 1 affording only isomer meta 13b. It is noteworthy to mention that the steric or/and electronic nature of the dienophile substituent is/are playing significant roles in the regioselectivity and isomers ratio.
Funding
King Saud University-Researchers Supporting Project Number (RSP-2020/64).
Acknowledgments
The authors would like to extend their sincere appreciation to Researchers Supporting Project Number (RSP-2020/64), King Saud University, and Riyadh, Saudi Arabia. M.A.S, M.S.A.G and M.A and thank Aljanad University of Science and Technology, Taiz, Republic of Yemen for facilities access.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- (4+ 2) Cycloaddition Reactions of 9-Substituted Anthracene Compounds. Orient. J. Chem.. 2013;29(3):1033-1039.
- [Google Scholar]
- Simple structured color tunable white organic light-emitting diodes utilizing an ambipolar anthracene derivative with low-lying LUMO. Org. Electron.. 2020;76:105454
- [Google Scholar]
- Hybrids of a 9-anthracenyl moiety and fluorescein as chemodosimeters for the detection of singlet oxygen in live cells. Org. Biomol. Chem.. 2019;17(46):9883-9891.
- [Google Scholar]
- The antidepressants maprotiline and fluoxetine have potent selective antiproliferative effects against Burkitt lymphoma independently of the norepinephrine and serotonin transporters. Leukemia & lymphoma. 2010;51(3):523-539.
- [Google Scholar]
- The antidepressants maprotiline and fluoxetine induce Type II autophagic cell death in drug-resistant Burkitt's lymphoma. Int. J. Cancer. 2011;128(7):1712-1723.
- [Google Scholar]
- Intermolecular hydrogenation of a C C bond during π-cyclopentadienyliron complexation of 1, 8-dichloro-9, 10-dihydro-9, 10-ethenoanthracene. J. Organomet. Chem.. 1999;577(1):24-30.
- [Google Scholar]
- Microwave-assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res.. 2014;47(4):1338-1348.
- [Google Scholar]
- Dihydroethanoanthracene derivatives reverse in vitro quinoline resistance in Plasmodium falciparum malaria. Med. Chem.. 2008;4(5):426-437.
- [Google Scholar]
- Synthesis and pharmaceuticals of novel 9-substituted-1, 5-dichloroanthracene analogs. Google Patents. 2002
- [Google Scholar]
- Synthesis of chlorinated tetracyclic compounds and testing for their potential antidepressant effect in mice. Molecules. 2016;21(1):61.
- [Google Scholar]
- Exceedingly efficient deep-blue electroluminescence from new anthracenes obtained using rational molecular design. J. Mater. Chem.. 2008;18(28):3376-3384.
- [Google Scholar]
- Diverse size approach to incorporate and extend highly fluorescent unnatural nucleotides into DNA. Bioorg. Med. Chem.. 2017;25(14):3591-3596.
- [Google Scholar]
- Complementary Lock-and-Key Ligand Binding of a Triplet Transmitter to a Nanocrystal Photosensitizer. Angew. Chem. Int. Ed.. 2017;56(20):5598-5602.
- [Google Scholar]
- Design and synthesis of novel anthracene derivatives as n-type emitters for electroluminescent devices: a combined experimental and DFT study. Photochem. Photobiol. Sci.. 2014;13(2):342-357.
- [Google Scholar]
- Synthesis and antiproliferative action of a novel series of maprotiline analogues. Eur. J. Med. Chem.. 2014;71:333-353.
- [Google Scholar]
- Diels-Alder Reactions of 9-Substituted Anthracens. 1 IV. 9-Nitroanthracene and 9-Anthramide. J. Am. Chem. Soc.. 1960;82(10):2566-2569.
- [Google Scholar]
- Dihydroethanoanthracene derivatives as in vitro malarial chloroquine resistance reversal agents. Antimicrob. Agents Chemother.. 2004;48(7):2753-2756.
- [Google Scholar]
- Use of phenyl vinyl sulfoxide as an acetylene equivalent in Diels-Alder cycloadditions. J. Am. Chem. Soc.. 1978;100(5):1597-1599.
- [Google Scholar]
- Efficient soluble deep blue electroluminescent dianthracenylphenylene emitters with CIE y (y≤ 0.08) based on triplet-triplet annihilation. Sci. Bull.. 2019;64(11):774-781.
- [Google Scholar]
- Dexterity of gold catalysis in controlling the regioselectivity of cycloaddition reactions. Catalysis Rev.. 2019;61(3):406-446.
- [Google Scholar]
- Super acid catalysed sequential hydrolysis/cycloisomerization of o-(acetylenic) benzamides under microwave condition: Synthesis, antinociceptive and antiinflammatory activity of substituted isocoumarins. J. Chem. Sci.. 2013;125(1):71-83.
- [Google Scholar]
- Synthesis, theoretical studies and molecular docking of a novel chlorinated tetracyclic:(Z/E)-3-(1, 8-dichloro-9, 10-dihydro-9, 10-ethanoanthracen-11-yl) acrylaldehyde. J. Mol. Struct.. 2017;1150:358-365.
- [Google Scholar]
- Sultan, M.A., U. Karama, 2016. Substituent effects on regioselectivity of the Diels-Alder reactions: Reactions of 10-allyl-1, 8-dichloroanthracene with 2-chloroacrylonitrile, 1-cyanovinyl acetate and phenyl vinyl sulfone, J. Chem. 2016.
- Theoretical Study on Regioselectivity of the Diels-Alder Reaction between 1, 8-Dichloroanthracene and Acrolein. Molecules. 2016;21(10):1277.
- [Google Scholar]
- Synthesis, Characterization and DFT Calculations of 4, 5, 12-and 1, 8, 12-trichloro-9, 10-dihydro-9, 10-ethanoanthracene-12-carbonitriles. Crystals. 2017;7(9):259.
- [Google Scholar]
- Optical performance of efficient blue/near UV nitropyridine-conjugated anthracene (NAMA) based light emitting diode. Org. Electron.. 2016;31:25-30.
- [Google Scholar]
- Assignment of configurations to adducts of 2-substituted anthracene with maleic anhydride by NMR spectroscopy. Aust. J. Chem.. 1976;29(6):1215-1222.
- [Google Scholar]
- Structural elucidation with nuclear magnetic resonance spectroscopy. Diels-Alder adducts of 1-aminoanthracene and maleic anhydride: restricted rotation about the aryl C (1)-N bond and intrinsic asymmetry about the imide (Nsp2-Csp3) system. J. Organic Chem.. 1977;42(23):3736-3740.
- [Google Scholar]
- Phase I and II agents in cancer therapy: I. Anthracyclines and related compounds. J. Clinical Pharmacol.. 1986;26(7):491-509.
- [Google Scholar]
- The impact of microwave-assisted organic chemistry on drug discovery. Drug Discovery Today. 2002;7(6):373-380.
- [Google Scholar]
- Synthese und Eigenschaften von 1-Aminoalkyl-dibenzo [b, e] bicyclo [2.2. 2] octadienen. Helv. Chim. Acta. 1969;52(6):1385-1395.
- [Google Scholar]
- A family of multi-color anthracene carboxyimides: synthesis, spectroscopic properties, solvatochromic fluorescence and bio-imaging application. Dyes Pigm.. 2017;139:166-173.
- [Google Scholar]
- Highly regioselective Diels-Alder reactions of 9-substituted anthracenes and 2-acetamidoacrylate: synthesis of conformationally constrained α-amino acids. Tetrahedron Lett.. 2005;46(16):2857-2860.
- [Google Scholar]
- Discovery of novel dihydro-9, 10-ethano-anthracene carboxamides as glucocorticoid receptor modulators. Bioorg. Med. Chem. Lett.. 2009;19(8):2139-2143.
- [Google Scholar]
- Bisanthracene bis (dicarboxylic imide) s as soluble and stable NIR dyes. Chem. Eur. J.. 2009;15(37):9299-9302.
- [Google Scholar]
- Anthracene-based derivatives: Synthesis, photophysical properties and electrochemical properties. Chem. Res. Chin. Univ.. 2017;33(4):603-610.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.10.002.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







