Translate this page into:
Mechanistic investigation of photodynamic therapy using Visudyne in human KB carcinoma cells
⁎Corresponding authors.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Photodynamic therapy is a therapy that utilizes a specialized drug, a photosensitizer, which is not toxic by itself, but upon activation with light, is activated and leads to the production of singlet oxygen species. This production of singlet oxygen species starts a cascade that leads to cell death. Photodynamic therapy is a potential treatment for cancer patients, and it might be helpful to promote human health by providing alternative treatment to cancer. In-vitro study with Visudyne (VD) in KB carcinoma cells unveiled the mechanism of cell injury to cell death upon photodynamic therapy (PDT). In this study, we investigated real time monitoring of the production of singlet oxygen (SO), reactive oxygen species (ROS), mitochondria integrity and chromatin integrity in VD treated live cells by irradiating with a mercury lamp light source and 665–675 nm and 480–560 nm emission filter. Upon PDT we also observed cytoskeleton remodeling and cytochrome –C release using immunofluorescence techniques. We investigate the cytotoxicity of PDT and performed quantitative analysis for SO, ROS and mitochondrial potential change using colocalization analysis between VD and mitochondria.
Keywords
Visudyne
Photosensitizer
Chemotherapy
Photodynamic therapy
KB Carcinoma Cells
Cancer Treatment
1 Introduction
Photodynamic therapy (PDT) is a type of cytotoxic therapy that uses a specialized drug, the photosensitizer, and light (Brown et al., 2004). The photosensitizer is introduced to the patient, but is not toxic by itself. The photosensitizer becomes activated by light and reacts with oxygen to produce singlet oxygen species, which leads to cell death. Currently, PDT has been approved for use in several diseases including actinic keratosis (Patel et al.,2014), skin cancer (Zeitouni et al., 2013), esophageal cancer (Fayter et al.,2010) and non-small cell lung cancer (Fayter et al., 2010). One major limiting factor for the approval of PDT use in more diseases is its toxicity (Huang, 2005).5 Once the photosensitizer enters the patient’s blood stream, it will not only localize to abnormal tissue but to normal tissue as well. Since the drug is activated with light, tissue toxicity to light exposed areas such as skin and eyes is of major concern. One way to prevent these toxicities is to have the patient completely separate from light exposed areas until the photosensitizer has been cleared from their body. But, this is impractical and is an area of concern in our research. Decreasing the amount of dose given to patient may reduce the amount of photosensitizer localized to normal tissue and reduce toxicities. A way to accomplish this is to improve the potency and specificity of the drug at diseased tissues. Therefore, the development of modified photosensitizer that can target specific cells in tissue and organelles once it enters the cell are of paramount interest. During drug development process to find the most optimum drug, comparative parameters must be defined in order to evaluate different efficacy of modified photosensitizers at the cellular level. These parameters should be those which are part of the mechanism of action of the drug, because these are relevant to the toxicity of the drug to the cell. As stated before, it is well known singlet oxygen production is produced (Dolmans et al., 2003). by the reaction of the photosensitizer, light and oxygen. However, the process in between singlet oxygen production and cell death in solid tissue is not well understood. In this paper, Visudyne (verteporfin,) benzoporphyrin derivative (BPD) (Fig. 1), a photosensitizer which has been FDA approved for treatment of wet age-related macular degeneration, (Arnold et al., 2002; Bressler et al., 2005) was used to treat human KB head and neck cancer cells to study the mechanism of action of this photosensitizer. The parameters obtained from our study of VD mechanism of action will greatly facilitate the discovery and optimization of novel therapeutics. Furthermore, elaborate knowledge of VD (Pogue et al., 2001) mechanism of action may also be helpful in targeted PDT (Mahalingam et al., 2018, Celli et. al., 2011) in which the photosensitizers may show improved potency by targeting to certain cellular organelles.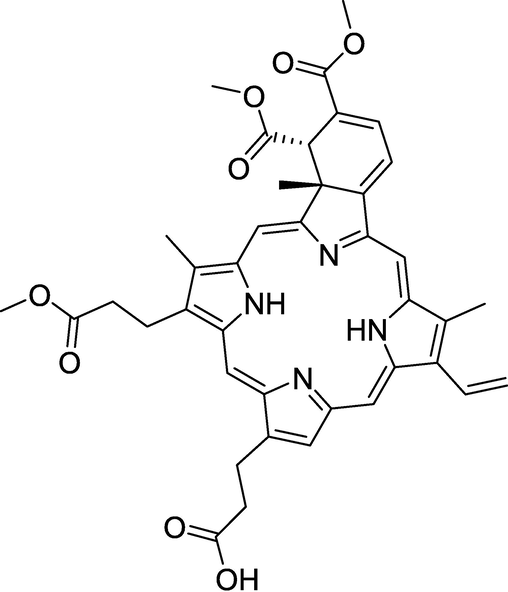
Structure of Visudyne (verteporfin,) benzoporphyrin derivative.
2 Materials and methods
2.1 Cell culture, plating
KB cells were cultured using RPMI media (GibcoTM, USA) with 1% Penicillin/streptomycin (GIbcoTM, USA) and 5 % FBS (Atlanta biologicals, USA). After reaching about 90% confluence of the cells the dividing took were used to plate the cells in 500 µL media to reach around 90% confluence for 2–3 days. Due to the light sensitive nature of VD as a photosensitizer drug, the incubations were performed for all dye and drug in the dark.
2.2 Cell death experiment
KB cells were incubated with 50 nM of VD diluted in RPMI media for 1 h. Cells were washed 2X with PBS (Gibco, USA) followed by incubation in 5 µM ROS and 5 µg/mL Hoechst diluted in RPMI for 30 min. Cells were again washed 2X with PBS and kept in CO2 independent media and irradiated using 690 nm light. ROS were captured at 488/520 nm followed by Hoechst at 350/461Cells were then incubated with 1 µg/mL propidium iodine for 30 min in RPMI. Cells were washed 2X with PBS and then imaged at 535/617 nm.
2.3 Mitochondria membrane potential experiment
1 h incubation of KB cells with 50 nM VD drug in RPMI media followed by washing twice with PBS. 7.665 µM JC-1 (Life Technologies, USA) in RPMI media was prepared and cells were incubated for 10 min and washed twice with PBS and kept in CO2 independent media. JC-1 was captured before irradiation at 590 nm (J-aggregates) and 529 nm (green monomers). Visudyne drug was activated by irradiation of light at 690 nm. JC-1 imaging was captured immediately for 2-minute intervals at 590 and 529 nm for 12 min.
2.4 Microscopy and imaging analysis
The colocalization tool in Bioimage XD software was used for colocalization analysis (Dunn et al., 2011). Singlet oxygen production (SOP) was measured using Image J to obtain SOSG intensities. Image J was used to map fluorescence intensities. Image J was used to map fluorescence intensities and also for the analysis of Mitochondria membrane potential by calculating the ratio of green to red channel intensities. Experiments of colocalization, Singlet Oxygen, ROS, Cytoskeleton, mitochondria and DNA structure, Apoptosis and Statistics has given in Supplementary section.
3 Results
3.1 Analysis of cell death
To evaluate the VD toxicity with KB cells, we performed viability assays using propidium iodide (PI) by VD treated KB cells irradiation with 690 nm light. We observed two different groups after irradiation, control group which was KB cells without VD treatment (Fig. 2a) and VD treated cells (Fig. 2b). As shown in Fig. 2, after irradiation, PI staining was observed only in VD treated KB cells (Fig. 2b).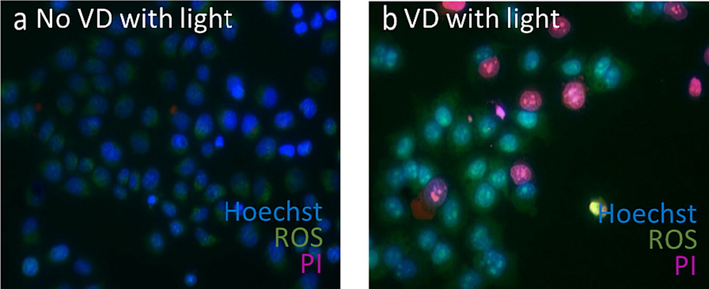
VD-PDT reduces cell viability of human KB carcinoma cells. Merged image of Hoechst (blue), PI (pink) and ROS (green) of: (a) Untreated KB cells shows only signal from Hoechst and ROS, (b) VD treated KB cells shows PI (pink), Hoechst (blue) and ROS (green).
3.2 Cellular localization of Visudyne
The sub cellular fate of VD was determined by fluorescent confocal microscopy. Initial subcellular localization appeared to exhibit a mitochondrial distribution, so KB cells were treated with VD and mitotracker. As shown in Fig. 3, when the mitotracker (green channel) and VD (red channel) were combined, an intense colocalization signal was detected. This result strongly supports the subcellular localization of VD to the mitochondria.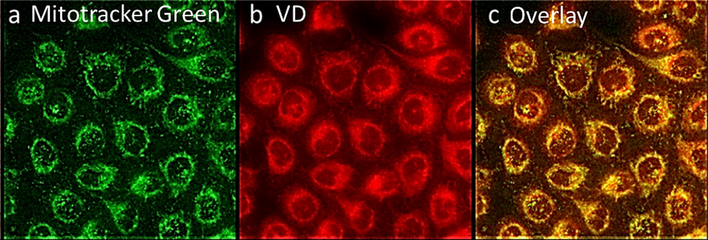
VD subcellular localization in the mitochondria. Human KB cells stained with; (a) Mitotracker green (b) VD drug. (c) colocalization (yellow) between mitochondria (green) and VD (red) in human KB-cells.
3.3 Singlet oxygen species production
The effect of VD on cellular reactive species generation was investigated. A higher intensity of SOSG (Singlet Oxygen Sensor Green) fluorescence, which corresponds to an increase in singlet oxygen species production, was observed in VD treated cells (Fig. 4b) when compared to a VD treated non-irradiated control group (Fig. 4a). Cellular Intensity map of SOSG signal (Fig. 4d) corroborates our finding that VD elicits an increase of cellular singlet oxygen production. To further assign singlet oxygen generation, total intensity of fluorescence was quantified (Waters, 2009) employing image J (n = 25) (Fig. 5). We observed statistically higher singlet oxygen production in VD treated cells after light treatment (Fig. 5c) compared to no VD treated control group after light treatment (Fig. 5b) (P < 0.00001).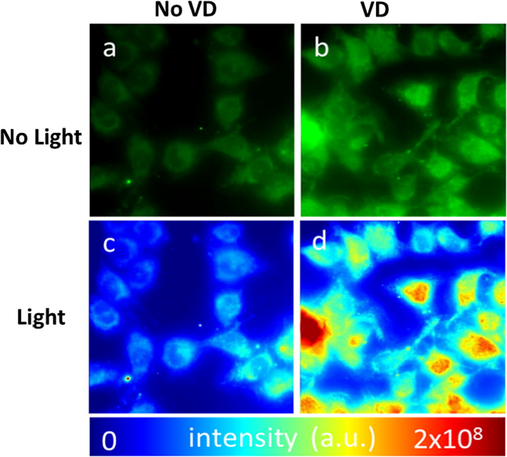
VD-PDT produce singlet oxygen species in VD treated human KB-cells after irradiation: (a) Before light treatment, (b) after light treatment. Intensity map of SOSG (Singlet Oxygen Sensor Green) signal of VD treated cells (c) before light treatment and (d) after light treatment.
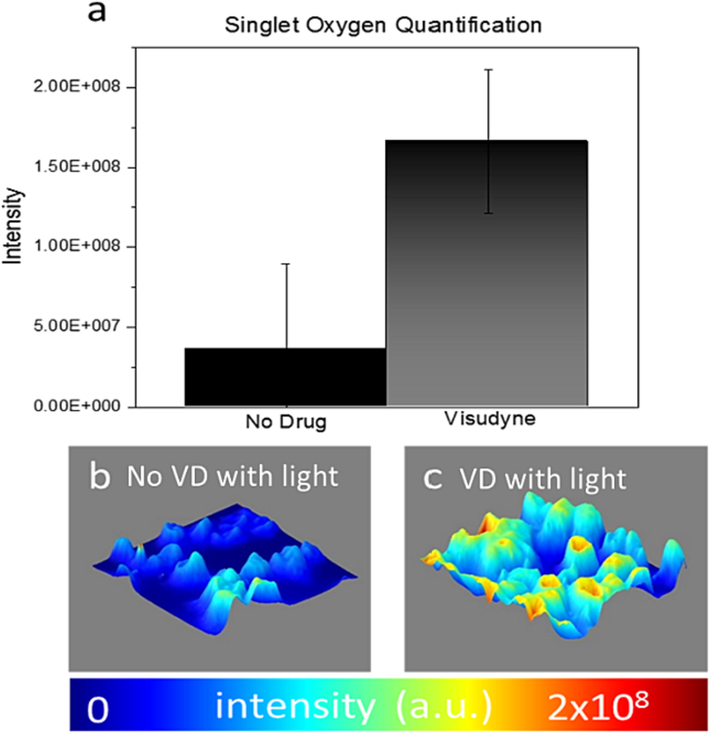
Singlet oxygen quantification in VD-PDT KB cells after light treatment shows statistically significant increase (P < 0.01) compared to control (a) 2.5 D fluorescence intensity map of SOSG sensor in KB cells after light treatment (b) without VD treatment (c) VD treatment.
3.4 Reactive oxygen species production
The next step in determining the mechanism by which PDT works was by studying the production in ROS following VD-PDT. We observed that ROS (Fig. 6) was predominantly present in mitochondria before and after irradiation. However, upon irradiation, significant increase of ROS in VD treated cells was noted (Fig. 6c, d, i) compared to control group (no VD with light) (Fig. 6 g, h, i). Interestingly, intensity map (Fig. 6, b, d, f, h) and 2.5 D plot (Fig. 6 i, j, k, l) show that upon irradiation, ROS signal was primarily observed in the nucleus, demarcated from cytoplasmic localization.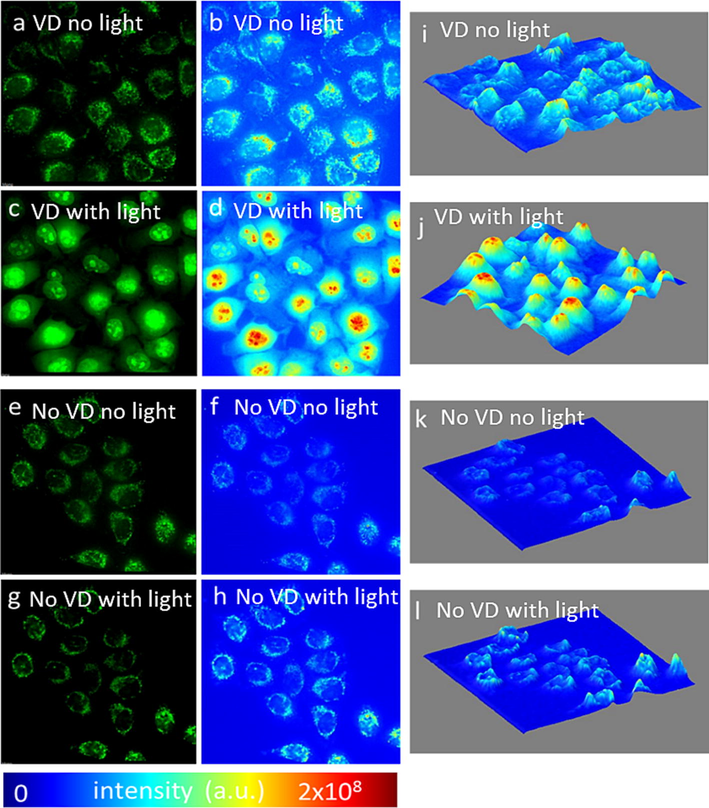
PDT of KB cells treated with VD increases ROS generation, especially in the cell nucleus. ROS mostly generated in mitochondria of cells before light treatment in (a) VD treated, (e) no VD treatment). Increasing ROS signal in cells after light treatment in (c) VD treated cells compare to (g) no VD treatment. Intensity heat map before (b, f) light treatment of (b) VD treated, (f) no VD and after (d, h) light treatment (d) VD, (h) no VD. 2.5D intensity plot of ROS intensity before (I, k) light treatment (i) VD, (k) no VD, and after light treatment (j, l), (j) VD, (l) no VD.
3.5 Changes in mitochondria membrane potential
Based on our results of VD mitochondria cellular fate, we further studied the effect of VD on mitochondria membrane potential (Δψm). We observed red aggregate of JC-1 dye, a sign of normal polarized mitochondria membranes, in both; no VD, no irradiation control group (Fig. 7a) and VD treated group prior to irradiation (Fig. 7b). The same ratio of green monomer (green channel) to red complex (red channel) of JC-1 dye was preserved before (Fig. 7a) and after irradiation (Fig. 7c) in cells not treated with VD, indicating light treatment does not directly affect mitochondrial membrane potential. (Fig. 7a, c). In the presence of VD, green monomer was elevated, and red aggregate was decreased after irradiation, indicating the decline of mitochondrial membrane potential (Perry et al., 2011) (Fig. 7d). Membrane potential was investigated using ratiometric analysis (Salido et al., 2007) of green monomer to red aggregate. We observed four-fold decrease of Δψm after 12 min from irradiation in VD treated cells compared to control (Fig. 7e).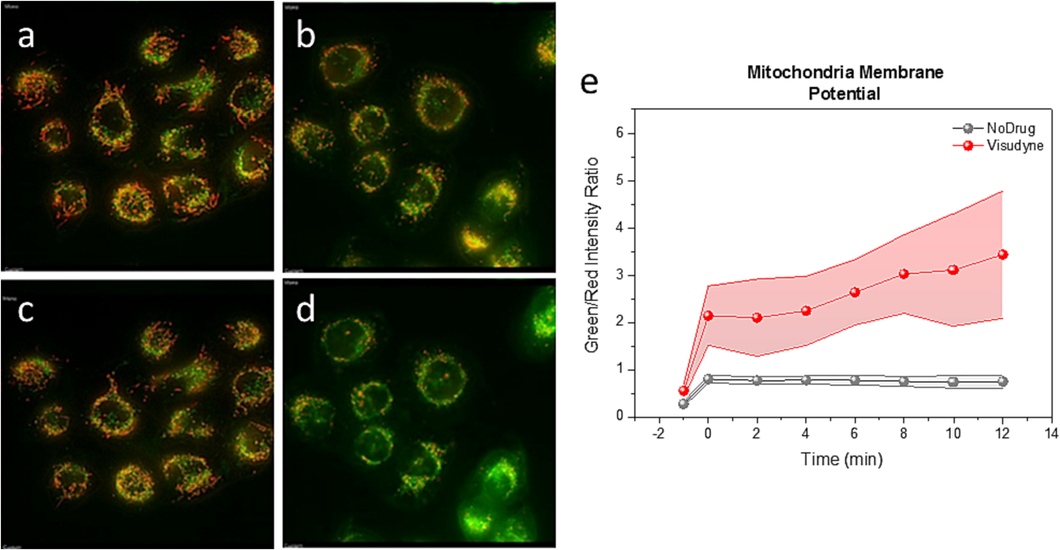
VD-PDT reduce mitochondria membrane potential in human KB-cells. JC-1 signal in control cells (a, c), (a) before (c) after light treatment and VD treated cells (b, d), (b) before and (d) after light treatment. (e) Green to red channel ratio during longitudinal study for 12 min upon light treatment on VD treated KB-cells (red) and control (no VD) cells (grey). The difference in each point is statistically significant (P < 0.001).
3.6 Structural changes in mitochondria, actin, and DNA
We investigated the effect of VD-PDT on morphological changes of actin cytoskeleton, mitochondria and cell nucleus. We observed morphological alteration of mitochondria in VD treated cells after light treatment (Fig. 8b) compared to VD treated cells before light treatment. (Fig. 8a). The VD-treated cells show a more diffuse staining and mitochondria swelling, which is a sign of cell damage. As shown in Fig. 8b, mitochondrial aggregates and mitochondria swelling are identified after light irradiation in VD treated cells (Fig. 8b). Disintegrated network of actin cytoskeleton (Fig. 8d) was observed after light irradiation in VD treated cells compared to (specifics) control group (Fig. 8c) indicating actin depolymerization after VD-PDT treatment. We also observed structural changes of the nucleus in VD treated cells (Fig. 8f) compared to control cells (Fig. 8g). In VD treated cells (Fig. 8f), the nucleus shows pyknosis which is also a sign of cell damage.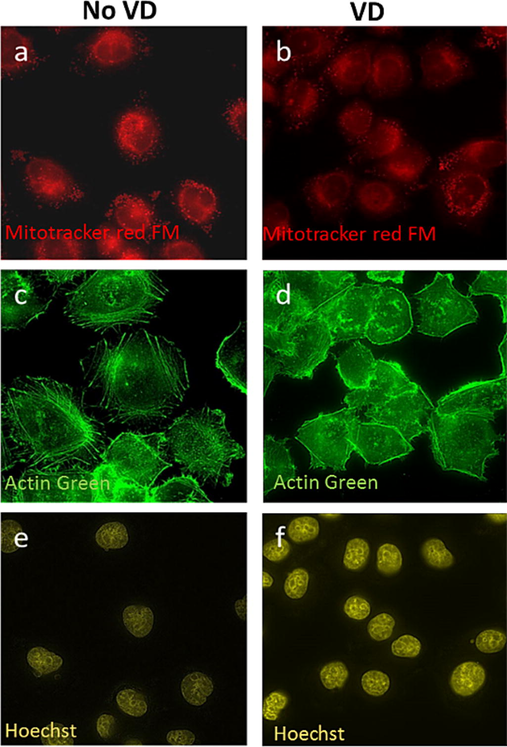
VD-PDT treatment in KB cells leads to changes in intracellular structures after irradiation. Mitochondria morphology (a, b) and Actin cytoskeleton (c, d) show more diffused structure after light treatment in VD treated cells: (b) mitochondria, and (d) actin cytoskeleton compare to (a) mitochondria and (c) actin cytoskeleton in no VD treated cells after light treatment. DNA condensation is observed in VD treated cells after light treatment (f) compare to no VD treated cells after light treatment.
3.7 Apoptosis through Cytochrome c release from the mitochondria
As shown in Fig. 9, we identified mitochondria release Cytochrome C release in VD treated cells (Fig. 9b) compare to mitochondrial Cytochrome C in control cells (Fig. 9a). Moreover, we also observed cell blebbing, the hallmark event of cell apoptosis, in VD treated cells after irradiation (Fig. 9b inset).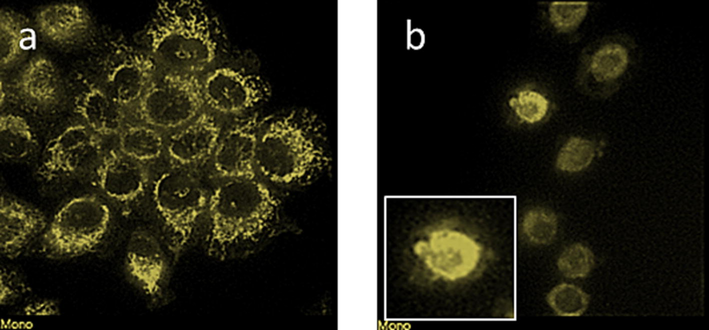
VD-PDT treatment leads to apoptosis in human KB cells. Anti-Cytochrome C in human KB-cells after light treatment in (a) no VD (b) VD treated cells show cell blebbing (inset).
4 Discussion
In this study, we used VD to propose a molecular mechanism for VD PDT in human KB cells. The identification of drug mechanism of action is a pertinent issue not only in the clinical efficacy but also in the development of novel targeted photosensitizers (Allison and Moghissi, 2013). Targeted PDT photosensitizers will allow us to improve its therapeutic efficacy and increase its clinical potency. This will diminish drug toxicity by reducing the dose needed for the same therapeutic effect. Our results demonstrate VD preferentially localizes to the mitochondria, which is in agreement with prior studies reported VD mitochondrial localization in pancreatic cancer cells (Celli et al., 2011), endothelial cells and prostate cancer cells (Fateye et al., 2015). Based on VD mitochondrial sub cellular fate, we hypothesized that mitochondria assume a pertinent role in VD-PDT mediated cell apoptosis (Wei and Li, 2020). Singlet oxygen species production is the hallmark of PDT mechanism (DeRosa and Crutchley, 2002). Light activates the reaction between photosensitizer and molecular oxygen, generating singlet oxygen species (Jarvi et al., 2011). We observe increase of singlet oxygen production after light irradiation. Based on its predominant cytoplasmic localization, we predict the singlet oxygen is mostly generated in mitochondria (Turrens, 2003). Because singlet oxygen species is only part of cellular reactive compounds (Lee et al., 2004). Our result suggests that VD-PDT initiates not only the production of singlet oxygen but also the other reactive species. Furthermore, its nuclear localization suggests the effect of VD-PDT in the nuclear reactive species production (Kotiadis et al., 2014) and or mitochondria nucleus retrograde signaling (Provost et al., 2010). Our finding strongly supports this prediction of initial signs of cell damage and pyknosis (Robbins and Cotran, 2014) upon VD-PDT. Highly reactive singlet oxygen is damaging to membranes (Blokhina et al., 2003) thus we assumed that the enhancement of singlet oxygen production in mitochondria will cause its membrane deterioration. Using JC-1 assay we proved this hypothesis by measuring the mitochondria membrane potential. In the normal polarized state consisting of a negative membrane potential, JC-1 will localize to the mitochondria and form aggregates that emit red fluorescence while a depolarized membrane hinders the entry of JC-1 into mitochondria thus increasing the green fluorescence of the JC1 monomer (Perelman et al., 2012). As expected, we observed the reduction in mitochondrial membrane potential upon irradiation of the VD-treated KB cells at 690 nm. We believe that this cascade mechanism begins with the localization and photoactivation of the VD drug in the mitochondria and the consequent production of oxygen species that the Induce depolarization of the mitochondrial membrane. The membrane potential of the mitochondria is also crucial for ATP production through oxidative phosphorylation (Huttemann et al., 2008). Therefore, we then investigated actin polymerization, because its structure is ATP dependent. Actin, which is essential for structural integrity (Stricker and Falzone, 2010), molecular signaling of cells (Obrdlik et al., 2008) and mitochondria motility (Boldogh and Pon, 2006; Olson and Nordheim, 2010) was found to be depolymerized upon VD-PDT treatment. Considering the roles of action network in sensing and mediating apoptosis (Desouza et al., 2012), we studied signs of cellular apoptosis VD-PDT treatment. We observed the release of mitochondrial cytochrome c to the cytoplasm upon VD-PDT and blebbing of cells, both signs of cell apoptosis (Jiang and Wang, 2004).
5 Conclusion
This study has not only shown the mechanism by which the PDT agent VD works, but deduced parameters by which photosensitizers can be compared in order to determine the most potent and efficacious drug. We conclude VD has a considerable localization to the mitochondria, and it mediates its damage through this organelle. Our mechanistic investigation suggests that the cell death in KB cells induced by VD-PDT is as follows: VD localizes in mitochondria (80% colocalization) which leads to increasing SO (60%) and ROS (70%) generation. PDT with VD decreases (50%) mitochondrial membrane potential over time and leads to mitochondrial deformation. These phenomena suggest the disruption in mitochondrial retrograde signaling which is marked with nuclear changes in VD treated cells and also the depolymerization of the actin cytoskeleton ultimately leading to cell death. The mechanism of cell death via PDT in VD treated KB cells begins with VD localization to the mitochondria, therefore exploration of better mitochondria targeting drugs for use in PDT therapy is recommended. Therefore, designing drugs that increase localization to the mitochondria is an area of future research. We suggest parameters; Singlet oxygen species, reactive oxygen species, mitochondrial membrane potential change, morphological studies and Cytochrome C release to be considered for in vitro studies on modified compounds to determine efficacy and potency improvement and select the drugs, which are to be further investigated in animal models.
Acknowledgement
The project was funded by Researchers Supporting Project Number (RSP2023R143), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 20 VRa, Therapy VP, 20 VRa, Therapy VP. Guidelines for using verteporfin (Visudyne (R)) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina. 2002;22:6-18.
- [Google Scholar]
- Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot.. 2003;91:179-194.
- [Google Scholar]
- Interactions of mitochondria with the actin cytoskeleton. Biochim. Biophy Acta.. 2006;1763:450-462.
- [Google Scholar]
- Guidelines for using verteporfin (visudyne) in photodynamic therapy for choroidal neovascularization due to age related macular degeneration and other causes: update. Retina- J. Retinal Vitreous Diseases. 2005;25:119-134.
- [Google Scholar]
- The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol.. 2004;5:497-508.
- [Google Scholar]
- Verteporfin-based photodynamic therapy overcomes gemcitabine insensitivity in a panel of pancreatic cancer cell lines. Lasers Surg. Med.. 2011;43:565-574.
- [Google Scholar]
- Photosensitized singlet oxygen and its applications. Coord. Chem. Rev.. 2002;233:351-371.
- [Google Scholar]
- The actin cytoskeleton as a sensor and mediator of apoptosis. BioArchitecture. 2012;2:75-87.
- [Google Scholar]
- A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol.. 2011;300:C723-C742.
- [Google Scholar]
- Comparison between endothelial and tumor cells in the response to verteporfin-photodynamic therapy and a PI3K pathway inhibitor. Photodiagn. Photodyn. Ther.. 2015;12:19-26.
- [Google Scholar]
- A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett's oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technol. Assess.. 2010;14:1-288.
- [Google Scholar]
- A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat.. 2005;4:283-293.
- [Google Scholar]
- Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J. Bioenerg. Biomembr.. 2008;40:445-456.
- [Google Scholar]
- The influence of oxygen depletion and photosensitizer triplet-state dynamics during photodynamic therapy on accurate singlet oxygen luminescence monitoring and analysis of treatment dose response. Photochem. Photobiol.. 2011;87:223-234.
- [Google Scholar]
- Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. BBA. 2014;1840:1254-1265.
- [Google Scholar]
- Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf.. 2004;3:21-33.
- [Google Scholar]
- Targeting of a photosensitizer to the mitochondrion enhances the potency of photodynamic therapy. ACS Omega. 2018;6:6066-6074.
- [Google Scholar]
- The histone acetyltransferase PCAF associates with actin and hnRNP U for RNA polymerase II transcription. Mol. Cell Biol.. 2008;28:6342-6357.
- [Google Scholar]
- Linking actin dynamics and gene transcription to drive cellular motile functions. Nat. Rev. Mol. Cell Biol.. 2010;11:353-365.
- [Google Scholar]
- Efficacy of photodynamic therapy vs other interventions in randomized clinical trials for the treatment of actinic keratoses a systematic review and meta-analysis. JAMA Dermatol.. 2014;150:1281-1288.
- [Google Scholar]
- JC-1: alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis.. 2012;22:e430.
- [Google Scholar]
- Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98-115.
- [Google Scholar]
- 2001 analysis of the heterogeneity of pO2 dynamics during photodynamic therapy with verteporfin. Photochem. Photobiol.. 2001;74:700.
- [Google Scholar]
- Nitric oxide and reactive oxygen species in the nucleus revisited. Can. J. Physiol. Pharmaco.. 2010;88:296-304.
- [Google Scholar]
- Robbins and Cotran Pathologic Basis of Disease. Philadelphia: Elsevier Saunders; 2014.
- Loss of mitochondrial membrane potential is inhibited by bombesin in etoposide-induced apoptosis in PC-3 prostate carcinoma cells. Mol. Cancer Ther.. 2007;6:1292-1299.
- [Google Scholar]
- Mitochondrial formation of reactive oxygen species. J. Physiol... 2003;552:335-344.
- [Google Scholar]
- Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol.. 2009;185:1135-1148.
- [Google Scholar]
- Verteporfin inhibits cell proliferation and induces apoptosis in different subtypes of breast cancer cell lines without light activation. BMC Cancer. 2020;20:1042.
- [Google Scholar]
- A retrospective review of pain control by a two-step irradiance schedule during topical ALA-photodynamic therapy of non-melanoma skin cancer. Lasers Surg. Med.. 2013;45:89-94.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102871.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







