Translate this page into:
Mechanisms of attachment and distribution of Nitzschia and Fragilaria at different flow rates
⁎Corresponding author at: School of Materials Science and Engineering, Guangdong Ocean University, No. 1, Luoqin Road, Jiangcheng District, Yangjiang 529500, China. tie-di@hotmail.com (Di Tie) tie@gdou.edu.cn (Di Tie)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Algae in rivers will accumulate on the surface of bridges and other structures in water with the increase of time, which will pollute the water environment and cause serious corrosion on the surface of structures. Extensive literature studies have shown that flow rate as the basic hydrodynamic condition significantly affects the growth and aggregation of algae, yet how different flow rate influences the aggregation and distribution of attached algae is relatively unexplored. In this work, used microscopic observation and high-throughput sequencing combined with a field hanging test to compare the mechanism of attachment and distribution of Nitzschia and Fragilaria at the flow rates of 0.794 m/s and 0.538 m/s, respectively. Mechanistic study shows that the high flow rate reduced the species abundance ca.10 % under the same conditions by increasing the interaction between hydrodynamic and gravity. It found an improvement in species distribution uniformity in different places and periods showed by high flow rate when contrasted with low flow rate. This mechanism offers mechanistic insights into preventing corrosion and fouling of attached algae.
Keywords
Flow rate
Attached algae
Aggregation
Distribution
Mechanism
Corrosion and fouling
1 Introduction
Submerged objects such as ships, offshore structures, and othermarine structures are attached by fouling organisms such as algae, causing corrosion and pollution problems (Karimi et al., 2021; Krishna Mohan et al., 2022a; Krishna Mohan et al., 2022b; Krishna Mohan et al., 2024). The spatial density and distribution of attached algae in flowing waters is an important part of eutrophication and corrosion management (Flynn et al., 2013). Hydrodynamic conditions can directly or indirectly affect algal proliferation and bloom development (Song, 2023). Flow rate is a commonly used parameter to study the hydrodynamic impacts on algal blooms, as it is the most representative of hydrodynamic conditions. Intuitively, flow rate characterizes how rapidly water moves, and this can directly influence the algal distribution and transport (Song et al., 2018; Atazadeh et al., 2021). The importance of flow rate in algae bloom control caused by phytoplankton overgrowth had already been shown in extensive literature research (Huang et al., 2015; Matson et al., 2020; Xin et al., 2020; Zhu et al., 2019; Li et al., 2013; Caroppo et al., 2016), but how flow rate influences the aggregation and distribution of attached algae remains unclear. Here, used microscopic observation and high-throughput sequencing combined with a field hanging test to identify the dominant algae and explore the mechanism of attachment and distribution of them. The results show that a high flow rate disturbs algal aggregation and inhibits algal growth. Moreover, high flow rate decreases the algae deposition and species abundance per unit area, altering the topology of their distribution. These findings will lay a theoretical foundation for the prevention and control strategies of algae eutrophication and corrosion in water bodies.
2 Materials and methods
Experiment was conducted in MaLan River Basin (38°92′N,121°54′E) in the northern Yellow Sea, China. The measured river width is 5 m, the average flow rate of the high flow-rate zone and low flow-rate zone were 0.794 m/s and 0.538 m/s by buoy velocimetry. Eight glass slides (MeVid Life Science, Nantong, Jiangsu, China) with tool clamps and fixing ropes were used for the hanging test. River bottom and structure surface were selected as the hanging place in different velocity zones. According to the formation process of fouling biofilm, the experimental slides were taken out at the test period of 1d and 7d respectively (Menesses et al., 2017). Experimentation Figures are given in Online Resource 1. Algal morphology and distribution were observed using an optical biomicroscope (Sunny Optical, Yuyao, Zhejiang, China) at 400x and 200x magnifications. All images were processed and saved using Image View software. Then, the algal identification by compared with the book of algal atlas (Deng, 2012). The determination method and distribution density identification are based on relevant standards (ASTM International, 2012; British Standard, 2006).
Samples on the surface of the hanging piece were collected with cotton swabs put them into the sampling bottle. Sterile saline was then added, shaking sampling bottle, before taking out cotton swabs. All sampling bottles were named and refrigerated at −80℃. Among the process of high-throughput sequencing, DNA was extracted with the PowerSoil® DNA Isolation Kit (MOBIO, Palo Alto, CA, USA), and the quality of DNA extraction were checked by 1.2 % agarose gel electrophoresis (Online Resource 2). Before PCR amplification, all DNA extracts were standardized to a concentration of 20 ng/μl, using pre-primer sequence (5′-GGACAGAAAGACCCTATGAA-3′) and the post-primer sequence (5′-TCAGCC-TGTTATCCCTAGA-3′) as the target sequences. The amplified products were purified and recovered by magnetic beads, and the sequencing libraries were prepared using TruSeq Nano DNA LT Library Prep Kit (Illumina, San Diego, California, USA) for sequencing. For PCR conditions and complete methods are given in Online Resource 3 and Online Resource 4.
3 Results and discussion
3.1 Dominant species identification and sample sequencing results
Optical biomicroscope observed six algae species with high distribution density (Fig. 1a), they are Nitzschia, Fragilaria, Synedra, Melosira, Navicula, and Liagora, among which Nitzschia and Fragilaria were the most densely distributed and the most intensive communities. Sample DNA detection demonstrated that the experiment was not influenced by the outside sources and DNA extraction was satisfactory (Fig. 1b). PCR amplification results showed that the concentration of products recovered by magnetic bead purification was all higher than the basic concentration (0.5 ng/μl), and the actual DNA fragment length was 410 bp (Fig. 1c), which was consistent with the results of gel electrophoresis (Fig. 1b). All the above results meet the requirements of sequencing library construction. Shannon sparse curve of all samples is shown in Fig. 1d. Curve analysis demonstrated that the sequencing depth of the experiment had covered most of the species in the samples (Heck et al., 1975; Kemp and Aller, 2004; Shannon, 1948; Shannon, 2001; Shannon et al., 2003), with the horizontal coordinates being the number of randomly selected sequences and the highest point of the curve being the sample's Shannon exponent. In summary, the species identification and sequencing quality meet the requirements of subsequent experimental analysis.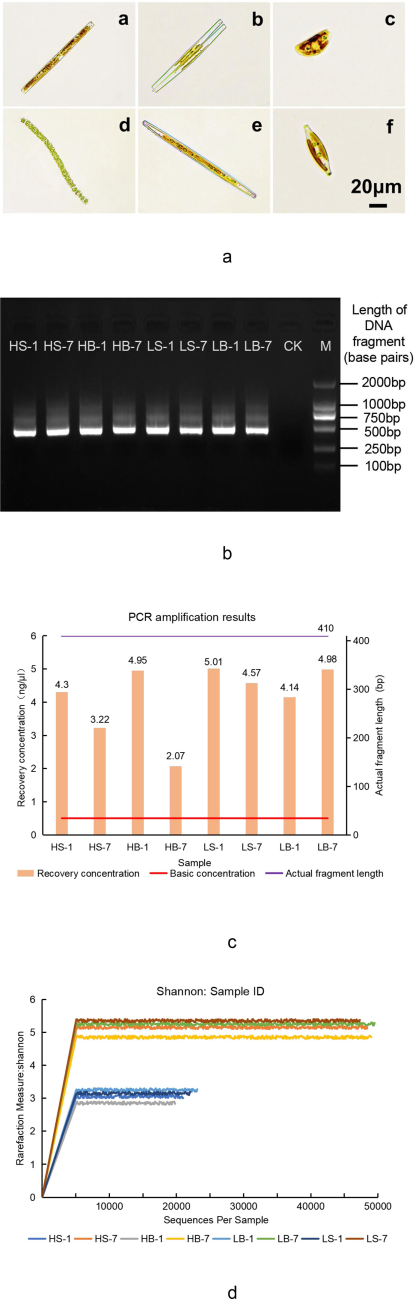
Identification results of dominant species and samples by sequencing. (a), which is six dominant algae genera, a- Nitzschia; b- Fragilaria; c- Synedra; d- Melosira; e- Navicula; f- Liagora, Bar = 20 μm. (b) shows the 1.2 % agarose gel electrophoresis, with ten lanes, where “M” stands for “Marker,” the length of the correct gene fragment is used as the reference of the length of the sample DNA fragment, and “CK” stands for “Check” to ensure that the experimental results are not interfered by the outside world. (c) is the Combination diagram of PCR amplification results. (d) shows the Shannon sparse curve, the curve tends to be gentle, and the depth of reaction sample sequencing is reasonable. Examples of sample grouping: H/L represents the high/low flow-rate zone, B/S represents the sampling point of the bottom/side wall, and 1/7 represents the test period 1d/7d.
3.2 Community observation and abundance analysis of dominant species
Dominant algal genera attached in all samples as a single individual after 1 day, but they formed microcolonies until 7 days later (Fig. 2a).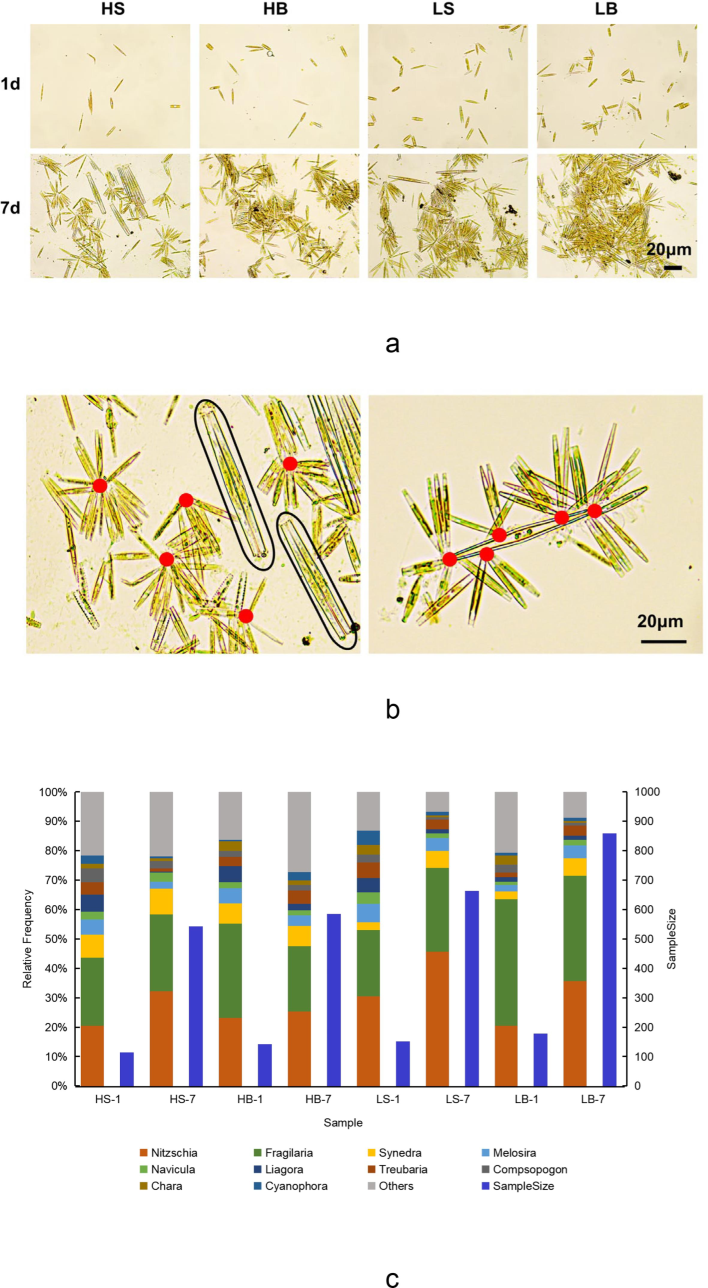
Effects of flow rate on dominant algal genera's attachment and aggregation behavior. (a) Dominant algal communities in each sample. Bar = 20 μm. (b) Mapping the attachment behavior of Nitzschia and Fragilaria, the attachment sites of Nitzschia are shown in red, and the black box selects Fragilaria. Bar = 20 μm. (c) Composition abundance and algae sample size, the legend marks the top 10 genera in the sample, and blue legends indicate the algae sample size.
By observing microcolonies, two distinct mechanisms for attaching of Nitzschia were found: One is the similar connected in a circular arrangement, and the other is attached to different algae (Fig. 2b). However, Fragilaria existed as a single individual or participated in the attachment process of Nitzschia. Algae sequence analysis (Fig. 2c) and microscopic observation results (Fig. 2a) demonstrated that the attach-ment density of algae in the high flow-rate zone was smaller compared to the low flow-rate zone at the same period and place, showing that high flow rate suppressed algal attachment. Further analysis of the sample species abundance showed that the average proportion of Nitzschia and Fragilaria in all samples was 58.35 %, with the maximum reaching up to 74.09 %. Under the same conditions, the species abundance at flow rate of 0.794 m/s was ca. 10 % smaller than that at flow rate of 0.538 m/s, suggesting that the high flow rate affected the community's species composition and the distribution of the dominant algal genera.
3.3 Distribution difference and mechanism of Nitzschia and Fragilaria
The variance of species abundance reflects the distribution difference of species in the community. Specifically, the larger the variance value, the more uneven the distribution of species in the community; The smaller the variance, the more uniform the distribution of species in the community (White et al., 2009). Methods for calculating the variance of species abundance value and comparing the uniformity of species distribution are given in online resource 5. For Nitzschia and Fragilaria, the species abundance distribution was more concentrated when the flow rate was 0.794 m/s (Fig. 3a). Moreover, the variance of species abundance values of the two dominant algae genera was 2.475 × 10-3 and 2.005 × 10-3 at flow rate of 0.794 m/s, which are both smaller than the corresponding variance values of 1.105 × 10-2 and 7.945 × 10-3 at flow rate of 0.538 m/s, indicating that the dispersion of species abundance values at high flow rate was low and the species distribution was more uniform. Algal force analysis (Fig. 3b) demonstrated that hydrodynamics and gravity affected the movement of algae together, as previously suggested (Kessler, 1985; Li et al., 2021), higher flow rate increased algal forces in the direction of the current, reducing their chances of attachment and community density per unit area. The analytical results of this study offer a rational framework to interpret how dominant algae attach and distribute at different flow rates.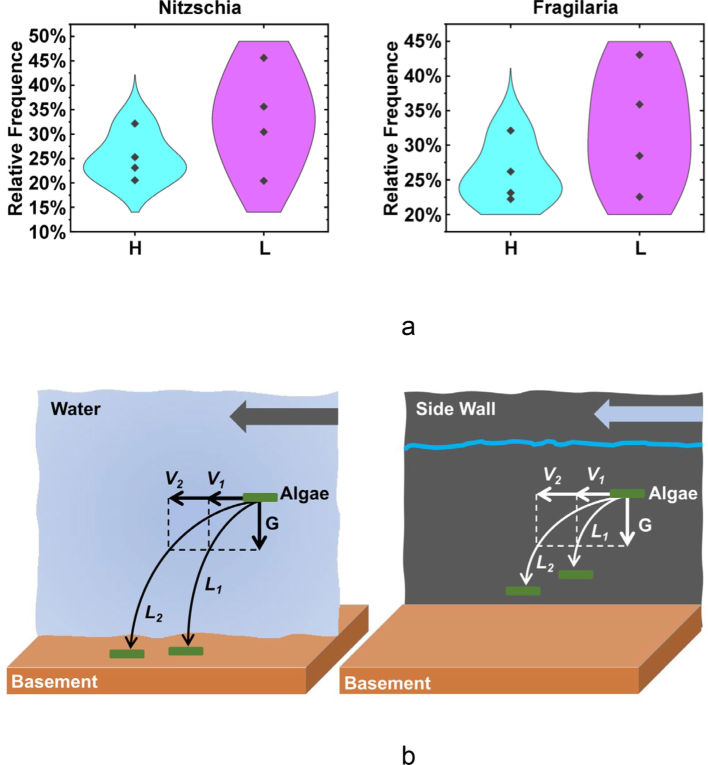
Distribution mechanism of Nitzschia and Fragilaria. (a) Comparing the distribution of high/low flow-rate dimensions of Nitzschia and Fragilaria, the abscissa naming rules refer to Fig. 1, and the ordinate indicates the species abundance of this species in the corresponding dimension. (b) Schematic diagrams of algal attachment to the basement and side walls at high and low flow rates. V1 and V2 represent low flow rate (0.538 m/s) and high flow rate (0.794 m/s), and the corresponding algae trajectories are L1 and L2.
4 Conclusion
The colonization and aggregation of single attached algae on the surface to form a micro-community is consistent with biofilm formation (Menesses et al., 2017), but different algae may form micro-communities in different ways. Microscopic observation results show that Nitzschia would complete the aggregation in a circular arrangement with its end or other algae as attachment points, while most Fragilaria existed in a single individual form (Fig. 2b). In addition, different flow rates may have a multitude of consequences for the aggregation and distribution of attached algae. On the one hand, a higher flow rate reduces the species abundance ca.10 % under the same conditions (Fig. 2a, Fig. 2c), as it increases algal forces in the direction of the current, reducing their chances of attachment and community density per unit area. On the other hand, a higher flow rate makes the distribution of attached algae more uniform in different periods and attachment sites (Fig. 2c, Fig. 3a). The interaction of flow rate and gravity could thus be an essential determinant of the aggregation and distribution of attached algae, which is consistent with the previous research results (Tan et al., 2019; Ren et al., 2021) about the effects of hydrodynamic and gravity on phytoplankton aggregation (Fig. 3b). This study showed that the dominant algae had a low tendency to attach and aggregate on the structure surface in high flow rate areas, and the distribution of different behaviors was more uniform over time. Moreover, the higher flow rate has a better effect on removing the communities of attached algae on the structure surface when the flow rate is controllable. Studying the influence mechanism of flow velocity on algae growth has a wide range of potential applications and influences in the field of ecological and environmental management and has important practical significance in water environment and environmental monitoring. On the one hand, it is helpful to find an effective method to suppress the occurrence of water bloom in static or slow-moving water bodies such as reservoirs and lakes by regulating the water velocity and improve the water environment. In addition, it can optimize the community structure of aquatic organisms, promote the restoration of biodiversity, and promote the restoration and reconstruction of the entire ecosystem. On the other hand, it is helpful to explore the possibility of using flow velocity as a new environmental monitoring index or auxiliary index, improve the accuracy and timeliness of environmental monitoring, predict the growth trend of algae and the risk of water bloom, provide scientific basis for timely prevention and control measures, and reduce the impact of pollution on the environment and human health.
Further research will control experiments to explore more accurate critical hydrodynamic characteristics. In the future, through the combination of systematic experiments and numerical simulation, more environmental factors will be considered to influence the behavior of bacteria, algae, and large fouling organisms in the water environment, which will provide theoretical guidance for the design of anti-fouling surfaces and the development of antibacterial agents.
Disclosure of Funding
The authors appreciate the financial support given by National Key Research and Development Program of China (2022YFE0126400), National Natural Science Foundation of China (52171235), Dalian Science and Technology Talent Innovation Support Program (2023RJ008), Yangjiang Alloy and Hardware Key Industry Talent Revitalization Program (RCZX2023004), and Liaoning Provincial Department of Education (LJKMZ-20220856).
CRediT authorship contribution statement
Kai Shen: Writing – original draft, Visualization, Methodology. Yi Li: Validation, Software. Zhanyong Zhao: Resources, Conceptualization. Weirong Li: Methodology, Formal analysis. Zhihui Liu: Validation, Investigation. Shukhrat Giyasov: Visualization, Data curation. Sapar Bayarmagnai: Resources, Project administration. Zhaoyang Lv: Validation, Formal analysis. Fei Gao: Resources, Project administration. Wenqing Shi: Resources, Conceptualization. Chao Lu: Software, Methodology. Di Tie: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- ASTM International. Standard Test Method for Analysis of Phytoplankton in Surface Waterby the Sedgwick-Rafter Method: ASTM D4148-82(2012).
- Community structure and ecological responses to hydrological changes in benthic algal assemblages in a regulated river: application of algal metrics and multivariate techniques in river management. Environ. Sci. Pollut. R.. 2021;28:39805-39825.
- [CrossRef] [Google Scholar]
- British Standard. Water quality—Guidance standard for the identification, enumeration and interpretation of benthic diatom samples from running waters: BS EN 15204:2006 [S].
- Phytoplankton dynamics with a special emphasis on harmful algal blooms in the Mar Piccolo of Taranto (Ionian Sea, Italy) Environ. Sci. Pollut. R.. 2016;23:12691-12706.
- [CrossRef] [Google Scholar]
- Atlas of Common Algae in Inland Waters of China. Wuhan (in Chinese): Changjiang Publishing House; 2012.
- Modeling the lateral variation of bottom-attached algae in rivers. Ecol. Model.. 2013;267:11-25.
- [CrossRef] [Google Scholar]
- Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology. 1975;56(6):1459-1461.
- [CrossRef] [Google Scholar]
- Impacts of hydrodynamic disturbance on sediment resuspension, phosphorus and phosphatase release, and cyanobacterial growth in Lake Tai. Environ. Earth Sci.. 2015;74:3945-3954.
- [CrossRef] [Google Scholar]
- Substrate properties as controlling parameters in attached algal cultivation. Appl. Microbiol. Biot.. 2021;105:1823-1835.
- [CrossRef] [Google Scholar]
- Bacterial diversity in aquatic and other environments: what 16S rDNA libraries can tell us. FEMS Microbiol. Ecol.. 2004;47(2):161-177.
- [CrossRef] [Google Scholar]
- Hydrodynamic focusing of motile algal cells. Nature. 1985;313(5999):218-220.
- [CrossRef] [Google Scholar]
- Antifouling paint formulations with ZnO and Fe2O3 nano-paints for marine applications. Sustain. Chem. Pharm.. 2022;30:100858
- [CrossRef] [Google Scholar]
- Al2O3 and CuO nano particulate-based paints for marine applications. Eng. Res. Express. 2022;4:035056
- [CrossRef] [Google Scholar]
- Impact of titanium and silicon metal oxide nanoparticles on surface coatings for marine vehicles to mitigate biofouling. Particul. Sci. Technol.. 2024;42(6):915-928.
- [CrossRef] [Google Scholar]
- Effect of hydrodynamics on autoflocculation and gravity sedimentation of Chlorella vulgaris. J. Water Process Eng.. 2021;43:102259 16/j.jwpe.2021.102259
- [Google Scholar]
- Effect of flow velocity on phytoplankton biomass and composition in a freshwater lake. Sci. Total Environ.. 2013;447:64-71.
- [CrossRef] [Google Scholar]
- Physical drivers facilitating a toxigenic cyanobacterial bloom in a major Great Lakes tributary. Limnol. Oceanogr.. 2020;65(12):2866-2882.
- [CrossRef] [Google Scholar]
- Measuring a critical stress for continuous prevention of marine biofouling accumulation with aeration. Biofouling. 2017;33(9):703-711.
- [CrossRef] [Google Scholar]
- Processes and mechanisms of phosphorus mobility among sediment, water, and cyanobacteria under hydrodynamic conditions. Environ. Sci. Pollut. R.. 2021;1–15
- [CrossRef] [Google Scholar]
- A mathematical theory of communication. Bell Syst. Tech. J.. 1948;27(3):379-423.
- [CrossRef] [Google Scholar]
- A mathematical theory of communication. ACM SIGMOBILE mobile computing and communications review. Nokia Bell Labs.. 2001;5(1):3-55.
- [CrossRef] [Google Scholar]
- Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res.. 2003;13(11):2498-2504.
- [CrossRef] [Google Scholar]
- Hydrodynamic impacts on algal blooms in reservoirs and bloom mitigation using reservoir operation strategies: a review. J. Hydrol. 2023 129375
- [CrossRef] [Google Scholar]
- Mechanism of the influence of hydrodynamics on Microcystis aeruginosa, a dominant bloom species in reservoirs. Sci. Total Environ.. 2018;636:230-239.
- [CrossRef] [Google Scholar]
- Mechanism underlying flow velocity and its corresponding influence on the growth of Euglena gracilis, a dominant bloom species in reservoirs. Int. J. Env. Res. Pub. He.. 2019;16(23):4641.
- [CrossRef] [Google Scholar]
- Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol.. 2009;5(4)
- [CrossRef] [Google Scholar]
- Algal blooms in the middle and lower Han River: characteristics, early warning and prevention. Sci. Total Environ.. 2020;706:135293
- [CrossRef] [Google Scholar]
- Algae growth distribution and key prevention and control positions for the Middle Route of the South-to-North Water Diversion Project. Water. 2019;11(9):1851.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103566.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







