Translate this page into:
In-vitro, in-vivo and in-silico exploration of different extracts of Justica adhatoda against Newcastle viral disease
⁎Corresponding authors. asmabinm@gmail.com (Asma Ashraf), mahboobchem@gmail.com (Mahboob Alam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Justicia adhatoda (family Acanthaceae) is an indigenous plant of Soan Skesar Valley with copious biological perspectives. In the present study, four methanolic, acetonic, chloroform & n-hexane extracts of J. adhatoda were checked for in vitro biological potentials by phytochemical profiling, bioactive contents, HPLC analysis, and antioxidant assays. Results showed that the plant exhibited a maximum TPC of 41.6 ± 0.4 mg. GA.E/g and TFC as 52.3 ± 0.3 m.g QE/g by methanolic extract, which may show the elevated antioxidant potential of that extract. Through DPPH (2,2-Diphenyl-1-picrylhydrazyl) assay, Ferric ion reducing antioxidant power assay (FRAP), Hydroxyl (OH) radical scavenging, and Nitric oxide (NO) radical tests for scavenging, the J. adhatoda studies showed that all extracts contain antioxidant capacity. It also demonstrated the stronger antiradical property in the outline of methanolic > acetonic > chloroform > n-hexane. The plant’s efficacy against Newcastle viral disease was tested by in ovo in SPF- SPF-embryonated chicken eggs. This indicates that 400 µg/mL of methanolic extract showed a high survival rate with 0 % mortality. Further, in silico studies showed the binding interactions between receptor protein and phytoactive components of plants which provide the way for pharmacological and drug-likeness activities for future studies. The present studies highlighted that all extracts especially methanolic extract of J. adhatoda exhibited the best phytochemical profile, active phyto-contents, antioxidant, and antiviral properties that can be supplementary explored for new drug advancement.
Keywords
J. adhatoda
Phytochemicals
Antioxidant
Antiviral
Molecular docking
1 Introduction
Plants have been utilized in folk medicines in the past. It is known thing that plants include chemical constituents, the accomplishment of which is intended for many processes, and incoming complex organismic communications (Ghalloo et al., 2022). The plant’s medicinal characteristics are caused by the existence in their active substances organs (glycosides, alkaloids, vitamins, flavonoids, coumarin, and tannins compounds) that have a physiological effect on animals and human beings too, or have biological action against many disease-causing pathogens (Kamkin et al., 2022). Pharmacological studies suggest that it has antibacterial, anti-inflammatory and diuretic properties. Interestingly, bromhexine (a key compound in Justicia adhatoda) has a synthetic derivative called Bromhexine HCl, which has expectorant properties (Mehta et al., 2023).
It has been concluded that up to 25 % of drugs approved in usual medicines are associated openly or ultimately with plant origin. Therefore, throughout the past few decades, there has been an enhanced curiosity in the research of medicinal plants and their deep-rooted use in various countries. On the other hand, nowadays it is necessary to pay for scientific evidence as to whether it is suitable to use the active principles of plants (Aumeeruddy and Mahomoodally, 2019). After being screened pharmacologically, modern medications need to be further characterized by looking at their pharmacokinetic and pharmacodynamic characteristics, as well as their toxicity. The World Health Organisation (WHO) estimates that 65–80 % of developing nations rely primarily on ethnomedicine due to its accessibility and affordability for treating their basic health issues (Shahzad et al., 2019).
Many researches have shown that cancer progression can be slowed by using the compounds of natural medicinal plants (Chaudhry et al., 2021). Radiation, chemotherapy, cure, or both may be used to treat 90 % of all early-stage liver cancers, while hepatocellular carcinoma is a type of advanced liver cancer. The compounds that are antioxidants act as free radicals scavengers created by the cells of the body because of many external factors and metabolic reactions. The most frequent reasons for cancer in the human body are the unstable free radicals, which cause severe damage to the DNA, RNA, lipids, and proteins (Venkatachalapathy et al., 2021).
In developing countries, the main reasons for illness and death are infectious diseases. It can have a destructive impact on communities because it is a serious health problem for the public (Joshi et al., 2020). An important infectious disease of poultry is Newcastle disease (ND) with a record of almost a century that has spread at least 4 panzootics worldwide (Miller and Koch, 2013). NDV is an enveloped virus with a negative-sense, nonsegmented RNA genome, which encodes 6 viral proteins: the matrix (M), nucleoprotein (NP), fusion (F), phosphoprotein (P), large (L) proteins and haemagglutinin‑neuraminidase (HN) [Office International des Epizooties (OIE, 2013)].
Live and inactivated vaccines have been used as standard poultry medicine for millennia, but disappointment is still occasionally recorded because of differences in antigenicity, the existence of maternal antibodies, uneven vaccination application, and chain cold disruption. Despite the many trial vaccinations that have been evaluated for many years, the disease continues to spread unabated in many parts of the world. From heightened onset with the highest death rate to minor bird disease, the potential of viral Newcastle disease as a source of transmission has been oppressed for the cure of dissimilar human vaccination and cancers (Naz et al., 2022).
Due to the prevalence of multidrug-resistant (MDR) diseases, researchers have been urged to hunt for novel molecules with antiviral action from a wide range of sources, including medicinal plants (Spengler et al., 2022). The antibacterial, antiviral, and antifungal activities of plant polyphenols are well-known in the medical world. This is because they have the potential to structurally or functionally destroy the membrane of a bacterial or viral cell. Multiple researches have shown that rich polyphenol plants have antiviral and antioxidant properties (Takó et al., 2020). The capabilities of polyphenols lie down in the research area for emerging anti-viral medications. The effort in many ways, like avoiding the entry of the virus entrance or affecting the replication of the virus. The widely accessible polyphenols and comparatively economical to make, making them a good option for the development of medication (Montenegro-Landívar et al., 2021).
Numerous plant species in Pakistan's diverse flora have enormous promise for the discovery of novel chemicals with significant immune system-healing properties. Because of their low cost, ease of accessibility, lack of negative side effects, potent antimicrobial properties, ability to lower blood cholesterol levels, and variety of uses for improving presentation feed conversion rate, weight gain in birds, and growth rate, medicinal plants are currently consumed and in high demand worldwide (Andleeb et al., 2020).
Justicia adhatoda belongs to the family Acanthaceae. J. adhatoda leaves have been widely used in Ayurvedic medicine for more than 2000 years, primarily for respiratory ailments. The plant's active ingredients, the roots, leaves, and flowers, have a variety of pharmacological qualities and are used in particular for respiratory issues like rheumatism, asthma, bronchial asthma, coughing, and chronic bronchitis (Pandey and Mishra, 2010). Due to the presence of tiny active dosages of chemicals that trigger physiological processes in both the human and animal body, the plant has therapeutic value. Essential oil and quinazoline alkaloids have been found in several fractions of J. adhatoda, among other important bioactive substances. As a result, J. adhatoda extract could be among the best choices for developing novel natural medicines (Dhankhar et al., 2011).
Building upon the previous discussion, molecular docking stands as a key computational method in drug discovery, used to predict and understand how small molecules interact with target proteins. There are two main methods: blind docking and active site docking. Blind docking explores the entire protein surface without knowing where the binding sites are, allowing the discovery of new interaction sites. Active site docking, however, focuses on known active sites on proteins, elucidating specific binding interactions that are critical for biological activity (Naveed et al., 2016; Naveed et al., 2022). Together, these methods provide a comprehensive strategy for studying ligand–protein interactions and guiding the design of therapeutically relevant compounds. This article discusses the importance of both approaches in enhancing our knowledge of molecular recognition and drug design.
In the current research work, the local medicinal plant, Justicia adhatoda, from Soan Skesar Valley was first screened for in vitro biological activities like phytochemical screening, TPC and TFC contents, and HPLC to identify the bioactive constituents and antioxidant activities. After in vitro assessment, the medicinal plant was evaluated for antiviral potential against Newcastle disease virus in SPF embryonated, 9-day-old chicken eggs (in ovo). Further, the efficient performance of phyto-components was checked by in silico review.
2 Material and methods
2.1 Collection and identification of plants
A clean and healthy medicinal plant was selected in Punjab, Pakistan's Soan Skesar Valley, close to the Khushab district. The plant was chosen based on a thorough analysis of beneficial medical characteristics that have been documented in the literature and the locals' familiarity with using the plant as folk medicine in this region. By using common descriptions and keys, the Botany Department, GCUF, officially confirmed the botanical identity of the selected plant, including its significant sections (Ingle et al., 2017).
2.2 Extract preparation
The plant material extraction procedure was carried out following the method established by Dipankar's research group (Dipankar and Murugan, 2012). The leaves of the J. adhatoda plant were desiccated in the shade and then pulverized into powder. For soxhlet extraction experiments, acetone, chloroform, methanol, and n-hexane were utilized as solvents. A thimble was filled with 20 g dry plant powder, which was then placed in the extraction chamber. A 250 mL solvent was used to weigh down the bottom round flask. At 55 °C, the extraction process was continued for 48 h. To obtain each crude solvent extract, the resultant liquid extracts were individually transferred to rotary vapor (at 40 °C). After being weighed, extracts were then stored in the refrigerator (up to 3 months) at 4 °C for further experiments.
2.3 Preliminary phytochemical profiling
Phytochemical quantitative evaluation of J. adhatoda extracts was tested by reported protocols (Sivanandham, 2015).
2.4 Total bioactive contents
2.4.1 Total phenolic content (TPC)
TPC of plant medicinal extracts was determined using the Folin-Ciocaltea assay according to the technique (Stanojevi et al., 2016). The stock solution was created using a 1 mL dilution of the plant extract in dimethyl sulfoxide (DMSO). Following the addition of the FCR (Folin-Ciocalteau) reagent (100 L) to 20 L of each extract, the mixture was incubated in the dark three additional times. The final combination was then mixed with a 100 mL solution of 10 % Na2CO3 before being left in the dark for an hour. To measure absorbance, a Dynamica Instruments (UK) Halo DB-20S UV spectrophotometer was used to measure absorbance at 765 nm. TPC was measured in triplicate. The TPC of plant extracts, expressed as gallic acid equivalents (GAE mg/g), was determined using a standard calibration curve generated with different concentrations of gallic acid (PE) (100, 200, 300, 400 and 500 μg/mL).
2.4.2 Total flavonoid content (TFC)
TFC of plant extracts was determined using the aluminum chloride (AlCl3) colorimetric method as described by Stanojević et al. (2016). 1 mL of each extract was mixed with 0.1 mL of 5 % NaNO2 solution and incubated for 5 min. Then, 0.6 mL of NaOH solution was added to the mixture. Dilute to 10 mL with distilled water and measure the absorbance at 510 nm with a UV spectrophotometer. A quercetin standard curve was prepared using quercetin solutions at concentrations of 100, 200, 400, 600, 800, and 1000 µg/mL. Results are expressed as quercetin equivalents (QE) per gram of plant extract (mg QE/g PE).
2.5 High-performance liquid chromatography (HPLC)
The extract samples were processed through high-performance liquid chromatography using a reverse phase procedure that has been verified (Perkin Elmer HPLC Equipment, Series 200 LC Pump/UV Detector). The Waters Spherisorb ODS2 stationary phase was applied to a reverse column (250/4,6 mm, 5-m). A constant flow rate of 1 mL/min was utilized for the analysis. The chromatographic experiments were carried out with mill-Q water and HPLC-grade solvents. The mobile phases for 254 nm in phytochemical contents have been calculated to be pure acetonitrile (A) and phosphoric acid (H3PO4) (B). The gradient system was altered in 0/95, 20/86, and 70/65 (min/percent B). Through the comparison of experimental and reference retention times, the correlation of chromatographic peaks was accomplished. Their quantities were measured by a standard curve, with findings represented as the mg/L extract. The procedures were performed in duplicate at room temperature (Menezes et al., 2016).
2.6 Antioxidant activities
J. adhatoda’s antioxidant activities integrated determining of free scavenging radicals and power-reducing properties. Ascorbic acid was used as a standard for DPPH, FRAP, and OH free radical scavenging activities, and curcumin was used as a standard for NO radical scavenging activity. All of these activities were performed according to the literature with minor modifications (Benarfa et al., 2019).
2.6.1 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
To evaluate the free radical scavenging capacity, plant extracts at different concentrations (62.5, 125, 250, 500, and 1000 mg/mL) were evaluated using DPPH assay. Add freshly prepared 0.1 mM DPPH solution to each extract concentration. The mixture was then incubated for 30 min at 27 °C in the dark to allow the extracts to scavenge free radicals. After incubation, absorbance was measured at 517 nm using a BioTek SynergyHT microplate reader. Control experiments containing only DPPH solutions without plant extracts were also performed for comparison.
2.6.2 Ferric reducing antioxidant power assay
The extract was added to newly made buffer phosphate (0.2 M, pH 6.6) and 1 % K3Fe(CN)6 at concentrations of 62.5, 12.5, 25.0, 50.0, and 100.0 g/mL for power reduction tests. For 20 min, the reaction mixture was set to 50 °C. 10 % w/v (TCA) was combined after 20 min of incubation (10000 rpm for 10 min). The resultant supernatant was subsequently diluted with freshly produced FeCl3 (0.1 w/v) and deionized water. A BioTek Synergy HT microplate reader was used to determine the reaction mixture's optical density at 700 nm.
2.6.3 Hydroxyl (OH) radical scavenging assay
Plant extracts were treated for four hours at 37° C with sodium buffer phosphate (pH 7.0, 0.2 M), EDTA, 10-mM 2-deoxyribose, and 10-mM H2O2. To the reaction mixture, TCA (2.8 % w/v) and TBA (1 % w/v in NaOH) were added. A reaction control mixture devoid of plant extract was produced. To evaluate the optimal procedure for manufacturing TBARS, the optical density of the reaction mixture was measured at 532 nm using an HT microplate reader, the BioTek Synergy.
2.6.4 Nitric oxide (NO) radical scavenging assay
The NO was generated via the Greiss reaction and computed from sodium nitroprusside. Nitric oxide synthase activation is restricted by curcumin, which is also a strong scavenger of naturally occurring nitric oxide. As a result, there is less sodium nitroprusside-derived nitrite present between nitric oxide and oxygen. The BioTek Synergy HT microplate reader assessed the absorbance to be 596 nm.
2.7 In-ovo antiviral assay
2.7.1 Source of virus
Avirulent (LaSota strain) signified live vaccine of NDV was obtained from a commercial Poultry feed market, Faisalabad, Pakistan. The titre HA was passed out in embryonated chicken eggs (ECEs) specific pathogen free (SPF) to find the median infectious dose (EID50).
2.7.2 Assortment of eggs
A day-old chicken eggs were kept in the incubator for nine days for the formation of the embryo. The temperature and humidity were constant throughout the 9 days. On the ninth day, the eggs were candling to observe embryonic development. Candling can help identify fertilized eggs (eggs containing developing chicks) and infertile eggs. Finally, after careful selection, fertilized eggs were separated from unfertilized eggs for further experiments.
2.7.3 Egg inoculation
For the inoculation of eggs, the eggs were grouped and labeled according to the inoculated extract they received. Seven groups (n = 5) were formed for each extract and inoculated with the different concentrations of J. adhatoda extracts and the LaSota strain of ND virus. The eggshells over the blunt end were cleaned with 70 % alcohol. A hole was pinched on the opposite side of the embryo position at the air sac, with a sterile needle. The first four groups (G1 to G4) received 300, 400, 500, and 600 µg/mL of (0.2 mL) extracts and 0.2 mL of virus (10-4.5) with injection. The G6 served as extract control, as this group of eggs was inoculated with extract (400 µg/mL) only. G5 served as virus control, these eggs were inoculated with 0.2 mL of 4 HA LaSota virus. The G7-grouped eggs were left uninoculated (negative control). The layout is given in Table 1. Then paraffin wax was to seal the holes and kept at 37 °C in the incubator. The eggs were. Candled daily after 24 h to assess the position of the embryo.
Sr. #
Group (G)
Treatment
1
G1
Methanol/Acetone/chloroform/n-hexane extract of J. adhatoda300 µg/mL + 0.2mL 4 HA virus
2
G2
Methanol/Acetone/chloroform/n-hexane extract of J. adhatoda400 µg/mL + 0.2mL 4HA virus
3
G3
Methanol/Acetone/chloroform/n-hexane extract of J. adhatoda500 µg/mL + 0.2mL 4HA virus
4
G4
Methanol/Acetone/chloroform/n-hexane extract of J. adhatoda600 µg/mL + 0.2mL 4HA virus
5
G5
Virus control
6
G6
Extract control
7
G7
Uninoculated embryonated eggs
2.7.4 Candling
Candling is a method that involves using light to inspect the interior of eggs while they are being incubated. It assists in monitoring the growth, well-being, and mobility of the embryo to detect any issues at an early stage. The survival of embryos was examined by candling of eggs every day for 72 h. The mobility of embryos was examined. Dead embryo eggs were chilled out for 1 h and their allantoic fluid was collected, for the assessment of virus titres. After 96 h of inoculation, the allantoic fluid was collected from the eggs of all experimental groups and tested by a standard HA assay to detect virus titers after 96 h of inoculation.
The titre HA test was used by double dilutions for the quantification of the total virus. Allantoic fluid was harvested from surviving and dead embryonated eggs by the allantoic fluid. In the 96-well round bottom-based microtiter plate, 50 μL of allantoic fluid collected from each group of eggs, was added at the first well of the microplate. Then fluids were serially diluted by 50 μL of normal saline from wells 1 to 11 along the rows. Then 1 % (freshly gathered from the chicken) of RBCs was added to each 50 μL from wells 1 to 12 of the microplate and gently mixed. Let them stand at room temperature for 25 min. After a certain period, virus titre which indicated as reciprocal of the highest dilution caused the chicken RBCs to aggregate.
2.8 In silico studies
2.8.1 Ligand preparation
Justica adhatoda's phytochemicals 2D structures were retrieved from PubChem (https://pubchem.ncbi.nlm.nih.gov) and run through Chem3D Pro to optimize ligands and minimize costs. For ligand optimization and minimization that results in the lowest energy ligand conformer, an energy force field is passed through the module.
2.8.2 Receptor preparation
Receptor proteins obtained from the Protein Data Bank (RCSB PDB) (https://www.rcsb.org) were prepared using Maestro (Gold v 5.3.0) and used for molecular docking with high-resolution X-ray structures. The software processes proteins by removing solvents, adding hydrogen atoms, creating disulfide bonds, assigning bond orders, filling in missing side chains and loops, and generating protonation states at pH 7.4 ± 0.5 using Epik tools. Subsequently, constraint minimization was performed at pH 7.0 using OPLS3e field forces and GOLD to achieve energy minimization and protein structure optimization.
2.8.3 Molecular docking
The settings of the molecular docking program were used to carry out the docking molecular experiments (https://www.ccdc.cam.ac.uk). Wets local search techniques were used to simulate docking. The orientation, starting position, and torsion of the ligand molecules were all determined at random. Each docking experiment was taken from 10 different runs that were meant to last for a maximum of 1.5 assessments.
Utilizing molecular docking experiments, it was found that ligands and proteins may interact or bind. Receptor protein (ID: 1G5G) has 3-dimensional structures obtained from the PDP protein data repository. Docking calculations have been carried out using BIOVIA Discovery Studio and GOLD version 5.3.0 for visualizing and modeling (https://www.3dsbiovia.com). The main location, molecular torsion, and ligand orientation were chosen at random. Ten different runs with a maximum of 1.5 evaluations each were used to extract one docking experiment.
3 Results
3.1 Preliminary phytochemical profiling
All the J. adhatoda’ extracts were tested for significant medicinal phytochemicals such as Alkaloids, Anthraquinone, Phenols, Flavonoids, Phlobatannins, Terpenoids, Tannins, Reducing sugars, Saponins, Cardiac glycosides, and Steroids. Table 2 shows the presence of these metabolites. + = present,_= absent.
Sr. #
Extracts
Alkaloid
Anthraquinone
Flavonoids
Phenols
Phlobatannin
Terpenoid
Tannins
Reducing sugars
Saponin
Cardiac glycosides
Steroid
J. adhatoda
1
Methanol
+
_
+
+
_
+
+
+
+
−
+
2
Acetone
+
_
+
_
_
+
+
_
_
+
_
3
Chloroform
+
_
+
_
_
+
_
_
+
_
_
4
n-hexane
+
_
_
+
_
+
+
_
+
_
+
3.2 Total bioactive contents
3.2.1 Total phenolic content
Phenolic chemicals are important plant components with redox properties that are responsible for antioxidant activity. The hydroxyl groups in plant extracts are what enable free radical scavenging. The Folin-Ciocalteu technique was used to resolve TPC, with gallic Acid serving as the standard. The absorption readings (10.0–1000 g/mL) obtained at different galactic acid concentrations were used to create a calibration curve. The results were calculated using the regression equation of galacturonic acid (Y = 0.0037x + 0.319; R2 = 0.993) and shown as mg of galacturonic acid equivalents (GAE) per gram of pigment extract (mg/g) (Fig. 1a). Methanolic extracts had the highest phenolic levels, according to Table 3’s findings. The differences in polarity among the extraction solvents may result in a significant variation in the concentration of bioactive chemicals in the extract. In comparison to the highly polar solvents, larger yields were seen in the methanol extract, and in the comparative acetone, chloroform, and n-hexane extracts.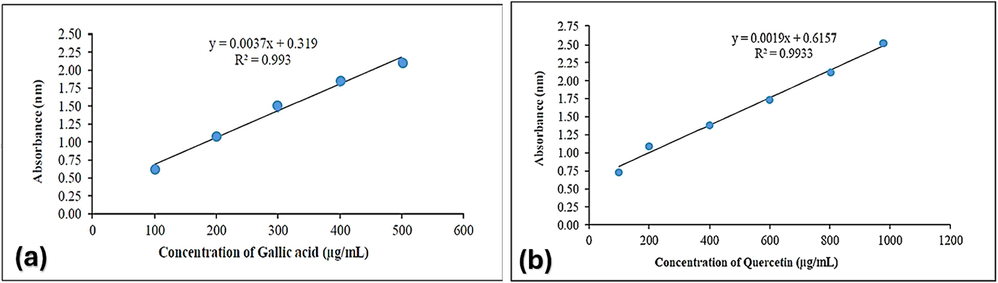
Standard curve of (a) gallic acid for the determination of total phenolic contents, and (b) quercetin for the determination of total flavonoid contents.
J. adhatoda
Plant extract
TPC (mg GAE/g)
TFC(mg QE/g)
Methanolic extract
41.6 ± 0.4
52.3 ± 0.3
Acetonic extract
35 ± 0.5
42 ± 0.5
Chloroform extract
33.6 ± 1.4
29.5 ± 1.5
n-hexane extract
29.5 ± 0.5
22 ± 1.5
3.2.2 Total flavonoid content
Aluminum chloride was used as a starting point in a colorimetric approach to measure the flavonoid contents in specific plant extracts. The results were calculated using the quercetin (100–1000 g/mL) calibration curve (Y = 0.0019x + 0.6157; R.2 = 0.9933) and reported as mg quercetin equivalents (QE) per gram of plant extract (mg/g) (Fig. 1b). The TFC values showed patterns that were similar to the TPC values. The quantity of phenols and flavonoids is also influenced by the polarity of the extraction solvents.
3.3 High-performance liquid chromatography (HPLC) of different extracts of J. adhatoda
High-performance liquid chromatography (HPLC) with a column size of 250 mm × 4.6 mm was used to identify the chemical components of the methanol extract of J. adhatoda. The test found that gallic acid, quercetin, caffeic acid, vanillic acid, benzoic acid, chlorogenic acid, syringic acid m-coumaric acid, p-coumaric acid, and cinnamic acid were present. Fig. 2(A) presents comprehensive data for the identified chemical components.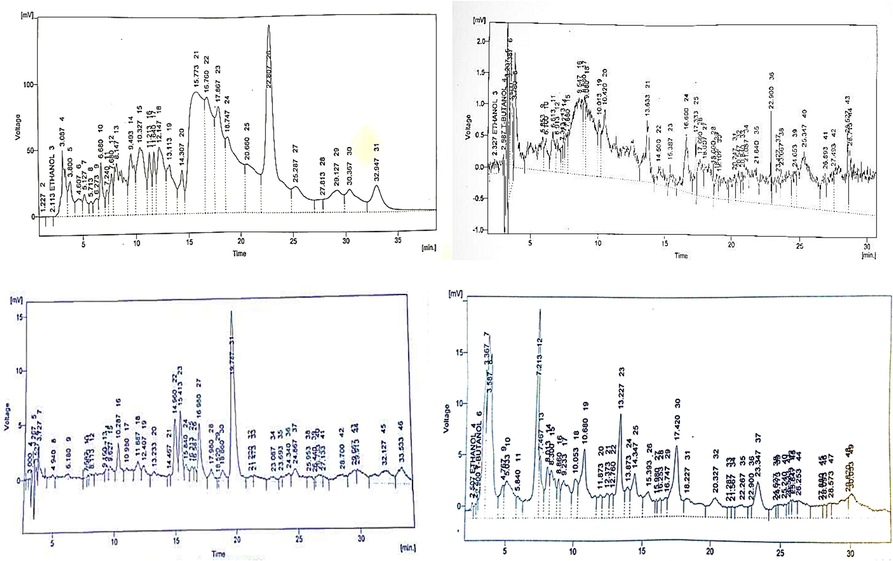
(A-D). HPLC chromatograms of methanol, acetone, chloroform & n- hexane extracts of J. adhatoda.
HPLC chromatogram Fig. 2(B) was used to define the chemical components of J. adhatoda plant in an acetone solvent extract. The test found that ethanol, quercetin, caffeic acid, vanillic acid, benzoic acid, chlorogenic acid, syringic acid, m-coumaric acid, p-coumaric acid and cinnamic acid, ferulic acid were present. The test found that ethanol, caffeic acid, vanillic acid, benzoic acid, chlorogenic acid, syringic acid, m-coumaric acid, p-coumaric acid and cinnamic acid were present. Fig. 2(C) displayed comprehensive data on the identified compounds.
HPLC test for J. adhatoda n-hexane extract revealed the existence of gallic acid, quercetin, caffeic acid, vanillic acid, benzoic acid, chlorogenic acid, syringic acid, m-coumaric acid, p-coumaric acid and cinnamic acid compounds on peaks defined in Fig. 2(D) with retention time showed for the identification of compounds, compared with standards used.
3.4 Antioxidant activities
All scavenging techniques supported the antioxidant activity data, which showed that the extracts exhibited a dependent concentration–response. The graphical representation demonstrated that J. adhatoda extracts exhibited the highest levels of radical scavenging in the following order: methanol > acetone > chloroform > n-hexane extract as displayed in Fig. 3(part A-D). In the case of scavenging radical potentials, the DPPH assay results recommended the methanolic extract exhibited the maximum IC50 i.e.65.4 ± 13.8 µg/mL. RFAP, OH radical, and NO radical scavenging resulted in 56.3 ± 9.7, 39.14 ± 6.5, 62.7 ± 12.5 µg/mL. The outcomes of FRAP and DPPH activities of the extracts recommended a direct correlation with the polyphenol content.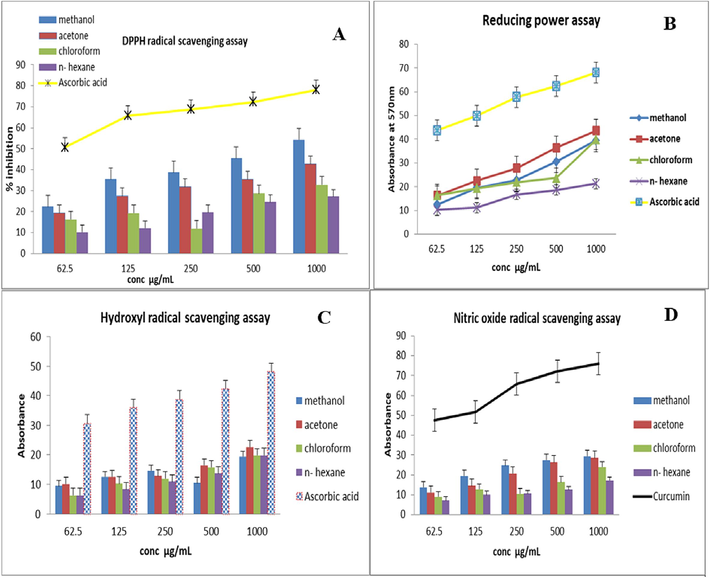
In vitro antioxidant activities of J. adhatoda leaves extracts. A: DPPH assay; B: RFAP assay; C: OH radical scavenging activity; D: NO scavening antioxidant activity.
3.5 In-ovo antiviral activity
3.5.1 Median percent embryo infectious dose
Applying the index formula to the dilution immediately resulted in the infection rate above 5.0 per.cen.t=0.10.-3.5. In 0.2 mL of viral solution dilution, one virus EI.D50 unit was present. One mL of viral solution is contained ten times the observed dilution reciprocity. The results of titration are shown in Table 4.
Median embryo infectious dose
No. of eggs
No. of eggs alive
No. of eggs dead
Percentage mortality
Dilutions
10-1
5
0
5
1.00 %
10-2
5
2
3
60 %
10-3
5
3
2
40 %
10-4
5
5
0
0%
10-5
5
5
0
0%
10-6
5
5
0
0%
10-7
5
5
0
0%
10-8
5
5
0
0%
10-9
5
5
0
0%
10-10
5
5
0
0%
Virus control
5
0
5
100 %
P.B.S control
5
5
0
0 %
From this data, the percentage figures entered in the Reed and Muench formula are as follows.
Therefore, infectivity titre of virus suspension in EID50/mL = 10 x 10 -3.5 = 10-4.5 EID50/mL.
3.5.2 In, ovo antiviral activity of J. adhatoda extracts
The antiviral efficacy of J. adhatoda extracts against NDV in ovo is shown in Table 5.1. Only 400 µg/mL of methanolic extract showed a high survival rate with 0 % mortality. But it's HA titre. The other three extracts did not inhibit the virus replication completely. Results of mortality percentage and HA titres are shown in Tables 5.1–5.2. A lower mortality rate was shown in the methanolic group. The acetonic extract showed the highest mortality rate 40 % at 400 µg/mL. 60 % death was displayed by 300 µg/mL of chloroform extract and the other 3 concentrations showed 40 % mortality passed out in embryonated e for virus replication was shown at 64 for 300 µg/mL and 16 was shown for 600 µg/mL of chloroform. On the other hand, n-hexane showed low mortality at 300 µg/mL, and the mortality rate was high as the concentration of extract increased.100 % mortality was shown in the virus control (VC) group which serves as positive control. There is 0 % mortality shown in the uninoculated eggs group and extract control (EC) group. The Titre HA explained values regarding their control of viral effects and aptitude. V.C. = virus control; E.C. = extract control.
Sr. #
Treatment
Concentration
µg/mL
Morality with time intervals
Mortality
%
24 h
48 h
72 h
1.
Methanolic extract
300
0
0
1
20 %
400
0
0
0
0 %
500
0
1
0
20 %
600
0
0
1
20 %
2.
Acetonic
Extract
300
0
0
1
20 %
400
0
1
1
40 %
500
0
1
0
20 %
600
0
1
0
20 %
3.
Chloroform extract
300
1
2
0
60 %
400
0
1
1
40 %
500
1
1
0
40 %
600
0
2
0
40 %
4.
n-hexane
extract
300
0
0
1
20 %
400
0
1
1
40 %
500
1
0
1
40 %
600
0
2
1
60 %
5.
VC
_
2
3
_
10.0 %
6.
EC
_
0 % mortality.
0%
7.
Un inoculated group
_
0 % mortality.
0%
Sr. #
Treatment
Concentration… µg/mL
HA titre
1.
Methanolic extract
300
8
400
4
500
8
600
16
2.
Acetonic extract
300
8
400
32
500
8
600
16
3.
Chloroform extract
300
64
400
16
500
8
600
16
4.
n-hexane extract
300
8
400
16
500
32
600
128
5.
VC
–
20.48
6.
EC
–
0
7.
Uninoculated group
–
0
3.6 In silico docking analysis
Chem 3D Pro was used in this method for the energy minimization of ligands in Gold docking. The docking molecular research evaluated the molecular interactions of phytocompounds from J. adhatoda, the principal pathogenic factor, with receptor proteins in order to determine the efficacy of these receptors. The PDB (protein data bank) provided the crystal coordinate structure of the receptor proteins. The GOLD suite version 5.3.0 with a resolution of 2.70 was used to load each structure one by one for docking. The 5.3.0 GOLD version has been used to screen various dock complexes based on docking fitness and GOLD score. The GOLD program yielded the best compound interacting with the receptor. The findings were reviewed on the basis of binding compatibility including fitness and docking score.
Table 6 showed that the greatest GOLD score, GOLD fitness along with retrieval IDs, and 3D structures (Naveed et al., 2023) were shown by the docked conformers of the synthesized 3 receptors. Every routine docking returned the top 10 ranked docked poses for every receptor. The highest ligand-receptor binding energy and interactions with the receptor (<6 Å bond lengths) were calculated to be the most efficient. The 2D view of Fig. 4 of the protein–ligand interactions of the best poses produced by the discovery studio.
Sr No.
Compound
retrieval IDs
3D structures
GOLD Score
kcal/mol
Gold binding fitness
kcal/mol
RMSD
Amino acid residue
Distance
Bond angle
1.
Betain
247
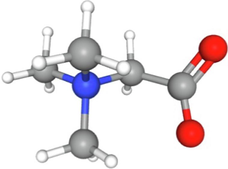
25.28
−1.02
4.87
A: ARG54
2.54
Electrostatic
C: LYS1063
4.58
Electrostatic
A: ARG54
1.70
Hydrogen Bond
C: LYS1063
2.35
Hydrogen Bond
C: ILE1065
2.67
Hydrogen Bond
2.
Vasicol
273,474,418
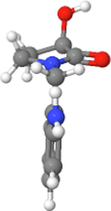
38.19
−0.96
3.98
C: ASN1064
2.41
Hydrogen Bond
C: LYS1063
2.64
Hydrogen Bond
C: ASN1064
2.37
Hydrogen Bond
3.
Peganine
72,610
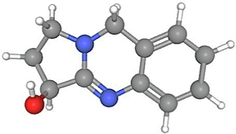
32.82
−0.03
3.76
A: ASN1064
1.77
Hydrogen Bond
C: GLN1062
3.07
Hydrogen Bond
A: THR58
2.75
Hydrogen Bond
C: LYS1063
3.95
Electrostatic
A: ARG54
2.71
Hydrogen Bond
C: LYS1063
4.86
Hydrophobic
4.
Anisotine
442,884
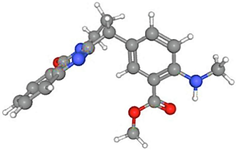
30.42
−0.04
3.45
C: GLN1062
2.92
Other
A: ARG54
5.39
Hydrophobic
A: ARG54
2.79
Hydrogen Bond
B: GLU469
2.18
Hydrogen Bond
5.
Scopolamine
3,000,322
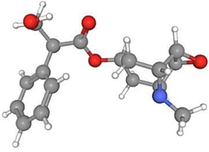
42.08
−7.35
1.76
A: LYS1063
2.38
Hydrogen Bond
A: GLN1062
2.06
Hydrogen Bond
A: THR58
2.05
Hydrogen Bond
B: GLU476
3.06
Hydrogen Bond
C: ASN1064
3.04
Hydrogen Bond
A: ARG54
5.34
Hydrophobic
6.
Vaiscoline
259,846
37.89
−2.40
8.98
C: GLN1062
2.01
Hydrogen Bond
C: LYS 1063
4.05
Hydrophobic
7.
Linolenic acid
5,280,934
48.28
−6.16
2.78
B: ASN500
2.33
Hydrogen Bond
A: ARG54
4.09
Hydrophobic
C: LYS1063
5.47
Hydrophobic
8.
Deoxyvasicine
68,261
38.98
−15.15
−5.87
C: ASN1064
1.75
Hydrogen Bond
C: LYS1063
2.66
Hydrogen Bond
C: GLN1062
2.00
Hydrogen Bond
9.
Vasicine
667,496
28.86
−1.80
−2.87
C: GLN1062
2.66
Hydrogen Bond
B: GLU476
2.27
Hydrogen Bond
C: LYS1063
4.50
Electrostatic
C: TYR1061
5.00
Hydrophobic
A: ARG54
4.90
Hydrophobic
C: LYS1063
2.07
Hydrogen Bond
10.
Lignoceric acid
11,197
40.09
−3.07
C: GLN1062
3.03
Hydrogen Bond
C: THR1060
2.10
Hydrogen Bond
A: ARG54
4.61
Hydrophobic
C: LYS1063
4.56
Hydrophobic
C: TYR1061
4.21
Hydrophobic
11.
Arachadic acid
10,467
0.09
−0.01
−0.00
C: LYS1063
5.04
Hydrophobic
C: TYR1061
3.72
Hydrophobic
12.
Ascorbic acid
54,670,067
32.50
−22.87
−4.87
A: ARG54
2.15
Hydrogen Bond
B: GLU476
2.62
Hydrogen Bond
B: GLU476
2.78
Hydrogen Bond
C: GLN1062
2.79
Hydrogen Bond
A: THR55
2.69
Hydrogen Bond
13.
Behenic acid
8215
31.45
−14.65
−6.98
C: GLN1062
2.45
Hydrogen Bond
C: TYR1061
2.54
Hydrogen Bond
A: ARG54
5.35
Hydrophobic
14.
Beta sitosterol
222,284
−9.54
−40.76
−0.00
A: THR58
2.72
Hydrogen Bond
A: ARG54
5.11
Hydrophobic
C: LYS1063
5.16
Hydrophobic
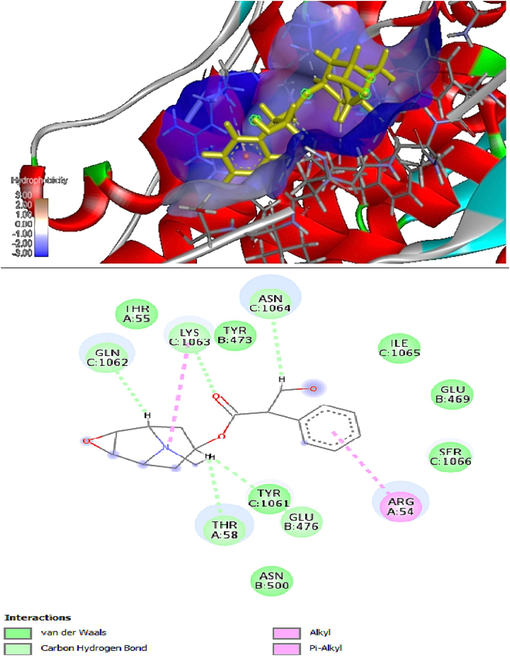
In silico molecular docking results of 3D and 2D (A&B) interaction between justica adhatoda with receptor proteins 1G5G.
4 Discussion
Modern medicine has become well known for its prompt and precise treatment. Notwithstanding notable progressions in contemporary medicine, a considerable fraction of presently accessible pharmaceuticals is expensive, harbor potential adverse effects, and possess restricted safety profiles (Soumya et al., 2019). The use of herbal medicines has increased in low-resource settings due to cost-effectiveness, cultural integration, and the potential for fewer side effects compared to traditional medicines. However, robust research is needed to confirm the efficacy and safety of these herbal products (Aminah et al., 2021). For this reason, the current study offers a foundation for creating a connection between regional traditional herbs and scientific institutions. The present research was implemented to collect the medicinal benefits from the indigenous plant J. adhatoda of Soan Skesar Valley, Salt Range, Punjab, Pakistan.
To evaluate the pharmacological and biological qualities of the medicinal plant J. adhatoda, research was conducted. According to the reported document (Dhanani et al., 2017), an extraction approach can produce extracts with a high yield and no alteration to the functional qualities of the extract. Various biological activities of extracts generated using various extraction techniques have been described in numerous research. The process of removing bioactive components from plants involves many steps, such as grinding, homogenization, and extraction. To collect and isolate bioactive compounds from plant material, extraction is a critical step. Under the same extraction conditions, solvent is recognized as an important parameter (Do et al., 2014). In this research, four solvents, methanol, acetone, chloroform, and n-hexane, were used to obtain the extracts from the selected plant.
In the current study, a higher extraction yield was observed in the methanolic and acetone extracts as compared to chloroform and n-hexane, which indicated that the extraction efficiency favors the polarity of the solvent.
The phytochemical composition of the extracts varied according to their polarity. The greatest amounts of phenolics, alkaloids, flavonoids, and terpenoids were found in methanolic extracts of all of the plants studied, consequential in the chief presence of the methanolic extract.
Because of the diversity of their constituent biological and structural elements, common bioactive chemicals, whether in their raw or pure form, offer unique opportunities for innovative drug detection. Alkaloids, saponins, terpenes, and polyphenols are only a few examples of the diverse secondary metabolites that are produced exclusively by plants for a variety of functions, including as a toxicant, a chemical defense against microbial attack, an attractant for pollinators, and an insect repellent (Jan et al., 2021). Among these, phenolics and flavonoids are the prevalent, most varied, and most considered group of phytochemicals. These are vital components of both animal diets and human are safe to be utilized (Phuyal et al., 2020).
In the same pattern of solvents like methanol, acetone, chloroform, and n-hexane, J. adhatoda also displayed a sizeable amount of bioactive components, TPC and TFC. Numerous phytochemical components with properties including antitussive, antibacterial, abortifacient, anti-inflammatory, cardiovascular protection, anticholinesterase, and other noteworthy actions have been isolated from J. adhatodain literature. Even though the presence of small amounts of active chemicals that cause physiological effects in both human and animal bodies is what gives this plant its medicinal value. Quinazoline alkaloids and essential oils are two notable bioactive substances that have been found in various parts of J. adhatoda. Thus, J. adhatoda extract could form one of the preeminent options for developing natural narrative medicine (Dhankhar et al., 2017).
Four extracts of medicinal plants were tested for chemical profiling using HPLC in this present investigation. Fig. 2(A-D) demonstrates quantitative and qualitative analyses of bioactive and phenolic acid constituents in plant extracts. Chromatograms HPLC of chosen medicinal plants showed the presence of. caffeic acid, gallic acid, coumaric acid, quercetin, ferulic acid, vanillic acid, sinapic acid and chlorogenic acid, in all the medicinal plants. Which are significant flavonoid and polyphenolics having strong antioxidant characteristics (Güleç and Demirel, 2016).
Results obtained revealed that the concentration of physiologically active phenolic acid and flavonoid in chosen medicinal plants was higher. Many of the polyphenolic and flavonoids derived from medicinal plant products have been reported in many studies as powerful, effective antioxidants in vitro in comparison with vitamins C and E. Bioactive chemicals could thus make a substantial contribution to in vivo protective effects (Siddique et al., 2022).
Free radicals are thought to contribute negatively to the etiology of several severe and chronic diseases, such as atherosclerosis, aging, diabetes, neurodegeneration, and immunological suppression, according to the literature (Nimse and Pal, 2015). Numerous studies have emphasized the presence of antioxidants in foods such as medicinal herbs, fruits, and vegetables, including phenolics, terpenoids, flavonoids, and tannins. These antioxidants defend and support human health by scavenging ROS (Vijayalakshmi and Ruckmani, 2016). Antioxidants are efficient ROS scavengers and they can serve as capable defensive mediations for many ailments (Gulcin, 2015).
The graphical representation showed that all extracts of J. adhatoda demonstrated substantial radical free scavenging activity in a concentration-dependent pattern, i.e., plant extracts demonstrated increased antioxidant capacity at enhancing concentrations of plant extracts. Previous studies have demonstrated dose-dependent effects on the antioxidant activity exhibited by various plant extracts (Andleeb et al., 2020a). Our results corroborate this observation, showing a similar trend. We observed increased levels of phenolic compounds, including phenolic acids, phenolic diterpenes, and flavonoids, which may contribute to enhanced antioxidant capacity. These phenolic compounds, especially O-dihydroxyl-rich phenolic compounds, are known for their powerful antioxidant properties (Andleeb et al., 2020).
Not a single report has been found on the determination of the in-ovo activity of the J. adhatoda against Newcastle disease in chicken embryonated eggs earlier. It was a pleasant experience to find these plants as antiviral agents against viral disease because of their myriad significance medically and economically.
At the current time, plant-related pharmaceuticals are rated highly for viral infections. The plant extract was found to be effective if it prevented viral replication in the embryo cells, allowing for embryo growth, and/or if it reduced the viral burden in ECE, preventing embryo mortality. The NDV replication inside the cytoplasm of embryo cells results in a disease. The NDV invasion into the embryo cell is made possible by the working of the spikes glycoprotein fringe found on the virus's envelope (Aati et al., 2022). Their methods of producing disease are always predicated on their ability to replicate via the metabolic pathways of embryo cells, which typically results in multicellular animals suffering death and cell death (Faeji et al., 2017).
The current studies demonstrated that the positive control (VC) showed 100 % mortality and the HA titre 2048 with serial dilutions, proving the presence of virus coagulation with RBCs. The extract control (EC) group cleared that the plant extracts did not have any toxic effect on the growth of embryos. The survival of uninoculated group eggs showed the environment was favorable for the growth of embryos. Only 400 μg/mL of methanolic dose-treated ECEs show a 100 % survival rate. Other extracts and concentrations were not able to inhibit the virus growth completely, as compared to control groups. Current findings are in line with the results of the antiviral activity of some plants in literature, Andleeb et al. (2020a) demonstrated that ethanolic extract at 400 mg/mL and acetonic extract at 300 mg/mL of I. herbstii controlled the NDV replication completely. It was evidenced by the absence of embryo death and HA titre. 600 mg/mL concentration was proved as lethal in all extracts and petroleum ether extract showed a dependent dose pattern to decrease the death.
It is conceivable that there are additional components in a plant extraction that aid in controlling the virus, including components that promote tissue healing and immune modulation, which explains why traditional treatments required the preparation of a concoction of various plants in order to maximize the beneficial effects of a medicinal plant arrangement. However, it has been discovered that certain conventionally used medical herbs for the treatment of viral infections contain a variety of compounds. These substances/ingredients include, but are not limited to, alkaloids, terpenes, coumarins, flavonoids, naphthoquinones, anthraquinones, and their subclasses (Sulaiman et al., 2011). These compounds put forth their effects by killing the virus and interfering with the replication of the virus (Andleeb et al. (2020b)). Particularly, some substances exhibit protease inhibition and cleavage of HN and F proteins. These proteins are essential for NDV replication and attachment.
The in-silico studies evaluating the binding efficacy between protein receptors and plant compounds showed that the 1G5G receptor protein exhibited the best interaction. The GOLD fitness was 42.08 and the docking score was −7.35, including the formation of hydrogen bonds (LYS1063, GLN1062, THR58, GLU476, and ASN1064). These findings highlight the potential of computational tools in drug design for compound optimization and virtual screening to discover bioactive molecules. However, further studies are needed to explore the pharmacokinetic characteristics and in vivo use of these compounds.
5 Conclusion
The consequences of the current study propose that the medicinal plant Justicia adhatoda, possesses significant phytochemical profiling responsible for pharmacological and therapeutic activities. The methanol extract showed good total phenolic content (TPC) and total flavonoid content (TFC). High-performance liquid chromatography (HPLC) analysis of all extracts revealed the putative presence of noteworthy phytochemicals, confirming their biological properties. Furthermore, the methanol extract showed significant antiviral potential against Newcastle disease virus La-Sota strain in embryonated eggs. The molecular docking of in-silico studies additionally explained the interactions between receptor protein (1G5G) and phytochemical. Based on the results of in-vitro, in-vivo and in-silico docking studies, further studies are needed to evaluate its clinical studies and toxicity profile. Ultimately, the results of this study may provide valuable insights to researchers actively engaged in developing effective innovative drugs derived from natural products to combat Newcastle disease. The observed biological potential and phytochemical composition of this plant indicate its importance for the subsequent isolation of bioactive compounds.
CRediT authorship contribution statement
Rahat Andleeb: Conceptualization, Data curation, Writing – original draft. Nimrah Zafar: Formal analysis, Validation, Visualization, Writing – review & editing. Muhammad Umar Ijaz: Data Collection, Resources, Software, Writing – original draft. Sarfaraz Ahmed: Data curation, Funding acquisition, Project administration, Resources, Software, Writing – review & editing. Derya Karataş Yeni: Investigation, Methodology, Resources, Writing – original draft. Aliza Mazhar: Conceptualization, Data curation, Methodology, Writing – original draft. Asma Ashraf: Conception, Investigation, Resources, Writing – review & editing, Supervision, Project administration. Mahboob Alam: Data curation, Formal analysis, Validation, Writing – review & editing.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R380), King Saud University, Riyadh, Saudi Arabia. This research work was accomplished with the funds provided by Higher Education Commission (HEC, Pakistan) under NRPU Project No. 5647/Punjab/NRPU/R&D/HEC/2016.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aati, H.Y., Anwar, M.;,Al-Qahtani, J., Al-Taweel, A., Khan, K.-u.-R., Aati, S., Usman, F., Ghalloo, B.A., Asif, H.M., Shirazi, J.H., 2022. Phytochemical profiling. In: Vitro Biological Activities, and In-Silico Studies of Ficus vasta Forssk.: An Unexplored Plant. Antibio. 11, 1155.
- Chemical constituents and their biological activities from Taunggyi (Shan state) medicinal plants. Heliyon.. 2021;7:e06173.
- [Google Scholar]
- Analysis of bioactive composites and antiviral activity of Iresine herbstii extracts against Newcastle disease virus in ovo. Saudi J. Biol. Sci.. 2020;27:335-340.
- [Google Scholar]
- Andleeb, R., Ashraf, A., Ijaz, M.U., Sultana, T., Asad, F., Islam, B., Wajid, S.A., 2020b. In vitro antioxidant, hemolytic, thrombolytic potencies of centratherum anthelminticum seed extractsand it’s in ovo antiviral efficacy. Pak. J. Agri. Sci. 57. 10.21162/PAKJAS/20.112.
- Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone: Combination therapy with phytochemicals. Cancer. 2019;125:1600-1611.
- [Google Scholar]
- Effect of seasonal and regional variations on phenolic compounds of Deverra scoparia (flowers/seeds) methanolic Extract and the evaluation of its in vitro antioxidant activity. Chem. Biodivers.. 2019;16:e1900420.
- [CrossRef] [Google Scholar]
- Xylocarpus moluccensis induces cytotoxicity in human Hepatocellular Carcinoma HepG2 cell line via activation of the extrinsic pathway. Asian Pac. J. Cancer Prev.. 2021;22:17-24.
- [Google Scholar]
- Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem.. 2017;10:S1193-S1199.
- [Google Scholar]
- A review on Justicia adhatoda: A potential source of natural medicine. Afr. J. Plant Sci.. 2011;5:620-627.
- [Google Scholar]
- The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf.B: Biointerfaces. 2012;98:112-119.
- [Google Scholar]
- Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal.. 2014;22:296-302.
- [Google Scholar]
- In-ovo biological activities of Phyllanthus amarus leaf extracts against Newcastle disease virus. J. Med. Plants Res.. 2017;11:419-425.
- [Google Scholar]
- Phytochemical profiling, in vitro biological activities, and in silico molecular docking studies of dracaena reflexa. Mol. 2022;27:913.
- [Google Scholar]
- Fe3+–Fe 2+ transformation method: an important antioxidant assay. In: Advanced Protocols in Oxidative Stress III. New York, NY: Humana Press; 2015. p. :233-246.
- [Google Scholar]
- Characterization and antioxidant activity of quercetin/methyl-β-cyclodextrin complexes. Curr. Drug Deliv.. 2016;13:444-451.
- [Google Scholar]
- Phytochemicals: Extraction methods, identification and detection of bioactive compounds from plant extracts. J. Pharmacogn. Phytochem.. 2017;6(1):32-36.
- [Google Scholar]
- Plant secondary metabolite Biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agron. 2021;11:968.
- [Google Scholar]
- Antibacterial, antifungal, antiviral, and anthelmintic activities of medicinal plants of Nepal selected based on ethnobotanical evidence. Evid. Based Complement. Altern. Med.. 2020;2020:1043471.
- [Google Scholar]
- Comparative analysis of the efficiency of medicinal plants for the treatment and prevention of COVID-19. Intern. J. Biomater.. 2022;2022
- [CrossRef] [Google Scholar]
- Evaluation of anti-diabetic activity of Justicia adhatoda (Linn.) leaves in diabetic wistar rats. J. Univ. Coll. Med. Sci.. 2023;11:1-5.
- [Google Scholar]
- Chemical and toxicological effects of medicinal Baccharis trimera extract from coal burning area. Chemosphere. 2016;146:396-404.
- [Google Scholar]
- Miller, P. J., Koch, G., 2013. Newcastle disease. Diseases of Poultry, 13th ed.(Swayne, DE, Glisson, JR, McDougald, LR, Nolan, LK, Suarez, DL and Nair, VL eds.), John Wilkey and Sons, Inc., Ames, 89-107.
- Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ.. 2021;801:149719
- [Google Scholar]
- In-silico analysis of non-synonymous-SNPs of STEAP2: to provoke the progression of prostate cancer. Open Life Sci.. 2016;11(1):402-416.
- [Google Scholar]
- A vaccine construction against COVID-19-associated mucormycosis contrived with immunoinformatics-based scavenging of potential Mucoralean epitopes. Vaccines. 2022;10(5):664.
- [Google Scholar]
- Artificial intelligence assisted pharmacophore design for Philadelphia chromosome-positive leukemia with gamma-tocotrienol: A toxicity comparison approach with Asciminib. Biomedicines. 2023;11(4):1041.
- [Google Scholar]
- Newcastle disease virus in poultry with an interface as a human vector. Vet. Vacc. 2022:100003.
- [CrossRef] [Google Scholar]
- Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv.. 2015;5:27986-28006.
- [Google Scholar]
- OIE (2013). Manual of diagnostic tests and vaccines for terrestrial animals. Office International des Epizooties, 7th ed. Paris, pp. 1404.
- Antibacterial activities of crude extract of Aloe barbadensis to clinically isolated bacterial pathogens. App. Biochem. Biotech.. 2010;160:1356-1361.
- [Google Scholar]
- Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. The Sci. World J.. 2020;2020
- [CrossRef] [Google Scholar]
- Study of antiviral potential of cholistani plants against New Castle disease virus. Pak. J. Zool.. 2019;51:1-4.
- [Google Scholar]
- Integration of in silico and in vitro approaches to evaluate antioxidant and anticancer properties of Tribulus terrestris extracts. Arab. J. Chem.. 2022;15:103984
- [CrossRef] [Google Scholar]
- Study of in vitro antioxidant and DNA damage protection activity of a novel luteolin derivative isolated from Terminalia chebula. J. Taibah Uni. Sci.. 2019;13:755-763.
- [Google Scholar]
- Evaluation of the antimicrobial and antivirulent potential of essential oils isolated from Juniperus oxycedrus L. ssp. Macrocarpa aerial parts. Microorganism. 2022;10:758.
- [Google Scholar]
- The effect of extraction techniques on yield, extraction kinetics, and antioxidant activity of aqueous-methanolic extracts from nettle (Urtica dioica L.) leaves. Sep. Sci. Technol.. 2016;51:1817-1829.
- [Google Scholar]
- In-ovo evaluation of the antiviral activity of methanolic root-bark extract of the African Baobab (Adansonia digitata Lin) Afr. J. Biotech.. 2011;10:4256-4258.
- [Google Scholar]
- Plant phenolics and phenolic-enriched Extracts as antimicrobial agents against food-contaminating microorganisms. Antioxi.. 2020;9:165.
- [Google Scholar]
- Assessment of chemopreventive potential of the plant extracts against liver cancer using HepG2 cell line. Molecules. 2021;26:4593.
- [Google Scholar]
- Ferric reducing anti-oxidant power assay in plant extract. Bangladesh J. Pharma.. 2016;11:570-572.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103163.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







