Translate this page into:
In vitro free radical scavenging activities of aerial parts’ aqueous extract and extract fractions of Ampelocissus latifolia (Roxb.) Planch. in relation to total phenolics and flavonoid contents
⁎Corresponding author. sray@zoo.buruniv.ac.in (Sanjib Ray)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Discovery of new antioxidants of plant origin is of current interest. Ampelocissus latifolia (Roxb.) Planch. is a traditionally used medicinal plant. The present study was aimed to investigate the in vitro free radical scavenging activities of aerial parts’ aqueous extract of A. latifolia (AAEAL) and its organic solvent extract fractions in relation to their total phenolics and flavonoid contents. The AAEAL was fractionated with the organic solvents like petroleum ether (AAEALPE), chloroform (AAEALCH), ethyl acetate (AAEALEA), and n-butanol (AAEALNB). For the determination of antioxidant activity of AAEAL and its extract fractions, Fe3+ ion reducing antioxidant power (FRAP), 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging, and total antioxidant assays were performed and the total phenolics and flavonoid contents were also estimated. The EC50 of the ferric ion reduction by AAEALEA and ascorbic acid were calculated as 90.8 ± 4.70 and 84.86 ± 7.22 µg/mL respectively. The IC50 of DPPH free radical scavenging with AAEALEA and ascorbic acid were calculated as 19.5 ± 0.5 and 18.35 ± 1.15 µg/mL respectively. The overall data indicate that the AAEALEA possesses the highest total antioxidant activity (37.85 ± 1.55 µg ascorbic acid equivalents/100 µg dry extract matter), the highest total phenolics (65.89 ± 1.33 mg tannic acid equivalent phenolics/100 mg dry extract) and flavonoid (30.67 ± 0.28 mg quercetin equivalent flavonoids/100 mg dry extract) contents. The antioxidant activities were also positively correlated with the total phenolics and flavonoid contents of the extract fractions. In summary, AAEAL may be considered as a natural source of antioxidants and its ethyl acetate extract fraction seems to be equally effective to the ascorbic acid as an antioxidant.
Keywords
AAEAL
Ampelocissus latifolia
Antioxidant
DPPH
Phenolics
1 Introduction
The excess of the free radicals may lead to the development of various oxidative stress-related disorders like cancer, anemia, cardiovascular diseases, diabetes, inflammation etc. (Llopiz et al., 2004). In aerobic organisms, the presence of various antioxidative enzymes like catalase, glutathione peroxidase, superoxide dismutase, and glutathione reductase protect the cells from free radical-mediated damage. When the production of the free radicals is increased, and the cellular antioxidant system cannot function properly, the cells fail to scavenge the free radicals and may cause DNA damage, lipid peroxidation, and oxidative damage to the cells. The research works are being conducted worldwide to identify the plant-derived antioxidants for their ability to scavenge free radicals. The plant-derived natural products are widely used as a source of traditional medicine to treat various disease conditions (Razali et al., 2008). The plant secondary metabolites are the rich source of antioxidants. It has been demonstrated that the regular consumption of various fruits, vegetables, cereals, and grains is associated with the low level of oxidative damages and exerts protective roles against the various chronic diseases like cardiovascular diseases (CVD), diabetes, cancers, neurodegenerative diseases etc. (Kruk, 2014; Kyro et al., 2013; Mursu et al., 2014; Yamada et al., 2011).
The plant-derived antioxidants are very important for their applications in functional foods and nutraceuticals. Several experiments have been done globally to determine the antioxidant and free radical scavenging activities of plant extracts to find out the safe and potent natural antioxidants of plant origin. The fruits like berries, grapes, dates, plums etc. and vegetables like broccoli, colored cabbages, cowpea, sweet potato, ginseng leaf, pepper leaf etc. are rich in antioxidants (Deng et al., 2012; Fu et al., 2011; Manganaris et al., 2014; Xia et al., 2010). The antioxidant compounds like polyphenols and flavonoids are widely distributed in nature and have gained a renewed interest because of their physiological functions like antimutagenic and antitumor activities (Li et al., 2009; Othman et al., 2007). The plant polyphenols are the most abundant antioxidants in human diets. Their free radical scavenging activities are attributed to their disease preventive actions (Rokayya et al., 2013). The various studies have revealed the fact that there is a positive correlation between the total phenolic content and antioxidant potentials of plant extracts (Dutta and Ray, 2015; Goswami and Ray, 2017; Sun et al., 2002).
Ampelocissus latifolia (Family: Vitaceae) is a native herb of Indian subcontinent and is widely prescribed in traditional medicine to treat dental troubles, ulcers, dysentery (Patil and Patil, 2012), gout, fractured bone, dyspepsia, indigestion, and tuberculosis (Prusti and Behera, 2007; Swarnkar and Katewa, 2008). It is used as an antidote for snake bite, applied on wounds, abscess, and for easy labor and delivery of a baby (Patil and Patil, 2005). Ampelocissus latifolia also exhibits antibacterial (Pednekar and Raman, 2013), antiproliferative, cytogenotoxic (Chaudhuri and Ray, 2014; 2015a), anti-inflammatory (Tamilarashi et al., 2000), and allelopathic activities (Chaudhuri et al., 2015; Chaudhuri and Ray, 2015b; Chaudhuri and Ray, 2016). There is a report on the antioxidant activity of the crude methanolic extract of this plant (Pednekar and Raman, 2013). The phytochemical analysis revealed the presence of various compounds like phenolics, flavonoids, terpenoids, anthraquinones etc. having antioxidant activities (Pednekar and Raman, 2013). Recently, a report on exhaustive analysis of A. latifolia fruit extracts has shown the presence of abundant phenolics and flavonoids imparting various bioactivities including excellent free radical scavenging and ferric reducing activities, anti-elastase, anti-collagenase, anti-tyrosinase, and anti-inflammatory activities using in vitro assays (Singh et al., 2015). However, in the present state of knowledge, the antioxidant activity of the aerial parts’ aqueous extract of A. latifolia (AAEAL) and identification of the most active fraction in terms of antioxidant activity is yet to explore. Keeping this in the background, the present study was undertaken to investigate the in vitro antioxidant potentials of AAEAL and to determine its antioxidant-active fractions. The novel aspect of the present study is that, here we have initially extracted phenolics with polar hot water (AAEAL) to determine antioxidant potentials and then AAEAL was successively fractionated using the different organic solvents. Moreover, the total phenolics and flavonoid contents in the aqueous extract and its extract fractions were quantified and were also correlated with their antioxidant potentials.
2 Materials and methods
2.1 Chemicals
Quercetin, DPPH, ascorbic acid, ferric chloride, and trichloroacetic acid were obtained from Sigma-Aldrich, St. Louis, M.O., USA. Tannic acid was purchased from HIMEDIA Laboratories Pvt. Ltd., Mumbai, India. Folin-Ciocalteu’s phenol and aluminum chloride were obtained from MERCK Specialities Pvt. Ltd., Mumbai, India. Other chemicals used here were of analytical grade.
2.2 Collection of aerial parts of A. latifolia
Fresh plant materials (leaf and creeper stem) were collected from The Burdwan University campus, West Bengal and identified by renowned Taxonomist, Prof. Ambarish Mukherjee, The University of Burdwan. The voucher specimens (No. BUGBAC012) are maintained in the Department of Zoology, The University of Burdwan for future reference. The collected plant material was washed, shade dried, powdered, and stored in an airtight container for future use.
2.3 Crude aqueous extract preparation and fractionation
The 20 g ground plant material was extracted in 400 mL of distilled water at low heat (50 °C) in a water bath and after every 2 h, the extract was filtered through No. 1 Whatman® filter paper. The process was repeated 3 times to get a bulk amount of the extract (coded as AAEAL for aerial parts’ aqueous extract of A. latifolia) which was then concentrated using a vacuum evaporator and stored at −20 °C for future use. The yield of AAEAL was 29.97 ± 1.30% of the dried powdered plant matter.
The AAEAL was fractionated sequentially with petroleum ether, chloroform, ethyl acetate, and n-butanol to get petroleum ether fraction, AAEALPE; chloroform fraction, AAEALCH; ethyl acetate fraction, AAEALEA; n-butanol fraction, AAEALNB, and the remaining aqueous fraction i.e. AAEAL remaining fraction respectively. The extract fractions were completely dried in a vacuum evaporator and then stored at 4 °C. The yields of the extract fractions were measured as 0.19 ± 0.004, 0.64 ± 0.002, 3.34 ± 0.04, 8.06 ± 0.02, and 86.03 ± 0.15% of the dried extract matter for AAEALPE, AAEALCH, AAEALEA, AAEALNB, and AAEAL remaining fraction respectively.
2.4 In vitro antioxidant assays
2.4.1 Ferric ion reducing antioxidant power (FRAP) assay
The reductive capability of AAEAL and its fractions were investigated and compared with the standard ascorbic acid by measuring the Fe3+-Fe2+ transformation (Oyaizu, 1986). Phosphate buffer (0.20 M, 1 mL), and K3Fe(CN)6 (1%, 1 mL) were added to the sample (0.50 mL) and incubated at 50 °C for 20 min. After that, trichloroacetic acid (10%, 1 mL) was mixed to it and then centrifuged at 3000 rpm for 10 min. 1.50 mL supernatant solution was added to 1.50 mL of distilled water and 0.10 mL of FeCl3 (0.10%) solution. The absorbance was recorded at 700 nm using a spectrophotometer (UV-1800 Series, Shimadzu, Japan).
2.4.2 DPPH free radical scavenging assay
The antioxidant capacity of AAEAL and its extract fractions were determined by DPPH free radical scavenging assay (Ayoola et al., 2008) and were compared with the standard ascorbic acid. Methanol (3 mL) and DPPH solution (0.002%, 1 mM, 0.50 mL) were mixed with 1 mL of different concentrations (5, 10, 15, 20, 25, 50 and 100 μg/mL) of the sample solution. The whole reaction mixture was kept in dark for 30 min and after that, the absorbance was measured at 517 nm using a spectrophotometer (UV-1800 Series, Shimadzu, Japan).
The decrease of the absorbance indicates an increase in the radical-scavenging activity. The IC50 values (concentration at which 50% scavenging occurs) were calculated from the graph between the inhibition (%) versus the concentrations of the samples. The DPPH free radical-scavenging activity was calculated as [A0 = absorbance of the control (without extract), A1 = absorbance in the presence of the extract].
2.4.3 Total antioxidant capacity assay
The phosphomolybdate assay is a widely accepted method for the determination of the total antioxidant capacity of plant extracts. The basic principle is that, here, Mo (VI) is reduced to Mo (V) by the sample analyte and a green colored phosphate Mo (V) complex forms at the acidic range of pH (Umamaheswari and Chatterjee, 2008). The total antioxidant potentials of the different extract fractions of AAEAL were measured and compared with standard ascorbic acid. The sample solution (100 μg/mL) was mixed with 3 times volume of the reagent mixture containing sulfuric acid (0.60 M), ammonium molybdate (4 mM), and sodium phosphate (28 mM) and incubated at 95 °C for 90 min. The absorbance was recorded at 695 nm using a spectrophotometer (UV-1800 Series, Shimadzu, Japan) and the data are represented in terms of µg ascorbic acid equivalents/100 µg dry extract matter.
2.5 Quantitative analysis of the antioxidant phytochemicals
2.5.1 Total phenolic content (TPC)
The total phenolic content of AAEAL and its extract fractions were estimated with Folin-Ciocalteu’s phenol reagent (FCP) (Makkar et al., 1993; Ray et al., 2013). 10 μL of sample solution of the extract/extract fractions was added to 990 µL distilled water and 0.50 mL FCP reagent (1 N) and were mixed thoroughly. 2.50 mL 20% Na2CO3 solution was added, mixed properly and kept for 40 min at room temperature (25 ± 2 °C). The absorbance was recorded at 725 nm using a spectrophotometer (UV-1800 Series, Shimadzu, Japan). The TPC was estimated as tannic acid equivalent (TAE) using the tannic acid (TA) standard curve (2.50–25 μg/mL). Data are represented as Mean ± SEM of nine sets of experiments.
2.5.2 Total flavonoid content (TFC)
The total flavonoid content of the extract/extract fractions was estimated by AlCl3 method (Chang et al., 2002; Chaudhuri and Ray, 2015a). Distilled water (1 mL) and NaNO2 (5%, 1.50 mL) were added to the sample solution (0.50 mL) and allowed to settle for 5 min. Later on, AlCl3 (10%, 0.15 mL) was added followed by the addition of NaOH solution (1 M, 1 mL). Finally, distilled water (0.85 mL) was added. The absorbance reading was taken at 510 nm using a spectrophotometer (UV-1800 Series, Shimadzu, Japan). Here, flavonoid content was estimated as quercetin equivalent (QE) using a standard quercetin calibration curve. The data are represented as Mean ± SEM of nine sets of experiments.
3 Statistical analysis
All the assays were performed in triplicate (unless stated otherwise) and the data points are expressed as Mean ± SEM (Origin software 6.0). The statistical significance of the differences was determined by one-way analysis of variance (ANOVA) at a confidence level of 95% (pα = 0.05) followed by the Post Hoc Tukey test (pα = 0.05). The correlation coefficient (R) and coefficient of determination (R2) between the total phenolics as well as flavonoids and the antioxidant activity was carried out using the Microsoft Office Excel 2007. Moreover, regression analysis was performed (pα = 0.05) to determine the level of significance of correlation.
4 Result and discussion
4.1 In vitro antioxidant assays
4.1.1 FRAP assay
The data indicate a concentration-dependent (10–100 µg/mL) increase in the FRAP activities of the AAEAL and its extract fractions as shown by their increasing OD values (Table SI). The half maximum effective reducing power concentrations (EC50) for ascorbic acid (84.86 ± 7.22 μg/mL) was found to be lower than that for AAEAL (193.06 ± 9.51 μg/mL) which is a crude extract having a combination of phytochemicals, some with oxidative or some with antioxidant actions (Chaudhuri and Ray, 2014; Dutta and Ray, 2015). The AAEAL was further fractionated sequentially with petroleum ether (AAEALPE), chloroform (AAEALCH), ethyl acetate (AAEALEA), and n-butanol (AAEALNB) to isolate the bioactive fractions (Li et al., 2009). The fractions were checked for FRAP activities and their EC50 values were compared (Table SI, Fig. 1). The EC50 values for AAEALPE, AAEALCH, AAEALEA, AAEALNB, and AAEAL remaining fraction were calculated as 367.02 ± 21.15, 259.67 ± 13.35, 90.8 ± 4.70, 104.80 ± 5.29, and 139.17 ± 6.99 μg/mL respectively.![EC50 values (μg/mL) of FRAP activity of ascorbic acid, AAEAL and its fractions. Assays were done in triplicate and the data are expressed as Mean ± SEM. The level of confidence was considered at 95% (pα = 0.05) (ANOVA) for the determination of differences among the groups, followed by Post Hoc Tukey test [*p < 0.05].](/content/185/2020/32/1/img/10.1016_j.jksus.2018.12.006-fig1.png)
EC50 values (μg/mL) of FRAP activity of ascorbic acid, AAEAL and its fractions. Assays were done in triplicate and the data are expressed as Mean ± SEM. The level of confidence was considered at 95% (pα = 0.05) (ANOVA) for the determination of differences among the groups, followed by Post Hoc Tukey test [*p < 0.05].
There was no significant difference in the antioxidant activities among the AAEALEA, AAEALNB, and the remaining fraction. The antioxidant activity of AAEALEA is significantly higher than the AAEAL, AAEALPE, and AAEALCH fractions, but no significant difference was present between the antioxidant activity of ascorbic acid and AAEALEA, indicating the AAEALEA is a potential fraction having ascorbic acid equivalent antioxidant activity (Fig. 1).
4.1.2 DPPH assay
The DPPH assay is used for the determination of the free radical scavenging activity of any agents. In the presence of an antioxidant compound, DPPH, a stable free radical, is reduced and it changes color from purple to yellow due to the formation of 1,1-diphenyl-2-picryl hydrazine from the hydrazyl crystal. The extent of discoloration indicates the free radical scavenging potentials. Results indicate a concentration-dependent increase in DPPH free radical scavenging activity of the extract/extract fractions. Here, the concentration of AAEAL was required nearly 2.40 times (Fig. 2) higher than that of the ascorbic acid (Ascorbic acid, IC50: 18.35 ± 1.15 µg/mL; AAEAL, IC50: 44.85 ± 4.95 µg/mL), which may be due to AAEAL being in a crude form. There are similar reports showing the antioxidant activity of various crude extracts (Ayoola et al., 2008; Deng et al., 2012; Dutta and Ray, 2015, 2020; Fu et al., 2011.).![IC50 values (μg/mL) of DPPH free radical scavenging activity of ascorbic acid, AAEAL, and different fractions of AAEAL. Assays were done in triplicate and the data are expressed as Mean ± SEM. The level of confidence was considered at 95% (pα = 0.05) (ANOVA) for the determination of differences among the groups followed by Post Hoc Tukey test [*p < 0.05].](/content/185/2020/32/1/img/10.1016_j.jksus.2018.12.006-fig2.png)
IC50 values (μg/mL) of DPPH free radical scavenging activity of ascorbic acid, AAEAL, and different fractions of AAEAL. Assays were done in triplicate and the data are expressed as Mean ± SEM. The level of confidence was considered at 95% (pα = 0.05) (ANOVA) for the determination of differences among the groups followed by Post Hoc Tukey test [*p < 0.05].
Comparison of the DPPH scavenging activities of fractionated extracts revealed that some of the fractions possess higher antioxidant activity than the AAEAL itself in the decreasing order of AAEALEA (IC50 = 19.50 ± 0.50 µg/mL) > AAEALNB (IC50 = 34.95 ± 1.05 µg/mL) > remaining fraction of AAEAL (IC50 = 36.15 ± 2.85 µg/mL) (Fig. 2). The AAEALCH and AAEALPE possess a lower free radical scavenging activity than the AAEAL (Figure S1), indicating a differential free radical scavenging activity of the different extract fractions of AAEAL. Here, AAEALEA showed the maximum free radical scavenging activity. The one way analysis of variance (ANOVA) at the confidence level of 95% (pα = 0.05), followed by the Post Hoc Tukey test [*p < 0.05] was done to test whether any significant differences were present among the free radical scavenging activities of the different extract fractions (Fig. 2). The plant crude extract contains many types of phytochemicals, while each fraction obtained by the fractionation method yields only a few types of molecules and this is mainly due to the solvent polarity and affinity of the compounds for specific solvents (Umamaheswari and Chatterjee, 2008). Here, the IC50 value of AAEALEA (19.5 ± 0.50 µg/mL) was found to be more than two times lower than that of the crude AAEAL (44.85 ± 4.95 µg/mL), indicating that the effective antioxidants were extracted in ethyl acetate fraction of AAEAL. Like FRAP assay, the data of DPPH assay also indicate that AAEALEA is the best depository of phytochemicals having antioxidant activity. A similar report also exists showing that fractionation of hydromethanolic extract of Coccinia grandis resulted in the differential antioxidant potential of the different extract fractions (Umamaheswari and Chatterjee, 2008).
4.1.3 Total antioxidant capacity assay
Here, the total antioxidant capacity was analyzed through the phosphomolybdate assay, which is a widely used method for the determination of the total antioxidant potential of plant extracts (Umamaheswari and Chatterjee, 2008). Data indicate a decreasing order of total antioxidant capacity of the extract fractions as AAEALEA > AAEALNB > AAEAL remaining fraction > AAEALCH > AAEALPE. The AAEALEA showed a significantly higher (*p < 0.05, analyzed by one way ANOVA followed by Post Hoc Tukey test) antioxidant capacity (37.85 ± 1.55 µg ascorbic acid equivalents/100 µg dry extract) than the other extract fractions (Fig. 3). Previously Umamaheswari and Chatterjee (2008) reported the differential total antioxidant activity of the petroleum ether, chloroform, ethyl acetate, and remaining extract fractions of the methanolic extract of Coccinia grandis. These differences in the total antioxidant property of the extract fractions may be due to the variation in chemical nature and the phenolics content.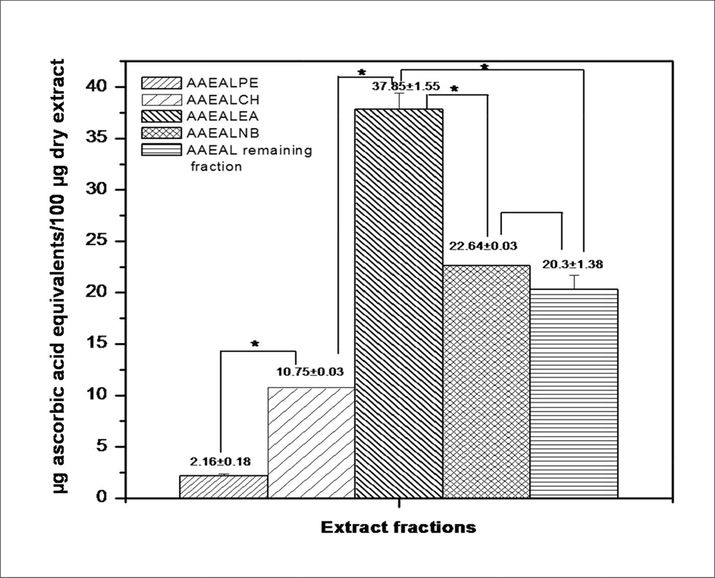
The total antioxidant activity (phosphomolybdate assay) of the successive organic solvent extracts of AAEAL. Assays were performed in triplicate and the data are expressed as Mean ± SEM. The level of confidence was considered at 95% (pα = 0.05) by ANOVA for the determination of differences among the groups. The Post hoc Tukey test shows a significant difference (*p < 0.05) between the two fractions.
4.2 Quantitative estimation of total phenolics and flavonoid contents
The high level of antioxidant property of the plant extracts largely depends on their polyphenols and flavonoid contents (Razali et al., 2008) and they act as hydrogen or electron donors, metal ion chelators, and also exhibit considerable free radical scavenging activities. The total phenolics and flavonoid contents of AAEAL and its extract fractions were estimated and the results indicate that AAEAL contains 22.58 ± 1.24 mg TAE TP/100 mg dry extract and 4.81 ± 0.77 mg QE flavonoids /100 mg dry extract (Fig. 4). The fractionation of AAEAL with the different solvents resulted in partitioning of phytochemicals in accordance with the polarity matching, where phenolics and flavonoids were mostly extracted in ethyl acetate fraction, AAEALEA, (65.89 ± 1.33 mg TAE TP/100 mg dry extract, 30.67 ± 0.28 mg QE flavonoids/100 mg dry extract) (significantly higher value from the other fractions as determined by one way ANOVA followed by Post Hoc Tukey test at *p < 0.05), followed by AAEALNB, AAEAL remaining fraction, AAEALCH, and AAEALPE. The results also reveal that there is no significant difference between AAEALNB and AAEAL remaining fraction in terms of their flavonoid contents.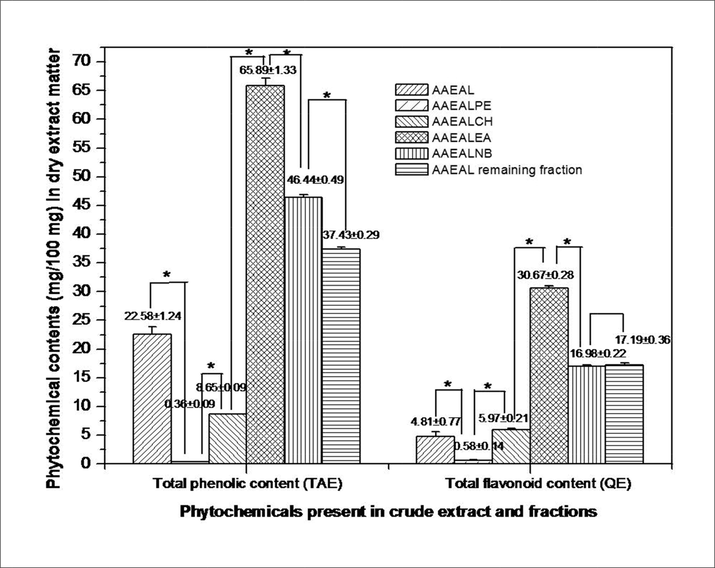
The total phenolics and flavonoid contents in AAEAL and its extract fractions. The data are expressed as Mean ± SEM of nine sets of experiments. The Post hoc Tukey test shows a significant difference (at *p < 0.05) between the two fractions regarding both total phenolics and flavonoid contents.
4.3 Correlation analysis
The polyphenols and flavonoids are the plant secondary metabolites that are ubiquitously present in almost all plants. There exists a positive correlation between the consumption of phenolic-rich plant products and the reduced incidence of cancer (Hollman and Katan, 1999). Here, the correlation and regression analysis revealed a linear negative correlation between the EC50 values of the FRAP activity of the different extract fractions of AAEAL and the total phenolics (R = 0.945, R2 = 0.893, p < 0.05) and flavonoid (R = 0.910, R2 = 0.829, p < 0.05) contents (Fig. 5). A linear positive correlation was found in the DPPH free radical scavenging activity of the different extract fractions of AAEAL with their total phenolics (R = 0.953, R2 = 0.908, p < 0.05) and flavonoid (R = 0.904, R2 = 0.818, p < 0.05) contents (Fig. 5). The data indicate that phenolics and flavonoid compounds contributed 90.80 and 81.80% respectively for the scavenging property of the extract fractions. Similarly, the total antioxidant activity of the extract fractions of AAEAL also exhibited a linear positive correlation with the total phenolics (R = 0.978, R2 = 0.957, p < 0.05) and flavonoid (R = 0.995, R2 = 0.990, p < 0.05) contents, suggesting that phenolics and flavonoids contributed 95.70 and 99% respectively for the total antioxidant effect of the extract fractions (Fig. 5). Thus, the presence of a higher amount of phenolics and flavonoid in the extract fractions may have contributed directly to the in vitro antioxidant activities. The outcome of the present study is in accordance with the existing reports indicating a linear positive correlation between the total phenolics and the antioxidant activities (Dutta and Ray, 2015; Li et al., 2009; Singh et al., 2015).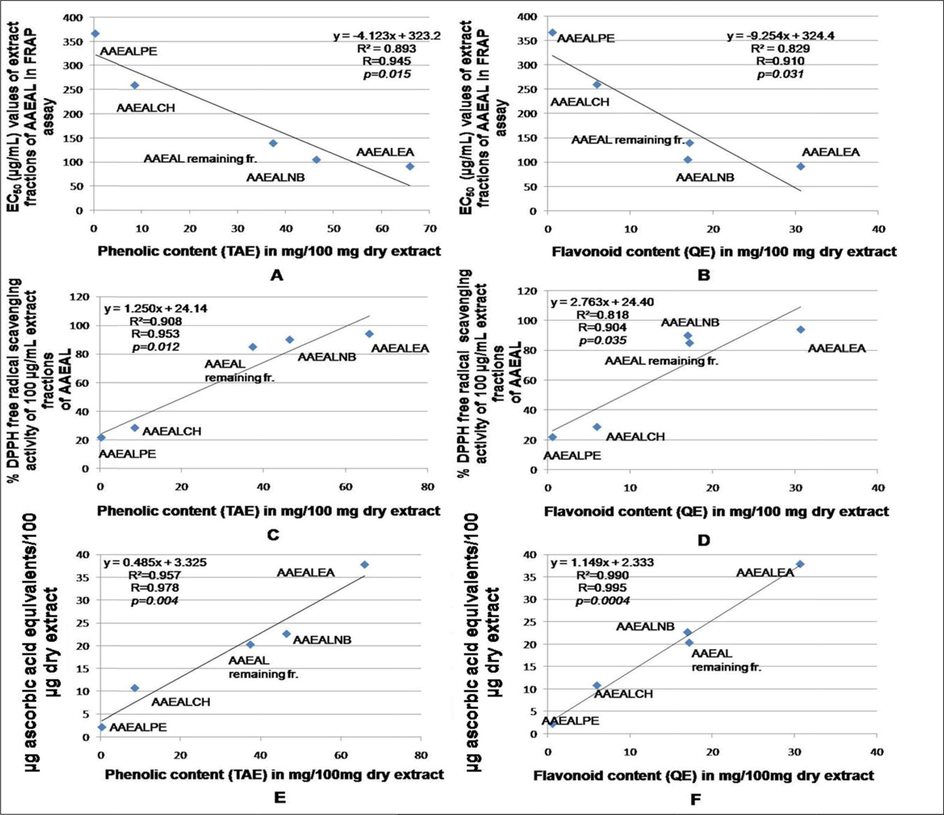
Correlation analysis between the phenolics and flavonoid contents and the in vitro antioxidant activities. Linear negative correlation between (A): total phenolics (X) and EC50 values of extract fractions in FRAP assay (Y); (B): total flavonoids (X) and EC50 values of extract fractions in FRAP assay (Y). Linear positive correlation between (C): total phenolics (X) and % DPPH scavenging activity (Y); (D): total flavonoids (X) and % DPPH scavenging activity (Y); (E): total phenolics (X) and µg ascorbic acid equivalents/100 µg dry extract (Y); (F): total flavonoids (X) and µg ascorbic acid equivalents/100 µg dry extract (Y) of the extract fractions.
5 Conclusion
At the present state of knowledge, this is the first report on the antioxidant properties of aqueous extract of Ampelocissus latifolia and its organic solvent extract fractions. The AAEAL and its extract fractions showed a concentration-dependent free radical scavenging activity. Amongst all the extract fractions of AAEAL, the ethyl acetate extract fraction exhibited the highest free radical scavenging and total antioxidant activities. Moreover, antioxidant activities of the AAEAL and its fractions were correlated positively with the total phenolics and flavonoid contents. The AAEAL may be considered as a source of antioxidants and its ethyl acetate fraction seems to be the most effective antioxidant fraction and is equally effective to the ascorbic acid as an antioxidant. Thus, the present study explores the antioxidant activity of the crude aqueous extract of A. latifolia and the relative antioxidant potentials of its extract fractions. However, before the therapeutic use of AAEALEA, a detailed investigation is required to explore the active principle(s), in vivo antioxidant validity, and toxicological assessment.
6 Disclosure statement
No conflict of interest was declared.
Acknowledgements
The authors acknowledge the financial support of UGC MRP [F.No. 42-563/2013 (SR) dt. 22.03.2013], the State Funded Fellowship [FC(Sc.)/RS/SF/ZOO./2011-2012/96(3) DATED 30.01.2012], and the DST-FIST, DST-PURSE, and UGC-DRS-sponsored infrastructural facilities in the Department of Zoology. Prof. A. Mukherjee has kindly authenticated the plant species and Dr. N.R. Datta has partly edited the language.
References
- Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res.. 2008;7(3):1019-1024.
- [Google Scholar]
- Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal.. 2002;10(3):178-182.
- [Google Scholar]
- Allelopathic effects of aerial parts’ aqueous extract of Ampelocissus latifolia (Roxb.) Planch. in apical meristem cells. Asian J. Plant Sci. Res.. 2015;5(3):11-16.
- [Google Scholar]
- Evaluation of phytotoxic and cytogenotoxic potentials of leaf aqueous extract of Ampelocissus latifolia (Roxb.) Planch. in relation to its total polyphenol content. Int. J. Pharma. Bio. Sci.. 2014;5(4):225-235.
- [Google Scholar]
- Antiproliferative activity of phytochemicals present in aerial parts’ aqueous extract of Ampelocissus latifolia (Roxb.) Planch. on apical meristem cells. Int. J. Pharma. Bio. Sci.. 2015;6(2):99-108.
- [Google Scholar]
- Determination of effective allelopathic (inhibitory) extract fractions of Ampelocissus latifolia (Roxb.) Planch. leaf. Euro. J. Exp. Bio.. 2015;5(8):1-7.
- [Google Scholar]
- Allelopathic potential of tannic acid and its equivalent phenolics extracted from aerial parts of Ampelocissus latifolia (Roxb.) Planch., IOSR J. Agric. Vet. Sci.. 2016;9(7):90-100. http://www.iosrjournals.org/iosr-javs/papers/vol9-issue7/Version-1/P09070190100.pdf
- [Google Scholar]
- Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods. 2012;4(4):906-914.
- [Google Scholar]
- Evaluation of in vitro free radical scavenging activity of leaf extract fractions of Manilkara hexandra (Roxb) Dubard in relation to total phenolic contents. Int. J. Pharm. Pharm. Sci.. 2015;7(10):296-301.
- [Google Scholar]
- Comparative assessment of total phenolic content and in vitro antioxidant activities of bark and leaf methanolic extracts of Manilkara hexandra (Roxb.) Dubard. J. King Saud. Univ. Sci.. 2020;32:643-647.
- [Google Scholar]
- Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem.. 2011;129(2):345-350.
- [Google Scholar]
- Relative total phenolics content, 1,1-diphenyl picrylhydrazyl free radical scavenging and total antioxidant potentials of seven Indian medicinal plant parts’ aqueous extracts. Int. J. Pharma. Bio Sci.. 2017;8(2):283-291.
- [Google Scholar]
- Dietary flavonoids: intake, health effects and bioavailability. Food Chem. Toxicol.. 1999;37(9–10):937-942.
- [Google Scholar]
- Association between vegetable, fruit and carbohydrate intake and breast cancer risk in relation to physical activity. Asian Pac. J. Cancer Prev.. 2014;15(11):4429-4436.
- [Google Scholar]
- Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Causes Control. 2013;24(7):1363-1374.
- [Google Scholar]
- Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Bioresour. Technol.. 2009;100(2):970-974.
- [Google Scholar]
- Antigenotoxic effect of grape seed procyanidin extract in Fao cells submitted to oxidative stress. J. Agric. Food Chem.. 2004;52(5):1083-1087.
- [Google Scholar]
- Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agric.. 1993;61(2):161-165.
- [Google Scholar]
- Berry antioxidants: small fruits providing large benefits. J. Sci. Food Agric.. 2014;94(5):825-833.
- [Google Scholar]
- Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: the Kuopio ischaemic heart disease risk factor study. Am. J. Clin. Nutr.. 2014;99(2):328-333.
- [Google Scholar]
- Antioxidant capacity and phenolic content of cocoa beans. Food Chem.. 2007;100(4):1523-1530.
- [Google Scholar]
- Studies on products of browning reactions, antioxidant activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet.. 1986;44(6):307-315. https://www.jstage.jst.go.jp/article/eiyogakuzashi1941/44/6/44_6_307/_pdf/-char/en
- [Google Scholar]
- Biodiversity of vulnerable and endangered plants from Jalgaon district of North Maharashtra. Asian J. Pharm. Life Sci.. 2012;2(2):144-150.
- [Google Scholar]
- Ethnomedicinal practices of Nasik district, Maharashtra. Indian J. Tradit. Knowl.. 2005;4(3):287-290.
- [Google Scholar]
- Antimicrobial and antioxidant potential with FTIR analysis of Ampelocissus latifolia (Roxb.) Planch. leaves. Asian J. Pharm. Clin. Res.. 2013;6(1):157-162.
- [Google Scholar]
- Ethnobotanical exploration of Malkangiri district of Orissa, India. Ethnobot. Leaflets. 2007;11(1):122-140.
- [Google Scholar]
- Antiproliferative activity of allelochemicals present in aqueous extract of Synedrella nodiflora (L.) Gaertn. in apical meristems and Wistar rat bone marrow cells. IOSR J. Pharm.. 2013;3(2):1-10.
- [Google Scholar]
- Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale) Food Chem.. 2008;111(1):38-44.
- [Google Scholar]
- Cabbage (Brassica oleracea L. var. capitata) phytochemicals with antioxidant and anti-inflammatory potential. Asian Pac. J. Cancer Prev.. 2013;14(11):6657-6662.
- [Google Scholar]
- Evaluation and comparison of polyphenols and bioactivities of wild edible fruits of North-West Himalaya, India. Asian Pac. J. Trop. Dis.. 2015;5(11):888-893.
- [Google Scholar]
- Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem.. 2002;50(25):7449-7454.
- [Google Scholar]
- Ethnobotanical observation on tuberous plants from tribal area of Rajasthan (India) Ethnobot. Leaflets. 2008;12(1):647-666.
- [Google Scholar]
- Phytochemical and pharmacological evaluation of Ampelocissus latifolia. Anc. Sci. Life. 2000;20(1&2):14-18.
- [Google Scholar]
- In vitro antioxidant activities of the fractions of Coccinia grandis L. leaf extract. Afr. J. Tradit. Complement. Altern. Med.. 2008;5(1):61-73.
- [Google Scholar]
- Biological activities of polyphenols from grapes. Int. J. Mol. Sci.. 2010;11(2):622-646.
- [Google Scholar]
- Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J. Epidemiol.. 2011;21(3):169-175.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2018.12.006.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







