Translate this page into:
In vitro and in silico validation of antibacterial potential of Pinus roxburghii and Cedrus deodara leaves’ extract against human pathogenic bacteria
⁎Corresponding authors at: Department of Environmental Sciences, COMSATS University Islamabad, Abbottabad Campus, 22060, Pakistan (A.M. Abbasi). drrafiq@cuiatd.edu.pk (Rafiq Ahmad), amabbasi@cuiatd.edu.pk (Arshad Mehmood Abbasi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The emergence of resistant pathogenic bacterial strains has threatened the human beings and already developed remedial measures. Based on the traditional herbal therapeutic history, present study is aimed to assess in vitro and in silico inhibition potential of leaves extracts of Pinus roxburghii and Cedrus deodara against human pathogenic bacteria Staphylococcus aureus, Salmonella typhi and Pseudomonas aeruginosa. Hexane, methanol and acetone extracts of both plants were evaluated against above mentioned bacterial strains employing agar well diffusion technique. While docking analyses were performed to analyze the interaction of vital bioactive compounds and bacterial virulence proteins to get an idea about potential candidates for drug discovery. Both plant extracts exhibited greater antibacterial activities against S. aureus as compared to S. typhi and P. aeruginosa. The activity of different extracts also portrayed the role of polarity of solvent and compound to be extracted in each solvent i.e., activity of hexane extract > methanol > acetone with some variations. MIC (minimum inhibitory concentration) values of P. roxburghii extracts were less than that of C. deodara against tested strains, while variation was observed in MBCs (minimum bactericidal concentrations). Furthermore, molecular docking of studied plants bioactive compounds and bacterial proteins showed strong interactions (binding affinity) i.e., taxifolin > nortrachelogenin > bisabolene > valencene > caryophyllene. Antibacterial efficiencies of P. roxburghii and C. deodara suggested their application as effective therapeutic agents against diseases caused by mentioned bacterial strains. In silico analysis suggests the isolation and usage of bioactive components as potential antibacterial agents/drugs after further experimentations on animals.
Keywords
Gymnosperm
Antibacterial
P. roxburghii
C. deodara
MIC
MBC
1 Introduction
The emergence and spread of antibiotic resistance in pathogenic bacterial strains has substantially threatened the present-day remedial measures (Manandhar et al., 2019). Multidrug resistant bacterial infections mainly increase cost of treatments and mortality rate. There are limited and expensive therapeutic preferences for these infectious agents with significant adverse effects (Nakagawa et al., 2016). The present-day exigence persuaded human to evaluate novel natural antimicrobial drugs with less side effects and greater efficiency (Salem et al., 2014). Therefore, current study was designed to employ plant extracts (P. roxburghii and C. deodara) for their antibacterial activity.
P. roxburghii (Chir pine) is one of the substantial pine species of Indo-Pakistan coniferous forests (Sadeghi et al., 2016). P. roxburghii is reported to have anti-inflammatory, hepato-protective antibacterial and anticonvulsant (Kumari et al., 2017). The beneficial properties of pine needles have also been portrayed in individuals with diabetes, rheumatism, obesity, cardiovascular diseases, liver and stomach infections, chronic bronchitis and cancer (Saad et al., 2017). Bark extracts of pines portrayed anti-mutagenic, anti-carcinogenic, anti-aging, anti-inflammatory and high antioxidant properties (Sood, 2018).
C. deodara (deodar), member of family Pinaceae is of immense ethnobotanical and therapeutic importance (Kumar et al., 2013). In China, C. deodara is one of the extensively exploited traditional medicinal herbs having anticancer effects with additional therapeutic capacities in relieving itches, removing dampness and destroying parasites (Shi et al., 2016). Needles have anti-inflammatory, anti-rheumatic and antimicrobial activities (Buneri et al., 2019).
Based on above findings in vitro antibacterial activity analyses of P. roxburghii and C. deodara crude extracts were evaluated against human pathogenic bacterial strains. In addition, to support results of this study, in silico interaction between bioactive compounds (already identified in crude extracts of selected plants) and virulent proteins of selected pathogenic bacterial strains was conducted to evaluate bioactive compounds that could be responsible for inhibition of pathogenic strains.
2 Materials & methods
2.1 Sampling and extract preparation
The fresh leaves of P. roxburghii and C. deodara plants were collected from Khyber Pakhtunkhwa Forest Department and Billion Tree Tsunami Afforestation Project Nursery Abbottabad, respectively. The selected plants were identified by Dr. Arshad Mehmood Abbasi, plant taxonomist at Department of Environmental Sciences, COMSATS University Islamabad (CUI) Abbottabad Campus, Pakistan, and the respective voucher specimens for P. roxburghii (CUHA-346) and C. deodara (CUHA-21) were submitted in the herbarium of COMSATS University Islamabad, Abbottabad Campus, Pakistan. Collected leaves were washed properly with distilled water followed by shade drying for 4–5 weeks (25 °C) to prepare their crude extract using standard methodologies with some modifications (Bhattacharjee et al., 2006). Dried leaves were ground into fine powder and soaked in hexane, methanol, and acetone solvents. 10 g of fine powder from both plants was soaked in 200 ml of each solvent in separate glass bottle placed on shaking incubator for overnight and finally filtered with Whatman No. 1 filter paper. The process of soaking the residues in distinct solvents, overnight incubation, and filtration to attain a clear filtrate was repeated 2 times and the resultant filtrates were evaporated and dried under reduced pressure at room temperature 25 ± 1 °C using rotary vacuum evaporator (Büchi® rotary evaporator Model R-200). Extracts were dried further using lypholizer and their yields were weighed and placed in air tight vials for future use. Percentage yields were calculated with following formula: Extract yield = R/S × 100.
R = Weight of extract, S = Weight of plant raw material (Mostafa et al., 2018).
Sample dilutions were prepared by dissolving different required quantities of dried powder of different extracts in 1 ml of dimethyle sulphoxide (DMSO).
2.2 Determination of antibacterial activity
To assess the effectiveness of selected plant extracts, S. typhi (ATCC 6539), P. aeruginosa (ATCC 9027) and S. aureus (KX262679) were collected from National University of Sciences & Technology, Pakistan. Antibacterial activity was evaluated employing agar well diffusion assay (Sen and Batra, 2012). Autoclaved nutrient agar media was poured in petri plates and placed in incubator (25 °C) for 3–4 h. Bacterial suspensions were prepared in autoclaved dH2O to get 0.5 OD at 600 nm (107–108 CFU/ml). 20 µl of each bacterial suspension was spread in petri plates and then wells were made with 6 mm cork borer, 30 µl of plant extract was poured in each well and plates were incubated at 37 °C for 18 h. Streptomycin (30 µg/well) and DMSO were also used as positive and negative control respectively. The diameter of clear zones of inhibition were measured as a sign of antibacterial activity (Chauhan et al., 2017).
2.2.1 Determination of MICs and MBCs
MICs were evaluated for those effective plant extracts which displayed antimicrobial activity at concentration of 50 mg/ml. While MBCs were determined for the lowest concentrations of plant extracts which did not exhibit any visible growth by streaking them on fresh media (Rehman et al., 2018).
2.3 Molecular docking analysis
To authenticate the outcome of in vitro antibacterial activity of crude extracts, in silico study was conducted to investigate the interaction of bioactive compounds in the targeted plant species with virulent proteins i.e., cysteine and serine proteases of selected bacterial strains. Corresponding 3D structures of the protein targets were obtained from RCSB Protein Data Bank. Bioactive compounds were actually selected from already available GC–MS data of studied extracts using aforementioned solvents. Ligand molecules were obtained from online database ZINC15 http://pubs.acs.org/doi/abs/https://doi.org/10.1021/acs.jcim.5b00559. Docking analysis was done using online tool CB-Dock and ligand–protein binding features were analyzed in Discovery Studio 4.1 (Dassault Systems Biovia) (Sampangi-Ramaiah et al., 2020).
2.4 Statistical analysis
Antibacterial activity was done in triplicates and the data was presented as Mean ± Standard deviation. Tukey’s HSD post hoc test following One-way ANOVA was carried out to investigate the significant difference in antibacterial activity of different concentrations of both plant extracts.
3 Results
3.1 Antibacterial activity of plant extracts
Investigated extracts of P. roxburghii and C. deodara displayed potential effectiveness in suppressing pathogenic bacterial growth. Variation in activity was observed which might be due to different type of pathogenic organisms and types of extracts. Overall, zones of inhibition for all the selected extracts ranged from 10.3 to 17.7 mm, 9.7–19.3 mm and 9.7–18.7 mm against P. aeruginosa, S. aureus and S. typhi respectively (Table S1).
P. aeruginosa was revealed as the most resistant strain to plant extracts followed by S. typhi while S. aureus was the most susceptible strain to the plants extracts. P. roxburghii extracts showed more activity than C. deodara extracts against P. aeruginosa, S. aureus and S. typhi while a little variation in zones of inhibition was observed by C. deodara hexane extract against S. typhi which showed larger zones of inhibition. Maximum zone of inhibition (17.7 mm) against P. aeruginosa was shown by P. roxburghii acetone extract at concentration of 100 mg/ml, while hexane and methanolic extracts (100 mg/ml) of P. roxburghii depicted maximum activity against S. aureus by showing zones of inhibition of 19.3 mm. Similarly, S. typhi was greatly inhibited by P. roxburghii methanol extract (100 mg/ml) with zones of inhibition of 18.7 mm. Streptomycin displayed zone of inhibition of 19.7 ± 0.6 mm against P. aeruginosa and S. typhi each, while 26.7 ± 0.6 mm of zone of inhibition against S. aureus. DMSO did not show any zone of inhibition (Figs. 1-3).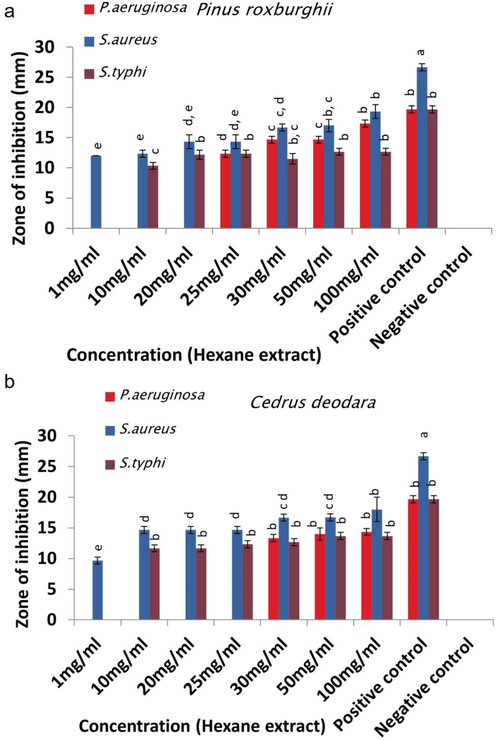
Antibacterial activity of (a) P. roxburghii and (b) C. deodara hexane extracts.
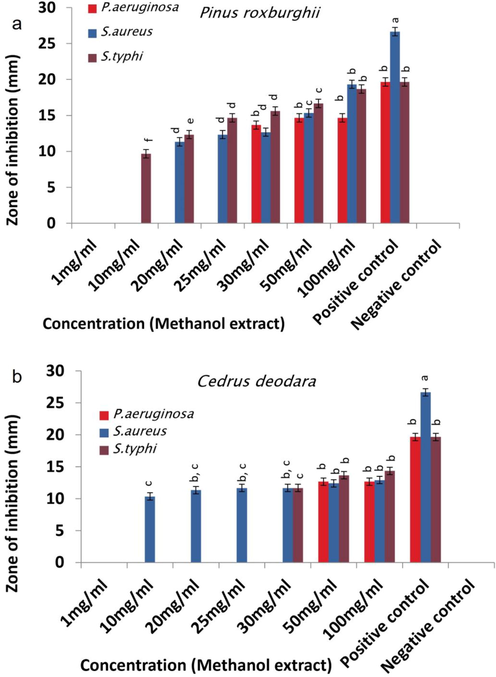
Antibacterial activity of (a) P. roxburghii and (b) C. deodara methanol extracts.
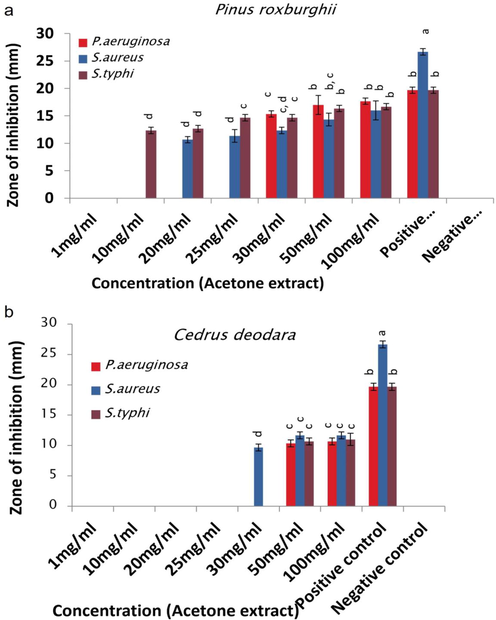
Antibacterial activity of (a) P. roxburghii and (b) C. deodara acetone extracts.
Hexane extracts (100 mg/ml) of P. roxburghii and C. deodara depicted greater activity against S. aureus followed by P. aeruginosa and S. typhi (Fig. 1 a & b/Table S1). Methanol extracts (100 mg/ml) of both plants showed different trend of antimicrobial activity. P roxburghii methanol extract inhibited S. aureus greater than S. typhi and P. aeruginosa, while C. deodara methanol extract portrayed larger zones of inhibition against S. typhi than other studied strains (Fig. 2 a & b). Acetone extract (100 mg/ml) of P. roxburghii inhibited P. aeruginosa followed by S. typhi and S. aureus, while C. deodara acetone extract (100 mg/ml) showed opposite trend of activity with maximum inhibition against S. aureus followed by S. typhi and P. aeruginosa (Fig. 3 a & b).
3.1.1 Mics and MBCs of the effective plant extracts
The inhibitory effects of P. roxburghii hexane, methanol and acetone extracts were started to be visualized at 25, 30 and 30 mg/ml with inhibition zones of 12.3 ± 0.6, 13.7 ± 0.6 and 15.3 ± 0.6 mm against P. aeruginosa strain respectively. Likewise, P. roxburghii hexane extract showed MIC of 1 mg/ml against S. aureus. The growth of S. typhi was suppressed by all tested strains of P. roxburghii at minimum concentrations of 10 mg/ml (Table 1, Figs. 1-3). Conc. Concentration (mg/ml).
Samples
Solvents
Inhibition zone (mm)
Conc.
P. aeruginosa
Conc.
S. aureus
Conc.
S. typhi
Pinus roxburghii
Hexane
20
ND
0.5
ND
1
ND
25
12.33 ± 0.6
1
12.00
10
10.33 ± 0.6
Methanol
25
ND
10
ND
1
ND
30
13.67 ± 0.6
20
11.33 ± 0.6
10
9.667 ± 0.6
Acetone
25
ND
10
ND
1
ND
30
15.33 ± 0.6
20
10.67 ± 0.6
10
12.33 ± 0.6
Cedrus deodara
Hexane
25
ND
0.5
ND
1
ND
30
13.33 ± 0.6
1
9.667 ± 0.6
10
11.67 ± 0.6
Methanol
30
ND
1
ND
25
ND
50
12.67 ± 0.6
10
10.33 ± 0.6
30
11.67 ± 0.6
Acetone
30
ND
25
ND
30
ND
50
10.33 ± 0.6
30
9.667 ± 0.6
50
10.67 ± 0.6
Positive Control
1
19.7 ± 0.6
1
26.7 ± 0.6
1
19.7 ± 0.6
Negative Control
1
ND
1
ND
1
ND
C. deodara extracts showed potentially less bacteriostatic activity against P. aeruginosa which was proved to be more resistant, and its MIC in hexane, methanol and acetone extracts reached to 30, 50 and 50 mg/ml respectively. C. deodara hexane extract suppressed growth of S. aureus and S. typhi at MIC of 1 and 10 mg/ml correspondingly (Table 1).
P. roxburghii hexane, methanol and acetone extracts showed potential bactericidal activity against P. aeruginosa with MBC value of 30 mg/ml for each extract while their MBC against S. aureus reached to 1, 20 and 20 mg/ml individually. P. roxburghii hexane, methanol and acetone extracts showed MBCs of 20, 20 and 10 mg/ml against S. typhi. Likewise, MBCs of C. deodara hexane, methanol and acetone extracts were 30, 50 and 50 mg/ml respectively against P. aeruginosa which was proved to be more resistant. C. deodara hexane, methanol and acetone extracts against S. aureus showed MBC of 10, 10 and 50 mg/ml respectively. MBCs of C. deodara extracts against S. typhi were observed to be 20, 30 and 50 mg/ml.
3.2 Molecular docking analysis
Docking analysis of monoterpenoids in hexane extract of both plants showed same pattern of inhibition potential as that of plants crude extracts i.e., P. aeruginosa > S. typhi > S. aureus by showing greater binding affinity. While sesquiterpenoids showed greater interaction with S. aureus with binding affinity ranged between −5.1 to −7.7 kcal/mol. Bioactive compounds of methanol extracts showed some variation in interaction and binding affinity with corresponding crude extracts activity. However, components of acetone extracts followed in vitro antibacterial activity pattern by showing greater binding affinity with P. aeruginosa and S. aureus respectively (Figs. 4-6).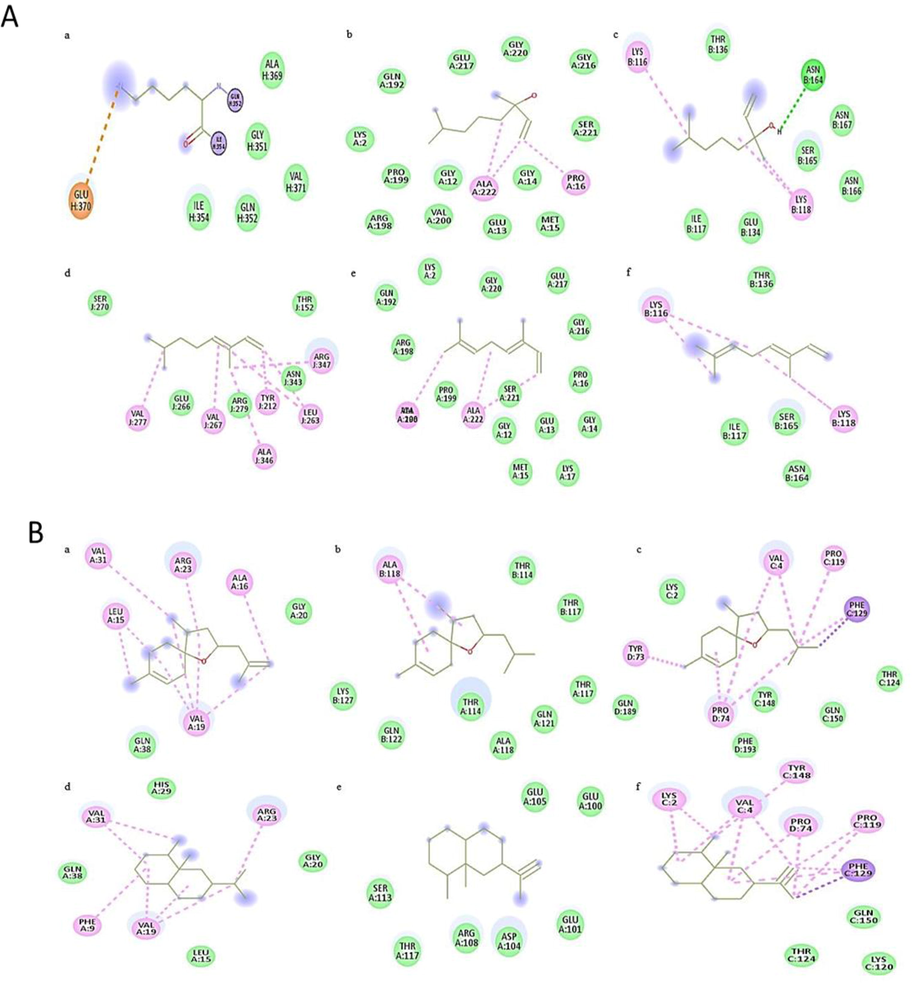
Docking analysis of bacterial proteases with A) monoterpenoids - Linalool (a, b, c), Ocimene (d, e, f) and B) sesquiterpenoids - Bisabolene (a, b, c), Valencene (d, e, f).
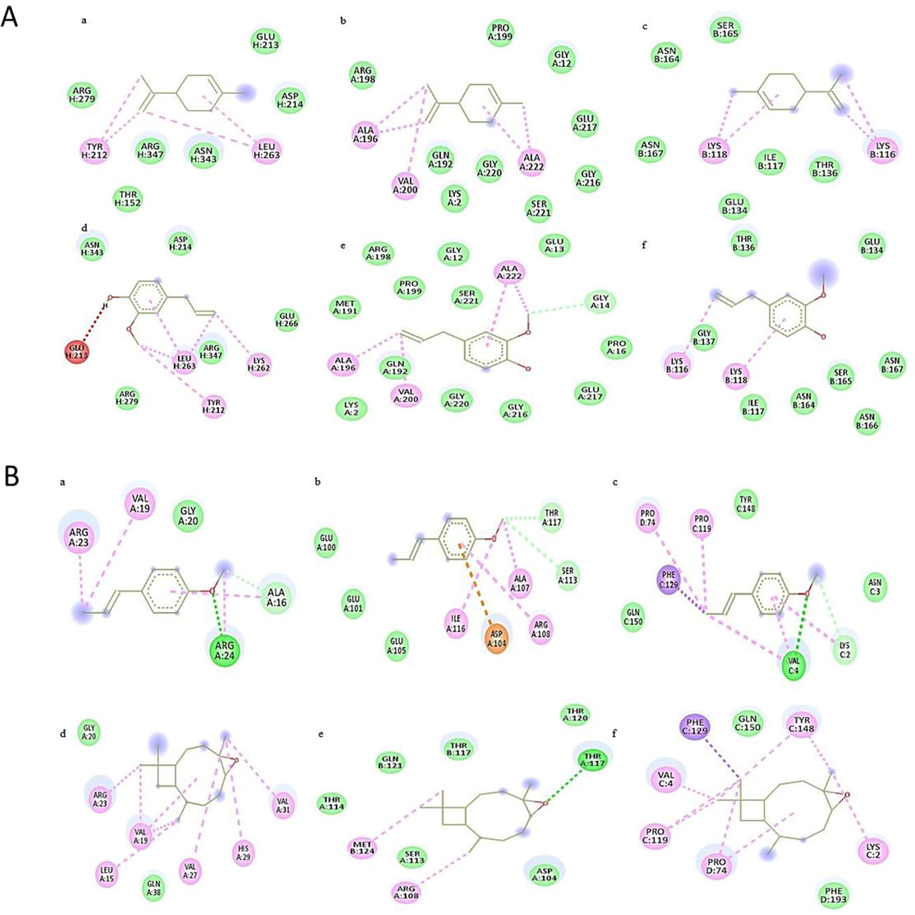
Docking analysis of bacterial proteases with A) Limolene (a, b, c) Eugenol (d, e, f) and B) Anethole (a, b, c), Caryophyllene (d, e, f).
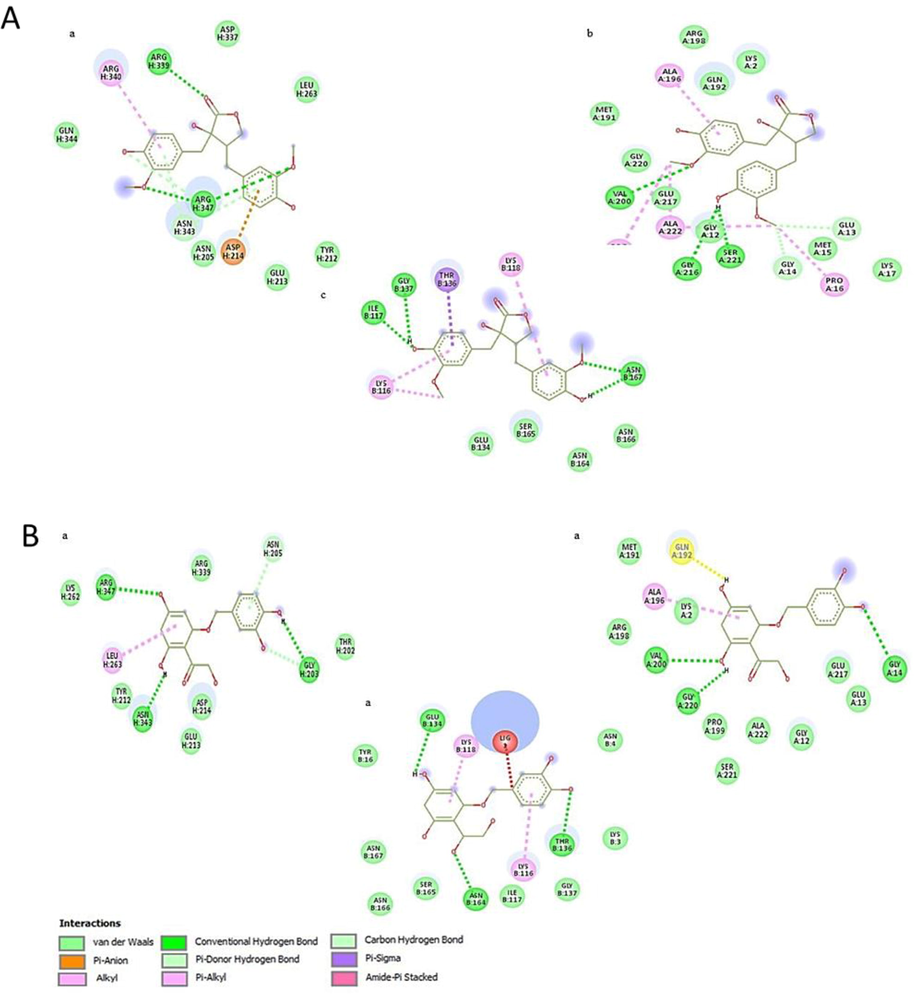
Docking analysis of bacterial proteases with A) Nortrachelogenin (a, b, c) and B) Taxifolin (a, b, c).
4 Discussion
Microbial infections always posed a threat with high morbidity and mortality in immune-compromised individuals, but the discovery of alternate traditional medicines is of prime importance to eliminate microbial infections and limit the use of toxic synthetic antibiotics. S. aureus is causative agent of skin infections and food borne diseases while gastroenteritis diseases in humans are caused by metabolites and toxins produced by S. typhi and P. aeruginosa (Siddiqui et al., 2009). Selected extracts portrayed potential antimicrobial activity against studied strains signified the occurance of maximum bioactive components in extracts of both plants.
Zafar et al. (2010) reported that oil extracted from pinus species inhibited the growth of S. aureus but has no inhibitory effect on S. typhi, like our current findings in which greater antimicrobial activity of P. roxburghii leaf extracts was observed against S. aureus than S. typhi. The constituents in respective crude extracts cause disruption of microbial cell membrane by interacting with its proteins and enzymes. A flux of protons disperse towards cell exterior might obstruct enzymes essential for amino acid synthesis or induce cell death (Burt, 2004).
Hexane extract of both plants showed greater inhibition against S. aureus and P. aeruginosa than S. typhi while variable potency was observed by C. deodara extract to inhibit P. aeruginosa (Fig. 1). These extracts comprised of monoterpenoids, diterpenoids and sesquiterpenoids which play key part in antimicrobial activity by posing toxic effects on structure and functions of bacterial membrane (Tsvetkov et al., 2019). The possible reason of showing a little variation in activity of both extracts could be the absence/less quantity of specific components in hexane extract of C. deodara due to which P. aeruginosa showed resistance at low concentrations and its high concentrations are required to inhibit bacterial strain. Concentration of bioactive compounds might be different in both extracts due to two different plant species, their age and growth environment as C. deodara plants were younger than P. roxburghii and both were collected from different locations (Yadav et al., 2017). Likewise, methanolic extracts of P. roxburghii inhibited S. aureus and S. typhi greater than P. aeruginosa. Whereas C. deodara methanolic extract inhibited S. typhi followed by S. aureus and P. aeruginosa (Fig. 2). Previous study showed the presence of α‐terpineol, linalool, limonene, anethole, caryophyllene and eugenol as bioactive components in methanolic extracts which are involved in dysfunction and disruption of the membrane, outflow of cytoplasmic constituents which lead to bacterial cell death (Gupta et al., 2011). P. roxburghii acetone extract prominently suppressed P. aeruginosa and S. typhi than S. aureus against which C. deodara extract showed action to a greater extent (Fig. 3). Literature presented 64.3 % of the acetone extract of P. roxburghii constituents secoisolariciresionol and nortrachelogenin, while C. deodara acetone extract consists of sesquiterpene, flavanoids, alkaloids, tannins, ferulic acid and beta-glucoside which are mainly involved in destruction of bacterial cytoplasmic membrane (Thapa et al., 2018). Greater activity of extracts against gram negative bacteria may be due to the presence of porin channels in their outer membrane, which facilitate transport of low-molecular-weight constituents, and lipophilic drugs have trouble to cross these channels (Guimarães et al., 2019). Hexane extracts of both plants showed less MICs against tested strains than methanol and acetone extracts, which might be due to the polarity of solvent. Other causes of variation in current MICs could be extracted constituents, extraction techniques and bacterial strains (Chaudhary et al., 2014).
Docking analysis of monoterpenoids showed more binding affinity with P. aeruginosa while sesquiterpenoids displayed greater interaction with S. aureus (Fig. 4, Table S2). The most common interacting amino acids in docked complexes of monoterpenoids and sesquiterpenoids were ALA, GLY, SER and LYS, ALA, VAL, and PRO respectively (Fig. 4). Docking analysis of some of the components of methanol extracts showed similar binding pattern with bacterial proteins as that of crude extracts, but others showed some variation. Common interacting aminoacids in docked complexes were ALA, VAL, LYS, ARG and LEU with conventional hydrogen bonds, Van der waals and Pi-alkyl interactions (Fig. 5, Table S2). In silico activity confirmed greater interaction of nortrachelogenin with P. aeruginosa and Taxifolin with S. aureus which make them ideal inhibitory components (Fig. 6, Table S2). Taxifolin from C. deodara acetone extract exhibited strong interaction with maximum binding affinity of −8.3 kcal/mol with S. aureus proteases. Interacting aminoacids involved in this docking complex were GLU, THR, ASN and LYS with conventional hydrogen bonds, Van der waals and Pi-alkyl interactions (Fig. 6B). Thus, flavonoids mainly taxifolin followed by Nortrachelogenin, bisabolene, valencene and caryophyllene proved to be potential candidates for possessing antibacterial activity as documented by Brown et al., 2015. Active compounds having antimicrobial activities restrain pathogenic bacterial strains by targeting significant constituents of bacterial metabolism including protein synthesis, cell wall, RNA polymerases. DNA gyrase & proteases. These bacterial constituents have direct impact in virulence by degrading virulence regulators and resisting adverse conditions in host (Human). In view of this, in silico analysis was done to identify the binding interaction of bioactive compounds present in studied crude extracts with the pocket of bacterial proteases. In silico analysis gave an idea to isolate and purify those components from crude extracts that showed strong interaction with bacterial virulence proteins and their usage as natural drugs against antibiotic resistant bacteria after more experimentation on animals.
5 Conclusion
Based on current findings, it could be concluded that all the tested extracts of selected plants possess the potential antibacterial activity which can be enhanced by increasing extracts concentration. P. roxburghii extracts having more antimicrobial potential than C. deodara extracts could be used as effective therapeutic agents against all tested strains and diseases instigated by them, limiting the use of health hazardous chemically synthesized antibacterial agents. Docking analyses further suggested the possible usage of selected natural compounds of P. roxburghii and C. deodara that showed strong interaction with bacterial virulence proteins could be isolated in purified form for potential drugs synthesis in future after in vivo experimentation.
Acknowledgements
The authors extend their appreciation to the researchers supporting project no. (RSP-2021/190), King Saud University, Riyadh, Saudi Arabia. Authors are also grateful to HEC-Pakistan for financial support under “Indigenous PhD Fellowships No 518-84211-2BS5-010. Moreover, authors are grateful to Alexander Marcel Asghar from Nottingham, UK, for proofreading.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antibacterial potentiality of Argemone mexicana solvent extracts against some pathogenic bacteria. Mem. Inst. Oswaldo Cruz.. 2006;101:645-648.
- [Google Scholar]
- A mass spectrometry-based assay for improved quantitative measurements of efflux pump inhibition. PLoS One.. 2015;10(5):e0124814.
- [Google Scholar]
- A comparative toxic effect of Cedrus deodara oil on larval protein contents and its behavioral effect on larvae of mealworm beetle (Tenebrio molitor)(Coleoptera:Tenebrionidae) Saudi J. Biol. Sci.. 2019;26(2):281-285.
- [Google Scholar]
- Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol.. 2004;94(3):223-253.
- [Google Scholar]
- Protective effect of Cedrusdeodara and Pinusroxburghii on experimentally induced gastric ulcers in rat. Int. J. Pharm. Pharma. Sci.. 2014;6(4):587-591.
- [Google Scholar]
- Antibacterial activity of Nerium indicum against some Gram-positive bacterial species. Int. J. Drug Res. Technol.. 2017;3(1):8-11.
- [Google Scholar]
- Antibacterial activity of terpenes and terpenoids present in essential oils. Mol.. 2019;24(13):2471.
- [Google Scholar]
- Phytochemistry and pharmacology of cedrus deodera: an overview. Int. J. Pharm. Sci. Res.. 2011;2(8):2010.
- [Google Scholar]
- Tubulin inhibitors from an endophytic fungus isolated from Cedrus deodara. J. Nat. Prod.. 2013;76(2):194-199.
- [Google Scholar]
- Phytoconstituent s and Pharmacological activity of Pinus roxburghiiSarg.: A Review. IJPDA; 2017. p. :241-249.
- Manandhar, S., Luitel, S., Dahal, R.K. 2019. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019.
- Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci.. 2018;25(2):361-366.
- [Google Scholar]
- Multiple uses of essential oil and by-products from various parts of the Yakushima native Cedar (Cryptomeria Japonica) J. Wood Chem. Technol.. 2016;36(1):42-55.
- [Google Scholar]
- Cystatin genes identification and antibacterial activity in medicinal plants used against clinical pathogens. SYLWAN. 2018;162:62-90.
- [Google Scholar]
- Anti-diabetes and anti-obesity medicinal plants and phytochemicals. Anti-Diabetes Anti-Obes. Med. Plants Phytochem 2017:59-93.
- [Google Scholar]
- Anti-pseudomonas activity of essential oil, total extract, and proanthocyanidins of Pinus eldarica Medw. bark. Res. Pharm. Sci.. 2016;11(1):58.
- [Google Scholar]
- Salem, M.Z.M., Ali, H.M., Basalah, M.O. 2014. Essential oils from wood, bark, and needles of Pinus roxburghii Sarg. from Alexandria, Egypt: Antibacterial and antioxidant activities. BioResources, 9(4), 7454-7466.
- Molecular docking analysis of selected natural products from plants for inhibition of SARS-CoV-2 main protease. Curr. Sci. 2020;118(7):1087-1092.
- [Google Scholar]
- Evaluation of antimicrobial activity of different solvent extracts of medicinal plant: Melia azedarach L. Int. J. Curr. Pharm. Res.. 2012;4(2):67-73.
- [Google Scholar]
- Extraction and purification of total flavonoids from pine needles of Cedrus deodara contribute to anti-tumor in vitro. BMC Complement. Altern. Med.. 2016;16(1):1-9.
- [Google Scholar]
- Phytosociology of Pinus roxburghii Sargent (chir pine) in lesser Himalayan and Hindu Kush range of Pakistan. Pak. J. Bot. 2009;41(5):2357-2369.
- [Google Scholar]
- Usefulness of multivariate analysis in forestry research: A case study of Pinus roxburghii. J. Pharmacogn. Phytochem.. 2018;7(2):3508-3509.
- [Google Scholar]
- Chemical profiling and biological activity analysis of cone, bark, and needle of Pinus roxburghii collected from Nepal. J. Intercult. Ethnopharmcol.. 2018;1(1):66-75.
- [Google Scholar]
- Chemical constituents of the extracts of the knotwood of Pinus roxburghii Sarg. and their antioxidant activity. Russ. Chem. Bull.. 2019;68(12):2298-2306.
- [Google Scholar]
- Qualitative phytochemical screening of some selected medicinal plants of shivpuri district (mp) Int. J. Life. Sci. Scienti. Res. 2017;3(1):844-847.
- [Google Scholar]
- GC-MS studies of needles essential oil of Pinus roxburghii and their antimicrobial activity from Pakistan. Elec. J. Environ. Agric. Food Chem.. 2010;9(3):468-473.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102518.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







