Translate this page into:
From kitchen to cosmetics: Study on the physicochemical and antioxidant properties of waste cooking oil-derived soap
⁎Corresponding authors. drmeenakshi973@gmail.com (Meenakshi Verma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
A large amount of used cooking oil (UCO) is improperly disposed of in sewage and rivers, leading to environmental pollution and posing health risks such as carcinogenic diseases. This study explores an eco-friendly approach to addressing this issue by repurposing UCO for laundry soap production, contributing to waste management and environmental decontamination. The soap was prepared by treating UCO with an alkaline solution (NaOH) through a simple saponification process, providing a greener alternative to traditional methods that rely on imported vegetable oils. The prepared soaps were evaluated for cleansing capacity as well as physical, chemical, and physicochemical properties. The results showed moisture content of 9.27 % to 10.34 %, pH ranging from 6.03 to 4.46, chloride percentage from 0.055 % to 0.29 %, free caustic alkali between 0.152 and 0.175, and total alkali content from 0.29 % to 0.73 %. These values meet the requirements of East African Standards (EAS), validating the quality of UCO-based soaps. By diverting UCO from waste streams and utilizing it in soap production, this approach supports waste management, minimizes environmental pollution, and contributes to sustainable production practices.
Keywords
Oil
Antioxidant
Soap
Saponification
Waste Management
Sustainability
1 Introduction
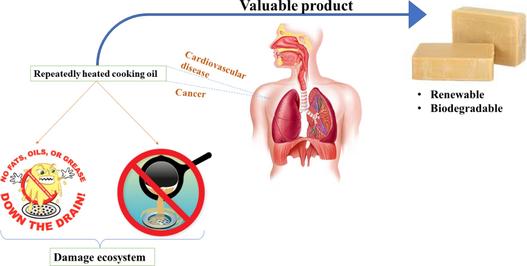
Indian cuisine is full of frying dishes, and cooking oil is the most essential thing used outside and inside for food-making purposes. In each household, approximate 2–5 kg of average cooking oil is utilized every month(Yacob et al., 2015) and the the major problem is associated with the repeated usage of the same oil various times in order to secure financial aspects. However, the repeatedly use of UCO leads to various kind of health hazardous problems including hypertension, elevate atherosclerosis, osteoporosis, and may affect liver and kidney structure and function. According to FSSAI (Food Safety and Standards Authority of India), heating cooking oil repeatedly causes the release of hazardous toxins as well as free radicals in the body which causes various carcinogenic diseases. Free radicals are unstable molecules that cause damage to healthy cells and other molecules like DNA, lipids, and proteins in our body and disrupt the functioning of cells. When oil is heated at high temperatures various reactions like (i) oxidation, (ii) hydrolysis, and (iii) polymerization occur which results in the formation of volatile compounds. At high temperature double and triple bonds start forming in a long carbon chain of fatty acids which are not good for human health and leads to an increase in the risk of cholesterol, cancer, liver disorder, and many heart diseases. Ganesan et al., highlightes that repeated heating of cooking oils at high temperatures leads to an increase in oxidative degradation products. These harmful compounds have been linked to a higher incidence of cardiovascular diseases, hypertension, and certain cancers(Ganesan et al., 2019). In India, research revealed that approximately 72 % of street vendors reuse cooking oil multiple times, which resulted in elevated concentrations of toxic aldehydes(Sehgal et al., 2023). Furthermore, the World Health Organization (WHO) has warned that prolonged consumption of such degraded oils may contribute to the development of serious health conditions, including atherosclerosis, diabetes, and obesity(World Health Organization, 2019). The), trans fat raises the risk of cardiovascular disease and death by 28 %. At present, nine of the sixteen countries having the most estimated percentage of cardiovascular heart disease deaths attributed to trans-fat consumption and lack a best-practice policy(“https://www.who.int/news/item/23-01-2023-five-billion-people-unprotected-from-trans-fat- leading-to-heart-disease,” 2023). Additionally, the improper disposal of waste cooking oil may result in several kinds of harmful environmental consequences as there is no specific practice for disposing of waste cooking oil. When used cooking oil (UCO) is directly discarded into the environment indoors and outdoors, it causes water contamination, air pollution due to its bad odor, and soil contamination(Murniati et al., 2024). Thus, it has become very crucial to address the aforementioned problems and to incorporate an alternative for managinging the above said problemThus, recycling of the UCOs has been proven as the best alternative, among them, preparation of soaps is considered as the most efficient and also fulfils the “Reduce, Reuse, Recycle” aspect of circular economy.(See Figs. 1–4 Table 1). The rating has been done from 1 star − 5 star according to cleansing action of soap.
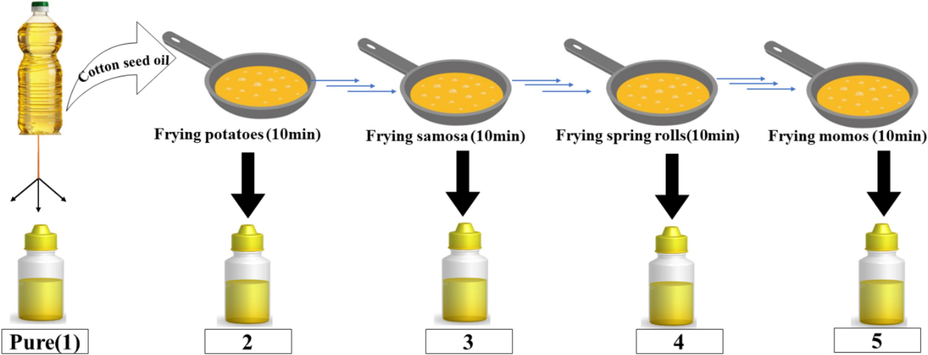
Schematic representation of frying protocol and collection of oil sample.
Schematic representation of strains on white clothes after 24 h of drying.

Soaps prepared from different oil samples.

After 24 h of washing with different soap samples.
Strains
Sample ❶
Sample ❷
Sample ❸
Sample ❹
Sample ❺
Lipstick
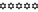
Coffee
Gravy
Grease
 Difficult to clean the strains, some are left.
Difficult to clean the strains, some are left. Takes time to clean.
Takes time to clean. strains clear.
strains clear. easy to clean the stains.
easy to clean the stains. Not take too much effort to clean the stains.
Not take too much effort to clean the stains.
Soap (RCOO-Na+ or RCOO-K+) also known as surfactant is a salt of the long chain of sodium or potassium salt of fatty acids used as a cleansing agent usually made by the action of alkali on fatty matter via saponification (Siram et al., 2019). For the formation of soap, the most commonly utilize base is sodium/potassium hydroxide as lye water and oil or fats as an acid. The composition of soap is the triglycerides that are the most important unit of fat/oils(Ukpebor et al., 2020). The number of small triglycerides units are present in the oil which is used for the formation of soap. The soap-making processes generally utilize vegetable oil, plant sources, and animal fats in large amounts. In worldwide, soap-making industries commonly employ palm oil in bulk obtained from plant sources. The studies indicate that this oil can be utilized as biodiesel production which is a green alternative to fuel production compared to the other methods(Panadare, 2015)(de Araújo et al., 2013). In various cases, UCOs are sometimes collected for the biofuel production. However, reprocessing of UCO into biofuels can be resource-intensive, often requiring significant chemical treatments and generating by-products that need further management. Thus, it is crucial to repurpose the UCO through a simpler, low-cost process. Moreover the availability of large amounts of oil/fats become a great concern for the soap-making industries so we are trying to apply an eco-friendly alternative for the formation of soap by utilizing UCO. Also, the prepared soap is cost-effective and user-friendly. The utilization of UCO for the preparation of soap bars is an environmentally friendly step towards circular economy and economic growth. Various studies have been done based on the utilization of UCO for the preparation of soap bars but no study has been discussed the difference in soap bars of different heating cycles of cooking oil so to check whether UCO is better in soap-making we have done the work with all different cycles of oil (Abera et al., 2023). Hence, the primary objective of this study is to assess the feasibility of utilizing used cooking oil (UCO) at different cooking cycles for soap production, focusing on the soap's physical, chemical, and physicochemical properties. Specifically, the research aims to determine the effect of multiple cooking cycles on the quality of the soap produced from UCO and to evaluate whether it serves as a viable, eco-friendly alternative to traditional soap-making processes. By comparing soap bars made from UCO subjected to different heating cycles, the study seeks to contribute to sustainable resource recovery while minimizing the environmental impact of UCO disposal.The repurpose of UCO not only avoids the environmental hazards of improper disposal but also reduces the need for energy-intensive processing, making it a greener alternative. The multiple-cycle use of WCO in soap production also highlights its potential for sustainable resource recovery.
2 Mechanistic insights of soap preparation
The mechanistic representation of formation of triglyceride then of soap is shown in Figure S1 (See Supplementary Information). The basic mechanism followed by the formation of soap is saponification. Saponification is the process where the esters bond breaks down to form carboxylate salt(soap) and glycerol by the action of triglycerides in fat/oil with lye water i.e., the mixture of NaOH with distilled water(Félix et al., 2017). This reaction results in the formation of soap, it is a type of alkaline hydrolysis. NaOH is widely used for the formation of lye water because it completely dissolves the fatty oils via saponification(Prabu et al., 2015). After saponification is completed, the soap has been prepared and during the curing time of soap, the alkali starts neutralizing and is no longer present in the soap after the weeks. So, NaOH is utilized in the formation of soap but is no longer evident in it.
3 Experimental
The detailed information about chemicals, equipments, sample colles has been provided in supplememntary information.
4 Physicochemical properties of UCOs and soap
For the preparation of soap, initially the physicochemical characteristics such as quality, iodine and saponification value of the collected oil samples were analysed.
4.1 Determination of quality of UCO
The quality tests like iodine and saponification value test have been done to know the properties of oil samples. The tests done follow the procedures discussed in several literatures and stepwise information has been provided as supplementary (Ajay Kumar, 2014) (Pocknell and Venni, 2010)(Adane et al., 2021).
4.2 Methodology for the preparation of laundry bar
In this study, cold press method is employed for soap preparation in which no external source of heat is applied for the production of soap(Zaroul et al., 2023). In this regard, initially approximate 50 % (w/w; 12.93 g NaOH in 25.87 g DI water) NaOH solution was prepared. Further, 100 g of collected oil samples were weighed and heated at 40–45 °C in separate flask. To the heated sample, NaOH solution was added with a maintained temperature of 45 °C as the dissolution of NaOH in water is an exothermic reaction. The reaction mixture was mixed thoroughly using the mixer blender until a uniform mixture was obtained. The mixture was then poured into the mold and let it in incubatorat the temperature of 40 °C for 24–48 h to settle them down completely. After that when it gets completely settled let it dry in racks for 28–30 days to see the transformation in the appearance of soap, hardness, and weight of soap. The general structure of soap shown in Fig S1.
4.3 Observance of weight loss of soap
Weigh loss is common for all the soap bars during the curing time as the soap dries out with time as the amount of water used to dissolve lye gets evaporates and the soap becomes harder or we can say more solid with time. During curing, all the leftover fatty matter was saponified and the remaining lye was neutralized. All the prepared samples of soap bars were weighed after 48 h of drying period and placed in racks for curing. The weights of all samples are observed on the first day and during the curing time.
5 Physicochemical tests for prepared soap
The physicochemical tests have been done to check the quality of the soap. It depends on several parameters including total fatty matter, pH, free alkali, and moisture percent. All the tests have been done by using the standard procedures reported in works of literature and detailed stepwise followed methodology has been provided in supplementary (Ajay Kumar, 2014), (Nurdiyanah et al., 2023).
6 Physicochemical properties of soap
The detailed methodology of Cleansing Capacity of Soap, Emulsification Test, Foam Ability Test, Texture and color, Acidity/Alkalinity of soap Test, Chemical Property has been provided in the supplementary information
7 Results and discussions
7.1 Properties of oil
7.1.1 Iodine test
The iodine value is used to determine the amount of unsaturation contained in fatty acids. The higher the content of unsaturated fatty acids, the larger will be the iodine value. Due to the presence of double bonds in oil/fats, the reaction occurs with iodine compounds. The higher iodine value of soap indicates the higher unsaturation of oil/fat resulting in a higher degree of heat and extent of oxidation during oil processing. The iodine value of UCO samples was investigated by using a procedure reported in the literature (Wali et al., 2015)(Legesse, 2020). The testing shows that oil samples have iodine values between the range of 1.0152–1.7766. due to high temperature(Akinola et al., 2010),(Tan et al., 2002). The iodine values of all oil samples are shown in Table S1.
7.1.2 Saponification value
The saponification value of oil is determined to know about the amount of alkali required to saponify a specific amount of oil/fats. The results obtained from the different heating cycle of the oil sample are shown in Table 2 from pure which is denoted as sample 1 to sample 5 lies under the range of 195 to 256 mg KOH/g which are comparable to each other and lies under the standard saponification value of oil. The studies revealed that the higher and lower saponification value of oil depends on the type of soap we desired to make(Nielsen, 1994). The obtained values illustrates moderate texturof soaps to be prepared having high cleansing and moisturizing ability and are highly efficient for soap-making process.
Sr. No.
% of the Antioxidant activity of soap solution
% of the Antioxidant activity of the oil sample
Control
−
−
Sample 1
21.63
25.12
Sample 2
31.07
24.46
Sample 3
29.34
41.66
Sample 4
37.52
40.48
Sample 5
61.29
53.41
7.2 Soap samples
7.2.1 Weight loss of soap
The weight loss of soap was observed during the curing time is the indication of all leftover contents gets evaporated. After a curing time of 1 month, the weight of the soap gets constant. The observance of weight loss is shown in Table S2. After 30 days of curing time, the weight of the soap sample gets constant and ready for further testing (Table S2).
7.2.2 Physical properties
Laundry soaps have been prepared in the current study, and both their physical and chemical properties and antioxidant activity were discussed. The results were evaluated against the various studies and literature reports on soap-making from UCO.
7.2.2.1 pH
The pH value is a measure of the degree of acidity or alkalinity of a solution. (Muhammed et al., 2022). The observed pH value of prepared laundry soap bars falls under the range of 9–10 which lies in the scale of standard pH value required for laundry soap bars. The higher pH of soap bars is due to incomplete saponification or if some residue of alkali is left unreacted. The observed data from the current work is consistent with the reports that stated pH value should be in the range of 9–11 is suitable for fabric and skin. The results of prepared soap bars shown in Table S3 are suitable for skin and fabric.
7.2.2.2 Moisture content
The moisture content is the amount of water present in soap, excess amount may leads to react with the unsaponified fat to form the number of free fatty acids and glycerol during the curing time, the process known as hydrolysis of soap. The amount of MCs in the prepared laundry bars ranged from 4.18 to 6.03, indicating an extended lifespan for the product(Table S3). The difference in prepared samples of soap bars are due to the variation of heating temperature of the oil and the found percentage and pH is below the EAS limit which is below 30 % for laundry soaps(African standard laundry soap-specification, 2013)(Legesse, 2020),(Atiku et al., 2014).
7.2.2.3 Free caustic alkali
The values obtained from this work reveals that the amount of free caustic alkali content of prepared laundry bars from UCO get from pure sample is denoted as sample 1 were 0.0558, sample 2 were 0.0744, sample 3 were 0.1116, sample 4 were 0.1178 and sample 5 were 0.2914, respectively shown in Table S3. These values are comparable and less than as compared to commercial soap bars discussed in the research work of (Legesse, 2020),(Mwanza and Zombe, 2020). Moreover, the values are also less and below than the limits set by EAS(0.2 %) for the laundry bars(African standard laundry soap-specification, 2013).This shows that the prepared soap bar is safe to use and UCO can be used for soap-making.
7.2.2.4 Total alkali content
The result from this work shows that total alkali content present in prepared bars from a pure sample which is denoted as sample 1 to sample 5 is in the range of 0.29 to 0.73 shown in Table S3. These values are comparable and below the limits of commercial soap bars (Legesse, 2020). The results of this research work are also below the ISO criteria, which says that soaps should contain no more than 2 % alkali(Vivian et al., 2014)(Zaria, 2021).
7.2.2.5 Total fatty matter
The total fatty matter observed from this work falls under the range of 75.58 to 88.21 whereas the other commercial detergent soap bars were fall under the range of 82.01 to 88.42 obtained from the study of Adane. All of the results are comparable to one another, as well as to ISO laundry soap requirements (76 %), showing that the manufactured soaps are of good or acceptable quality(Table S3).
7.2.2.6 Chloride content
The presence of high chloride content in soap samples is due to the use of chlorinated water to dissolve NaOH pallets. In this work, the percentage of chloride content was found to be comparable to each other and below the standard limits of EAS which is less than 1.5 %(African standard laundry soap-specification, 2013) (Table S3).
7.2.2.7 Cleansing action
The cleansing capacity of all the prepared soap samples has been checked by applying the strains of lipstick, sauce, and soup on white clothes. The strain of lipstick, grease, gravy, and coffee had easily removed. After 24 h of completely drying the strains, the clothes were washed to see the cleansing effect. The rate of effectiveness has shown in Table S1 according to cleansing action.
7.2.2.8 Physical properties
The results obtained from the prepared soap sample during the testing of physical properties are shown in the table S4 below.
The emulsification of soap occurs as a results of hydrophobic interactions between the water in soap solution where soap solution acts as a bridge between oil and water and form emulsions.
7.2.2.9 Texture and color
The color of soap bars is pale yellow and texture of as-prepared samples is moderate (not so hard, not soft) and the surface is smooth. The pictures of cutting soap to clearly seen its texture and color is shown in Fig. 5.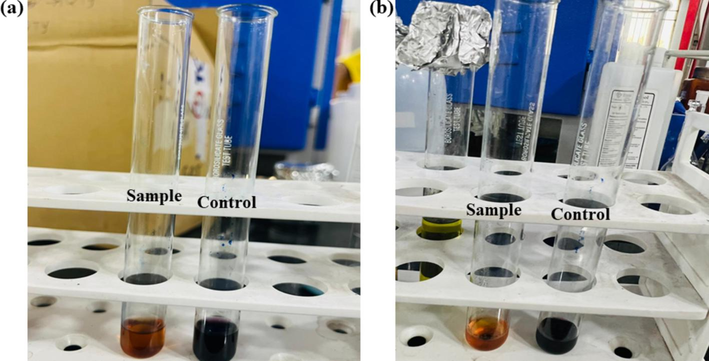
(a) Antioxidant activity of soap with DPPH (b) Antioxidant activity of oil with DPPH.
7.2.3 Chemical properties
From the reaction of soap with chloride salts, metal cations, and acid the following information were obtained shown in Table S5.
The soap is made up of sodium or potassium salts of fatty acid generally soluble in water.
The metal cations Ca2+ and Fe3+ are widely used to simulate the hard water conditions. Calcium chloride (CaCl2) and iron chloride (FeCl3) are insoluble in soap solution as they are component of hard water. When calcium ions react with fatty acids in soap solution, it forms insoluble calcium salts resulting in formation of white precipitates. The texture of precipitate depends on the number of fatty acids present. Also, Iron chloride reacts with soap solution in the presence of unsaturated fatty acids. The presence of iron ions catalyze the oxidation of unsaturated fatty acids results in the formation of colored precipitates.
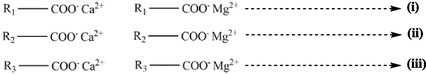
7.2.4 Antioxidant activity
DPPH assay was employed in order to assess the antioxidant activity of oils and soaps using equation.
The prepared soaps illustrate much better antioxidant potential (Table 2). The potential is attributed towards the presence of antioxidant activity in soap and oil. The calculated % antioxidant potential using above equation is found to be 21.636–61.29 % for soap solution and 25.21–53.41 for oil samples respectively. The oil and soap solutions possesses significant antioxidant potential.
8 Conclusion
A simple, cost-effective and green soap from UCO has been successfully prepared. The results concluded that we should select a soap that keeps a balance among the physicochemical, physical, and chemical properties. Soap that balances all these parameters is highly recommended as good quality soap. Analyses of all the soap properties were done to check the quality of the soap. All the data obtained from all samples are comparable to each other. The prepared laundry bars have less moisture content which increases the shelf-life of the product and the current study results in the bars are having low value of caustic alkali and chloride content which clarifies that soap had no adverse effect on fabric and skin. The prepared soaps show good cleansing power even towards the hard strains also the soap bars having good leather foaming capacity which is generally the demand of customers. The results obtained from laundry soap indicates that UCO is acceptable raw material to produced soap in bulk. Furthermore, the limitations of previous studies, which often focused on single-cycle WCO usage and its degradation during soap production, have been resolved in this work. It is observed that UCO, even after multiple cooking cycles, can be effectively used to produce high-quality soap with properties that meet East African Standards (EAS). This demonstrates the robustness of UCO as a raw material in soap production, extending its utility and promoting circular economy principles in waste management.
CRediT authorship contribution statement
Himanshi Soni: Writing – review & editing, Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Monika Bhattu: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Meenakshi Verma: Writing – review & editing, Validation, Software, Formal analysis, Data curation, Conceptualization. Manvinder Kaur: Writing – review & editing, Validation, Formal analysis, Data curation, Conceptualization. Abdullah A. Al-Kahtani: Writing – review & editing, Validation, Formal analysis, Data curation, Conceptualization. Irfan Hussain Lone: Writing – review & editing, Software, Resources, Formal analysis, Data curation. Ajar Nath Yadav: Writing – review & editing, Supervision, Resources, Formal analysis, Data curation. Mohd Ubaidullah: Writing – review & editing, Validation, Supervision, Project administration, Investigation, Data curation.
Acknowledgement
The authors extend their sincere appreciation to the Researchers Supporting Project number (RSP2024R266), King Saud University, Riyadh, Saudi Arabia for the support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The synergistic effect of waste cooking oil and endod (Phytolacca dodecandra) on the production of high-grade laundry soap. Heliyon. 2023;9:e16889.
- [CrossRef] [Google Scholar]
- Exploring of the potential of parthenium weed ash as substitute for commercial alkali for preparation of laundry soap: As a means to control invasion of parthenium. Front. Sustain.. 2021;2:1-12.
- [CrossRef] [Google Scholar]
- African standard laundry soap-specification, E., 2013. East African Community. EAS, Arusha, Tanzania.
- Ajay Kumar, A.K., 2014. Physico-chemical and natural products investigations of essential oil from the rhizomes of Kaempferia galanga L.
- Akinola, F.F., Oguntibeju, O.O., Adisa, A.W., Owojuyigbe, O.S., 2010. Physico-chemical properties of palm oil from different palm oil local factories in 8.
- Atiku, F., Fakai, I.M., Warra, A.A., 2014. Production of Soap Using Locally Available Alkaline Extract from Millet Stalk : Production of Soap Using Locally Available Alkaline Extract from Millet Stalk : A Study on Physical and Chemical Properties of Soap.
- de Araújo, C.D.M., de Andrade, C.C., e Silva, E. de S., Dupas, F.A., 2013. Biodiesel production from used cooking oil: A review. Renew. Sustain. energy Rev. 27, 445–452.
- Impact of consumption of repeatedly heated cooking oils on the incidence of various cancers-A critical review. Crit. Rev. Food Sci. Nutr.. 2019;59:488-505.
- [Google Scholar]
- Preparation of laundry soap from used cooking oils: Getting value out of waste. Sci. Res. Essays. 2020;15:1-10.
- [CrossRef] [Google Scholar]
- Formulation of laundry soap using locally made Palm Kernel Oil in Nigeria. Dutse J. Pure Appl. Sci.. 2022;8:51-57.
- [CrossRef] [Google Scholar]
- Murniati, D., Milama, B., Sutama, F.A., 2024. Development of students worksheet based on scientific approach in colloidal concept: Making soap from waste cooking oil, in: AIP Conference Proceedings. AIP Publishing.
- Mwanza, C., Zombe, K., 2020. Comparative Evaluation of Some Physicochemical Properties on Selected Commercially Available Soaps on the Zambian Market 7, 1–13. Doi: 10.4236/oalib.1106147.
- Nielsen, S.S., 1994. Introduction to the chemical analysis of foods. (No Title).
- Recycling waste cooking oil into soap_Knowledge transfer through community service learning. Clean. Waste Syst.. 2023;4:100084
- [CrossRef] [Google Scholar]
- Applications of waste cooking oil other than biodiesel: A review. Iran. J. Chem. Eng.. 2015;12:55-76.
- [Google Scholar]
- Pocknell, T.M., Venni, A.K., 2010. US Pat. 20100313839 A1. Tony Michael Pocknell, Adrian Kurt Venni.
- Prabu, S.L., Prakash, T.N.K.S., Thirumurugan, R., 2015. Cleaning Validation and Its Regulatory Aspects in the Pharmaceutical Industry, Developments in Surface Contamination and Cleaning. Elsevier Inc. Doi: 10.1016/B978-0-323-31303-2.00005-4.
- Sehgal, S., Roy, S., Mishra, N., 2023. Trans Fats in Street Foods-Sources, Health Risks and Alternative Sustainable Strategies, in: Sustainable Food Systems (Volume II) SFS: Novel Sustainable Green Technologies, Circular Strategies, Food Safety & Diversity. Springer, pp. 415–427.
- Siram, K., Habibur Rahman, S.M., Balakumar, K., Duganath, N., Chandrasekar, R., Hariprasad, R., 2019. Pharmaceutical nanotechnology: Brief perspective on lipid drug delivery and its current scenario, Biomedical Applications of Nanoparticles. Elsevier Inc. Doi: 10.1016/B978-0-12-816506-5.00005-X.
- Tan, C.P., Man, Y.B.C., Jinap, S., Yusoff, M.S.A., 2002. Effects of microwave heating on the quality characteristics and thermal properties of RBD palm olein 0–6.
- Manufacturing soap from waste cooking oil using cold and hot processes. Fulafia J. Sci. Technol.. 2020;6:32-36.
- [Google Scholar]
- Vivian, O.P., Nathan, O., Osano, A., Mesopirr, L., Omwoyo, W.N., 2014. Assessment of the Physicochemical Properties of Selected Commercial Soaps Manufactured and Sold in Kenya 433–440.
- Wali, F., Baloch, M.K., Nawaz, M., Khan, K., 2015. Comparison of Some Physico-Chemical Properties of Different Oils Available in the Local Market in Pakistan 2, 93–98.
- World Health Organization, 2019. The potential health impacts of reused cooking oil: A briefing note. [WWW Document].
- Households willingness to accept collection and recycling of waste cooking oil for biodiesel input in Petaling District, Selangor, Malaysia. Procedia Environ. Sci.. 2015;30:332-337.
- [CrossRef] [Google Scholar]
- Zaria, U.W., 2021. Volume 9 , Issue 2 , 2021 Determination of Alkali Content and Total Fatty Matter of Some Soaps 9, 334–339.
- Zaroul, M., Mohd, A., Zafiah, A., Rus, M., 2023. The Physical Characteristics of Handmade Soap Made Up Using Used Cooking Oil 4, 540–547.
Further reading
- Adelola, O.B., Ndudi, E.A., 2012. Extraction and Characterization of Cottonseed (Gossypium) Oil 398–402.
- Habib, A., Kumar, S., Sorowar, S., Karmoker, J., 2016. Study on the physicochemical properties of some commercial soaps available Study on the Physicochemical Properties of Some Commercial Soaps Available in Bangladeshi Market. Doi: 10.20431/2349-0403.0306002.
- Manji, A.J., Sarah, E.E., Modibbo, U.U., 2013. Studies on the potentials of Balanites aegyptiaca seed oil as raw material for the production of liquid cleansing agents 8, 1655–1660. Doi: 10.5897/IJPS07.049.
- Preparation of soap using different types of oils and exploring its properties submitted by Debesh Mishra Department of Chemical Engineering National Institute of Technology under the guidance of Dr. Susmita Mishra. J. Am. Oil Chem. Soc.. 2013;6:185-192.
- [Google Scholar]
- Profile, S.E.E., Profile, S.E.E., 2018. 8Vh Ri : Dvwh & Rrnlqj 2Lo Lq Wkh 0Dqxidfwxuh Ri 6Rdsv 2015–2016.
- Tijjani, Y.A., 2022. Study of the Physicochemical Properties of the Soap Prepared Using Oil Extracted from Neem Seed Collected from Kano Municipal, Kano State, Nigeria.
- Comparison of 3 kinds of test methods for determination of chloride content in soap products. J. Food Saf. Qual.. 2016;7:3403-3406.
- [Google Scholar]
- Zauro, S.A., Abdullahi, M.T., Aliyu, A., Muhammad, A., Abubakar, I., Sani, Y.M., 2016. Production and Analysis of Soap using Locally Available Raw-Materials Production and Analysis of Soap using Locally Available Raw-Materials.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103483.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







