Efficient and eco-friendly low-molecular-weight gelators based on l-phenylalanine as promising remediation tool for oil pollution
⁎Corresponding authors. mm.abdellatif@nrc.sci.eg (Mohamed Mehawed Abdellatif), ktnomura@tmu.ac.jp (Kotohiro Nomura)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
New low-molecular-weight gelators based on l-phenylalanine derivatives bearing long alkyl chains with different lengths were synthesized via one synthetic step. These gelators [i.e. 1 and 2] were nontoxic, biodegradable and prepared using naturally occurring starting materials. The gelation ability was investigated using different solvents and oils. 1H NMR and FTIR studies revealed that the intermolecular hydrogen bonding between the amide groups had a primary importance for supramolecular self-assembly process. In addition, the participation of the van der Waals interaction between the alkyl chains and π-π stacking supported the gelation process. The melting enthalpies were calculated. SEM was used for studying the morphology of the prepared xerogels. Moreover, the gelators succeeded to be used as an efficient tool for selective removal of several oils in their mixtures with water via gelation.
Keywords
Remediation
Low-molecular-weight-gelators
l-Phenylalanine
Oil spill
1 Introduction
Industrial waste oil and oil spill from various sources, may have a broad consequences on city ecological environments and human health for long period of time (Camilli et al., 2010; Gong et al., 2014; Guterman, 2009; Ohsedo, 2016; Peterson et al., 2003). These harmful consequences need an urgent efficient solution to remediate the oil pollution. Current oil treatment methods can be divided into chemical methods (Dewling & McCarthy, 1980) and physical methods (Fingas and Fingas, 2011). These methods have some concerns regarding its efficiency and the ability to recover the treated oils and reuse it. In some cases, the usage of some techniques can lead to more pollution without an efficient get rid of the oil from the water. Low-molecular-weight-gelators [LMWGs] can be used as a smart tool of oil spill remediation. The LMWGs [<2000 g. mol−1], (Meléndez et al., 2000; Steed et al., 2007; Zweep and Esch, 2013) have the ability to immobilize fluid phases i.e. water, organic solvents, oils, liquid crystals, ionic liquids, etc. (Dassanayake et al., 2011; de Loos et al., 2005; Estroff and Hamilton, 2004; George and Weiss, 2006; Kato et al., 2005; Le Bideau et al., 2011). This property is due to the formation of three dimensional fibrillar networks which formed through self-assembly process. This achieved intermolecularly through various physical interactions e.g. H-bonding, π-π stacking, van der Waals, etc. (Zweep and Esch, 2013). The applications of LMWGs in materials science as new soft materials are various and potent. The LMWGs offer unique property rather than other treatment techniques such as polymeric absorption materials. This property is the ability to easily recovery of the spilled oil and the used LMWG. The used LMWGs in oil spill remediation should fit some requirements such as selectivity for oils, non-toxicity, cheap price of raw materials and gelation ability at room temperature i.e. sea, river, collection tanks (Basak et al., 2012; Jadhav Swapnil et al., 2010).
Considerable efforts to design a new high-performance LMWGs for treatment of the spilled oil recently have been achieved. Bhattacharya and Ghosh reported firstly phase-selective gelation based on L-alanine (Bhattacharya and Krishnan-Ghosh, 2001). Suzuki and Hanabusa demonstrated LMWGs based on L-valine and L-isoleucine derivatives which could gel oil selectively from oil/water mixtures in efficient manner (Suzuki et al., 2006). Feng et al. also reported l-phenylalanine derivatives based LMWGs (Feng et al., 2014). Many other appreciated efforts were conducted (Abdellatif et al., 2018; Konda et al., 2014; Mukherjee et al., 2014; Rajkamal et al., 2014; Tsai et al., 2013; Yan et al., 2014).
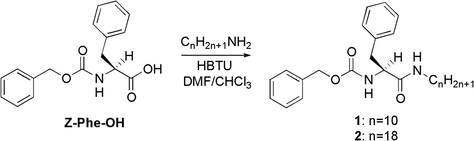
In this article, we would like to introduce two l-phenylalanine based LMWGs 1–2 [Scheme 1] as a powerful remediation tool for oil spill and oily liquid wastes that are important for development of new high performance LMWGs. These LMWGs are an efficient, eco-friendly, commercially available and easily synthesized [i.e. one synthetic step with no need for any chromatographic purification techniques].
- Synthesis of 1 and 2.
2 Experimental
2.1 Materials
Z-Phe-OH, decyl amine and octadecyl amine were purchased from TCI, Japan. HBTU was purchased from Iris, Japan. N,N-Diisopropylethylamine, solvents and all others Chemicals were purchased from Sigma-Aldrich.
2.2 Analysis
All the 1H and 13C NMR spectra were recorded on a Bruker AV500 spectrometer [500.13 MHz for 1H, 125.77 MHz for 13C]. Elemental analyses were performed by using an EAI CE-440 CHN/O/S elemental analyzer [Exeter Analytical, Inc.]. Atmospheric pressure chemical ionization [APCI] mass spectrometry was obtained on a Burker MicrOTOF II-SDT1.
The structure changes between gels samples were studied by ATR-FTIR spectroscopy [Perkin Elmer]. All spectra were obtained by 32 scans and 4 cm−1 resolutions in wavenumbers ranging from 4000 to 450 cm−1. The morphology and topography of the prepared samples were analyzed by scanning electron microscopy [SEM, Quanta FEG 250, FEI]. Differential scanning calorimetry [SETRAM DSC evo 131, scan rate 5 °C/min] was used to study the thermal behavior. All calculations were applied on the second heating/cooling cycle. The critical gelation concentrations [CGC] were determined by virtual checking in various fluids with different nature by test tube inversion (Suzuki yet al., 2006).
2.3 Synthesis of 1 and 2
In a 250 mL flask, Z-Phe-OH [5 g, 16.70 mmol] was dissolved in chloroform [50 mL], followed by addition of DIPEA [6.0 mL, 2.0 eq, 33.41 mmol] and decyl amine [3.56 g, 1.1 eq, 18.37 mmol] in case of gelator 1 or octadecylamine [3.56 g, 1.1 eq, 16.62 mmol] in case of gelator 2. The reaction mixtures were added to DMF [20 mL] containing HBTU [7.0 g, 1.1 eq, 18.37 mmol] and DIPEA [6.0 mL, 2.0 eq, 33.41 mmol]. The mixtures were stirred for 24 h at room temperature until completion of the reaction [monitored by TLC]. The reaction mixtures were diluted with chloroform [50 mL], washed two times consequently with 5% HCl, 10% K2CO3, brine and water. The resultant organic phase was dried over anhydrous MgSO4. The resultant solids were purified by dissolving in chloroform and reprecipitated by diffusion using petroleum ether and the precipitates were collected by filtration, dried in vacuo.
2.3.1 In case of gelator 1
1H NMR (500.13 MHz, CDCl3 at 25 °C): δ (ppm) = 7.22–7.36 (br, 10H), 5.55 (s, 1H), 5.40 (s, 1H), 5.13 (s, 2H), 4.34 (s, 1H), 3.00–3.18 (m, 4H), 1.18–1.33 (br, 16H), 0.91 (t, 3H). 13C NMR (125.77 MHz, CDCl3 at 25 °C): δ (ppm) = 170.45, 155.90, 136.61, 136.16, 129.31, 128.72, 128.56, 128.23, 128.04, 127.04, 67.04, 56.53, 39.55, 38.94, 31.93, 29.69, 29.67, 29.60, 29.51, 29,37, 29.29, 29.27, 26.77, 22.69, 14.13. Yield 5.6 g (75.0%).
Anal. Calcd. for C27H38N2O3
C, 73.94; H, 8.73; N, 6.39. Found: C, 74.03; H, 8.72; N, 6.29.
LRMS (APCI+) Calcd. for C27H38N2O3 [M + 1]: 439.61, Found: 439.30.
2.3.2 In case of gelator 2
1H NMR (500.13 MHz, CDCl3 at 25 °C): δ (ppm) = 7.21–7.35 (br, 10H), 5.60 (s, 1H), 5.44 (s, 1H), 5.11 (s, 2H), 4.35 (s, 1H), 3.00–3.19 (m, 4H), 1.18–1.33 (br, 32H), 0.91 (t, 3H). 13C NMR (125.77 MHz, CDCl3 at 25 °C): δ (ppm) = 170.45, 155.89, 136.60, 136.16, 129.31, 128.72, 128.56, 128.23, 128.04, 127.04, 67.04, 56.53, 39.55, 38.94, 31.93, 29.71, 29.67, 29.60, 29.51, 29,37, 29.29, 29.27, 26.77, 22.69, 14.13. Yield 5.6 g (75.0%).
Anal. Calcd. for C35H54N2O3
C, 76.32; H, 9.88; N, 5.09. Found: C, 76.07; H, 9.92; N, 5.04.
LRMS (APCI+) Calcd. for C35H54N2O3 [M + 1]: 551.83, Found: 551.40.
3 Results and discussion
3.1 Organogelation properties
The screening for the gelation performance was tested in 15 selected solvents: aromatics, linear and cyclic alkanes, alcohols, chlorinated, esters, ketones and different oils. The screening revealed that the prepared LMWGs exhibited good gelation behavior especially in aromatic and alkanes solvents [Table 1]. This behavior revealed the importance of the π-π stacking interactions between the LMWGs and the used aromatic solvent. In case of alkanes that may have some considerations regarding the solubility especially with presence of long alkyl chains. This may be appeared in gelification of many solvents. Gelator 2 had the ability for gelation in more solvents. This may be due to presence of excess and longer alkyl chain which enhanced the solubility that needed as a primary step for gelation. We observed also transparent gel formed in case of gelification of the aromatic solvents and oils rather than opaque gels in case of alkanes solvents. This may be attributed to small nanoscale structures formed through gelification of aromatic solvents and oils, whereas the opaque gels resulted from the formation of larger nano/microscale structure (Edwards et al., 2011).
| Solvents | 1 | 2 |
|---|---|---|
| n-Hexane | I | P |
| n-Octane | P | OG (0.11) |
| Cyclohexane | S | OG (0.52) |
| Ethyl Acetate | P | OG (0.50) |
| 1,2-Dichlorobenzene | S | TG (0.83) |
| Toluene | PG (2.00) | TG (0.62) |
| Benzene | PG (1.80) | TG (0.82) |
| P-Xylene | TG (1.67) | TG (0.70) |
| Dichloromethane | S | S |
| Chloroform | S | S |
| Methanol | S | S |
| Acetone | P | I |
| Paraffin oil | TG (1.00) | TG (0.14) |
| Soybean oil | TG (2.25) | TG (0.90) |
| Olive oil | TG (1.20) | TG (1.00) |
| Sunflower oil | TG (1.45) | TG (1.20) |
3.2 NMR spectroscopy
Fig. 1 showed visible and sharp signals for the gel of gelator 2 in d8-toluene [1 wt%] in1H NMR at 25 °C. That was consistent with what reported by Sakurai et al. regarding the wet gel possess a good thermal motion (Sakurai et al., 2003). Clear downfield shift for several protons were also observed; that referred to their participation in the hydrogen bonding and the deshielding of the protons in the gel state [i.e. Hc and Hd]. Moreover, deshielding shift was also observed for the aromatic protons [i.e. Ha and Hg]. This confirmed the primary importance of the hydrogen bonding formation for the gelation process with support of π-π stacking.
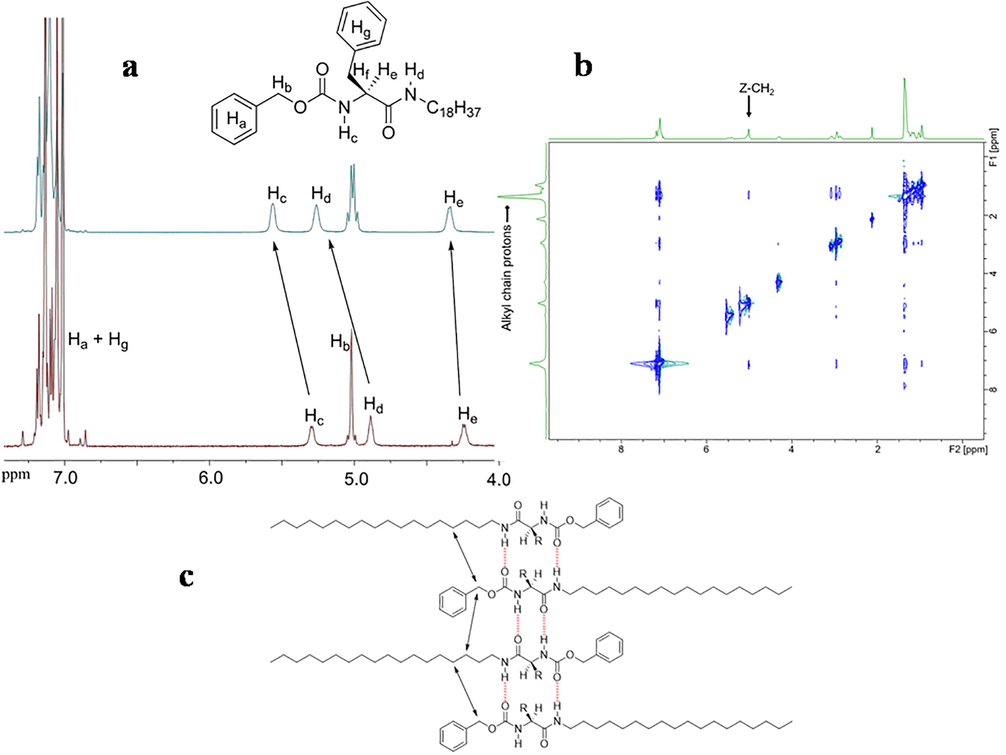
- a) 1H NMR spectra (in toluene‑d8 at 25 °C) of gelator 2 in 0.05 wt% solution (bottom) and 1 wt% gel state (top). b) 2D-NOESY 1H NMR spectra (in toluene‑d8 at 25 °C) of gelator 2 (1 wt%). c) Schematic representation of the proposed 1D columnar assembly of gelators.
3.3 Fourier-transform infrared investigation
To study the gelation process of the supramolecular network of gels that normally arranged via physical interactions, FT-IR spectra for gelators 1 and 2 were recorded in ethanol, toluene with various concentrations and solid [Figs. 6S–8S, see supporting information].
The measurements were performed in an ethanolic solution in which the gelators could not form gel; due to competition of the ethanol molecules and its participation in the hydrogen bonding rather than the gelator molecules. This provided the information of the non-hydrogen bonded amide groups; to assign the absorption bands corresponding to free amide groups. The characteristic bands of gelator 2 in an ethanolic solution were single bands at 3290, 1647 and 1531 cm−1, respectively, in the regions of —NH stretch region, the carbonyl amide I and the amide II which complied with literature. Moreover, absorption band at 1696 cm−1 was observed which assigned to C⚌O stretching band of the urethane unit.
More measurements were conducted in gel state using toluene at different concentrations 1, 3 and 5 wt%. The first observation showed the similarity between the gelation pattern of hydrogen bonding in solid and gel state. The results showed also that the absorption band 1647 cm−1 corresponding to the bonded carbonyl amide I in the solid state remain in the gel state even at 1 wt%. Additionally, appearance absorption bands corresponding to free carbonyl at 1652, 1655, 1661 cm−1, respectively, in case of 5, 3, 1 wt%. The same behavior was noticed the absorption band corresponding to the bonded C⚌O stretching and 1678 cm−1 of the urethane unit; absorption band corresponding to free C⚌O group appeared at 1688 cm−1.
As shown in Fig. 7S, the absorption bands assigned to symmetric and antisymmetric stretching vibrations of the long alkyl chains were observed at 2913 cm−1 and 2846 cm−1 in an ethanolic solution, but in the solid state and gel state these absorption bands shifted to 2924 cm−1 and 2855 cm−1, respectively, for symmetric and antisymmetric one. The observed shift could be assigned to the mobility decrease of the alkyl chains and its participate in strong aggregate formation by van der Waals interactions (Luo, Liu, and Liang, 2001; Suzuki et al., 2006).
The FT-IR study showed the intermolecular hydrogen bonding between the amide groups was highly important for the self-assembly process. In addition to, the clear participation of long alkyl chains in the gelation process.
3.4 Thermal stabilities study
The gel strength of the prepared organogels had evaluated using differential scanning calorimetry (DSC). The using of thermal stability as indication was due to its dependence on the nature, concentration of the gelators molecules and the nature of the gelified solvents. For better investigation we used different solvents (i.e. toluene and paraffin oil) and the evaluation was done at different concentrations of organogelators. The results [Fig 14S, see supporting information] showed that the values of Tgel for the organogels were increased by increasing the concentrations of the gelators, which may be due to increasing the density of 3D fibrillar network.
The values of gel-sol transition enthalpies (ΔH) indicated the strength of the intermolecular interactions. ΔH could be calculated from the following Schrader’s equation (Amanokura et al., 1998; Bielejewski et al., 2008; Seo and Chang, 2005):
The ΔH values were calculated from the slopes of the linear curves in [Fig. 14S right]. The values were found 127, 123 and 40 for 2 in paraffin oil, 2 in toluene and 1 in paraffin oil respectively. The melting enthalpy of organogels prepared using gelator 2 in paraffin oil was higher than analogous in toluene and in case of gelator 1 in paraffin oil, which confirmed the important of presence of long alkyl chain (i.e. 18).
3.5 Morphological study of the xerogel
The three-dimensional network which acquired the gelator the ability to immobilize the fluid phases was formed by the entanglement of self-assembled nanofibers. SEM was used for evaluating the porous structure of the prepared xerogels, obtained from drying of 2 organogels 1 wt% in toluene. The images of SEM showed a high entangled fibrillar network for the prepared xerogels [Fig. 2b]. Fig. 2c showed effect of gelator- solvent interactions on the fibre size, with a diameter of several hundred of nanometers long 256–569 nm. Formation of larger nano/microscale structure may be due to the decreasing of the interactions between the gelator and the solvent molecules (Edwards et al., 2011).
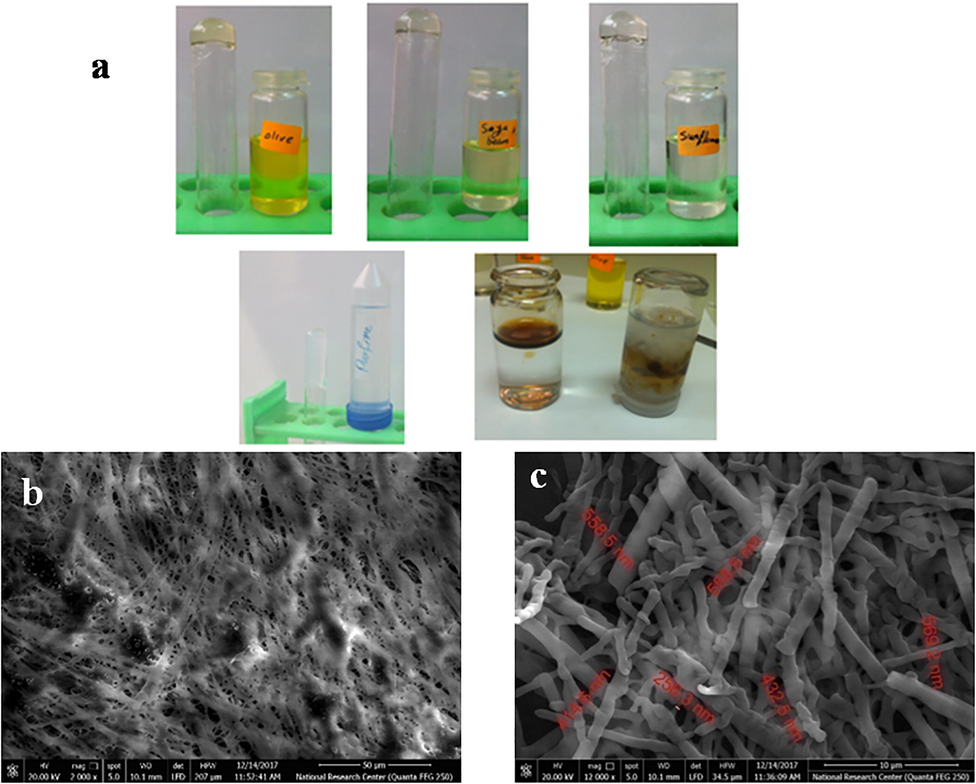
- a) Investigation of gelation ability and selective removal of various oils using 1 and 2. b, c) SEM images of xerogel of 2 prepared by drying of organogels 1 wt% in toluene.
3.6 Investigation of the 1-D self-assembly process (stacking-up)
The primary step for formation of a three-dimensional fibrillar network that responsible for immobilizing fluid phases was the stacking-up of the gelator molecules. The stacking up had two possibilities head-to-head (Yabuuchi et al., 2007) or head-to-tail (Iqbal et al., 2008). 2D-NOESY 1HNMR experiments provided information about which protons are spatially close and intermolecular correlated (Allix et al., 2010). The study of gel in d8-toluene provided us an evidence for long alkyl chain protons spatially correlated CH2 protons of Z unit [Fig. 1]. This referred to the stacking up of gelators 1 and 2 is head-to-tail one [Fig. 1].
3.7 Phase-selective gelation of different oils from mixtures with water
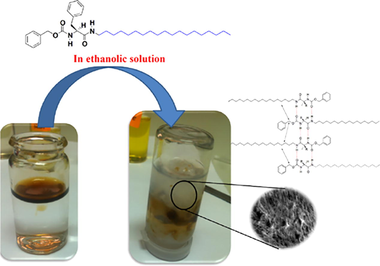
The gelation ability of the prepared gelators was investigated in various commercial oils, which used widely in daily life and in industrial sector. As shown in Fig. 2a, different oils (i.e. olive, soya bean, sunflower and paraffin oil) were tested by test tube inversion method. Fig. 2 demonstrated selective removal of waste mineral oil from its mixture with water by using an ethanolic solution of gelator 2 via formation of gel that can be easily removed by filtration.
The synthesis of these types of functional LMWGs is highly promising, to solve many problems in the river Nile [i.e. oil spill, oil leakage from different tankers, etc.] and in many industries which produce oily waste [i.e. cheese industry, cosmetics, oil refining, etc.]. The studies are running to modify the design of the gelators with more universal gel applications. Also, the scaling up for production of these LMWGs is under investigation.
4 Conclusions
Two new low-molecular-weight gelators based on l-phenylalanine derivatives bearing two different lengths alkyl chain (i.e. 10 and 18) have been successfully synthesized. The gelators showed high gelation efficiency mainly in aromatic, alkanes solvents and different oils. The gelator bearing longer alkyl chain showed better gelation efficiency with low CGC values in most solvents. The gels in paraffin oil had higher melting enthalpy than others in toluene. The study of the gelation process by 1H NMR and FT-IR showed the highly importance of the hydrogen bonding formation with support of π-π stacking. High entangled fibrillar network for the prepared xerogels was observed using scanning electron microscope. 2D-NOESY 1HNMR experiments revealed that gelator molecules were stacked-up head-to-tail. The two gelators also showed efficient selective removal of several oils in their mixtures with water.
Acknowledgements
Abdellatif thanks Follow-up Research Fellowship (Tokyo Human Resources Fund for City Diplomacy, TMU) for funding. Additionally, Abdellatif and Ibrahim have great thankful for nanomaterial investigation laboratory, Central lab. Network, National Research Centre for technical support and materials investigation.
Declaration of Competing Interest
None.
References
- New C-terminal hydrazide L-leucine derivatives as multi-solvent low molecular weight organogelators. J. Textiles, Color. Polymer Sci.. 2018;15(1):73-83.
- [Google Scholar]
- Evidence of intercolumnar π-π stacking interactions in amino-acid-based low-molecular-weight organogels. Langmuir. 2010;26(22):16818-16827.
- [Google Scholar]
- New sugar-based gelators bearing a p-nitrophenyl chromophore: remarkably large influence of a sugar structure on the gelation ability. J. Chem. Soc., Perkin Trans.. 1998;2(12):2585-2592.
- [Google Scholar]
- A new aromatic amino acid based organogel for oil spill recovery. J. Mater. Chem.. 2012;22(23):11658-11664.
- [Google Scholar]
- First report of phase selective gelation of oil from oil/water mixtures. Possible implications toward containing oil spills. Chem. Commun.. 2001;2:185-186.
- [Google Scholar]
- 1,2-O-(1-Ethylpropylidene)-α-d-glucofuranose, a low molecular mass organogelator: benzene gel formation and their thermal stabilities. Tetrahedron Lett.. 2008;49(47):6685-6689.
- [Google Scholar]
- Tracking hydrocarbon plume transport and biodegradation at deepwater horizon. Science. 2010;330(6001):201-204.
- [Google Scholar]
- Formation of oleogels based on edible lipid materials. Curr. Opin. Colloid Interface Sci.. 2011;16(5):432-439.
- [Google Scholar]
- Design and application of self-assembled low molecular weight hydrogels. Eur. J. Org. Chem.. 2005;2005(17):3615-3631.
- [Google Scholar]
- Solvent-gelator interactions-using empirical solvent parameters to better understand the self-assembly of gel-phase materials. Soft Matter. 2011;7(1):110-117.
- [Google Scholar]
- L-phenylalanine based low-molecular-weight efficient organogelators and their selective gelation of oil from oil/water mixtures. Soft Mater.. 2014;12(4):403-410.
- [Google Scholar]
- Chapter 12 – Physical spill countermeasures. In: Oil Spill Science and Technology. Boston: Gulf Professional Publishing; 2011. p. :303-337.
- [Google Scholar]
- Molecular organogels. Soft matter comprised of low-molecular-mass organic gelators and organic liquids. Acc. Chem. Res.. 2006;39(8):489-497.
- [Google Scholar]
- A review of oil, dispersed oil and sediment interactions in the aquatic environment: Influence on the fate, transport and remediation of oil spills. Mar. Pollut. Bull.. 2014;79(1):16-33.
- [Google Scholar]
- Biomimetic self-assembly of tetrapeptides into fibrillar networks and organogels. Eur. J. Org. Chem.. 2008;27:4580-4590.
- [Google Scholar]
- Sugar-derived phase-selective molecular gelators as model solidifiers for oil spills. Angew. Chem. Int. Ed.. 2010;49(42):7695-7698.
- [Google Scholar]
- Gelation of liquid crystals with self-assembled fibers. In: Low Molecular Mass Gelator. Heidelberg: Springer, Berlin; 2005. p. :219-236.
- [Google Scholar]
- A new class of phase-selective synthetic β-amino acid based peptide gelator: from mechanistic aspects to oil spill recovery. ChemPlusChem. 2014;79(10):1482-1488.
- [Google Scholar]
- Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev.. 2011;40(2):907-925.
- [Google Scholar]
- Self-assembled organogels formed by mono-chain l-alanine derivatives. Chem. Commun.. 2001;17:1556-1557.
- [Google Scholar]
- Controlling hydrogen bonding: from molecular recognition to organogelation. In: Molecular self-assembly organic versus inorganic approaches. Heidelberg: Springer, Berlin; 2000. p. :31-61.
- [Google Scholar]
- N-Acetylglucosamine-based efficient, phase-selective organogelators for oil spill remediation. Chem. Commun.. 2014;50(90):13940-13943.
- [Google Scholar]
- Low-molecular-weight organogelators as functional materials for oil spill remediation. Polym. Adv. Technol.. 2016;27(6):704-711.
- [Google Scholar]
- Long-term ecosystem response to the exxon valdez oil spill. Science. 2003;302(5653):2082-2086.
- [Google Scholar]
- Enantiomeric organogelators from d-/l-arabinose for phase selective gelation of crude oil and their gel as a photochemical micro-reactor. Chem. Commun.. 2014;50(81):12131-12134.
- [Google Scholar]
- Supramolecular structure of a sugar-appended organogelator explored with synchrotron X-ray small-angle scattering. Langmuir. 2003;19(20):8211-8217.
- [Google Scholar]
- Organogels from 1H-imidazole amphiphiles: entrapment of a hydrophilic drug into strands of the self-assembled amphiphiles. Chem. Mater.. 2005;17(12):3249-3254.
- [Google Scholar]
- Core Concepts in Supramolecular Chemistry and Nanochemistry. Wiley; 2007.
- Powerful low-molecular-weight gelators based on l-valine and l-isoleucine with various terminal groups. New J. Chem.. 2006;30(8):1184-1191.
- [Google Scholar]
- Biscalix[4]arene derivative as a very efficient phase selective gelator for oil spill recovery. Org. Lett.. 2013;15(22):5830-5833.
- [Google Scholar]
- Self-assembly of carbazole-containing gelators: alignment of the chromophore in fibrous aggregates. Tetrahedron. 2007;63(31):7358-7365.
- [Google Scholar]
- Dual-responsive two-component supramolecular gels for self-healing materials and oil spill recovery. Chem. Commun.. 2014;50(94):14839-14842.
- [Google Scholar]
- Functional Molecular Gels. Royal Society of Chemistry; 2013.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2019.06.003.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







