Translate this page into:
Deciphering the role of exogenously-applied vanillic acid in regulating drought stress tolerance in pea (Pisum sativum L.): Key growth and physio-biochemical attributes
⁎Corresponding authors. drnudrataisha@gcuf.edu.pk (Nudrat Aisha Akram), parvaizbot@yahoo.com (Parvaiz Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
To investigate the impact of exogenously applied vanillic acid (VA) in mitigating the adverse effects of drought stress, a greenhouse experiment was conducted on pea plants (Pisum sativum L.). The pea seeds were primed for 14 h in varying concentrations (0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 mM) of VA Then, thirty-five days old pea plants were subjected to control (100 % field capacity) and water deficit conditions (60 % F.C.). After thirty days of water stress treatments, the data showed a notable reduction in shoot and root fresh and dry weights, shoot and root lengths, and chlorophyll contents. While, water deficit stress led to an increase in leaf free proline, total phenolics, glycinebetaine (GB), ascorbic acid AsA) as well as the activities of catalase (CAT), superoxide dismutase (SOD) and peroxidase (POD) enzymes. We observed that seed priming with various concentrations of vanillic acid resulted in significant enhancement in shoot and root fresh and dry weights, shoot and root lengths, chlorophyll contents, proline, total phenolics, GB, AsA and the activities of POD, SOD and CAT enzymes of pea plants. Of varying concentrations of VA, 2.0 and 4.0 mM VA were more effective in improving the plant morphology and physio-biochemical metabolites of pea plants. So the results of the present study suggested that the improvement in growth and different physio-biochemical characteristics can be attributed to the VA-induced upregulation of osmoprotection and oxidative defense system of pea plants.
Keywords
Water stress
Pea (Pisum sativum L.)
Vanillic acid
Antioxidants
Osmoprotectants
1 Introduction
Often, the term “drought” refers to shortage of water compared to the demand of the plant according to the prevailing environmental conditions (Ali et al., 2016). Several regions of the world are currently experiencing drought stress due to unpredictable climate change (Javed et al., 2016; Lamaoui et al., 2018; Naumann et al., 2018; Seleiman et al., 2021). The drought −prone regions are increasing due to improper use of irrigations, so the crop productivity on such lands is hampering significantly (Naumann et al., 2018).
The condition of water shortage causes a significant reduction in leaf mass and area with a significant change in other morphological traits, such as the number of leaves per plant, and plant height (Seleiman et al., 2021; Yang et al., 2021). Plant developmental processes significantly slow down as drought duration and intensity increase (Duan et al., 2017). Moreover, during heat and drought stress, metabolic pathways experience significant alterations (Naz et al., 2014; Akram et al., 2016), and differentially regulated metabolism-related gene expression can be found, particularly in the cellular organelles such as mitochondria and plastids (Oleti, 2018). Water shortage has a significant impact on the functioning of a variety of metabolic processes, including the rate of photosynthetic activity, source-sink transport, and seed production (Aslam et al., 2013; Sehgal et al., 2018). For example, excessive generation of reactive oxygen species (ROS) generated by water stress deteriorates the chloroplast membranes, as the lipid peroxidation of cellular membranes is the result of over-production of ROS (Ashraf, 2009). In response to ROS, a variety of antioxidants (enzymatic/non-enzymatic) accumulate in the cells to reduce the destructive properties of ROS ((Razzaq et al., 2017; Koşar et al., 2022).
Pea (Pisum sativum L.) is an economical vegetable crop used all over the world. It is a very common model for genetic and physiological research. Pea has been the subject of countless scientific studies due to its simplicity of production, quick generation cycle, and significant morphological variation (Smýkal et al., 2012; Santos et al., 2019). However, it is categorized as a very sensitive crop in terms of its stress tolerance (Cernay et al., 2015; Devi et al., 2023). Although a variety of strategies are in vogue to enhance stress tolerance in plants, exogenous application of inorganic and organic chemicals has shown a promise in terms of improving plant stress tolerance. Thus, in the present investigation, vanillic acid (VA), one of the potential organic growth substances, was supplemented to drought-stressed pea plants to examine if this chemical could improve pea plants’ growth under water deficit conditions. Vanillic acid is de-scribed as a derivative of benzoic acid and an oxidized variant of vanillin is commonly used as a flavoring agent (Kim et al., 2010). It is a conjugated acid of vanillate and is a naturally occurring phenolic acid; vanillin is used widely in pharmaceuticals, cosmetics and the food industry as a flavoring agent (Imming et al., 2006). It was first reported in Melilotus messanensis (Macías et al., 1997), then in Chenopodium murale (Batish et al., 2007) and Dactylis glomerata (Parveen et al., 2011). Being a phenolic compound, vanillic acid in plants reduces the activity of root system by the allelopathy phenomenon (Chen et al., 2011). Different fruits (grapes, pomegranate, etc.), herbs and spices (cinnamon, tea, rosemary, sage, thyme, oregano, mint, ginger, etc.) and vegetables (pumpkin, broccoli, drumstick, curry, nettle, etc.) are the main source of vanillic acid (Ingole et al., 2021).
The influence of vanillic acid on the development and metabolism of different plants has been examined in different studies. For example, exogenously applied vanillic acid promoted alfalfa seedlings’ growth, particularly the development of plant aerial compo-nents (Khaleda et al., 2017). Likewise, another study, (Parvin et al., 2020) while assessing the impact of VA on tomato seedlings under saline stress, found a significant enhancement in growth of the tomato seedlings which was reported to be associated with reduced stress-induced oxidative stress as well as tissue Na+/K+ ratio. In the same crop, a marked increase in the actions of key antioxidative enzymes was observed (Ghareib et al., 2010; Singh et al., 2021). Moreover, working with maize (Stingu et al., 2011) showed 45 % improvement in the growth of maize seedlings supplied exogenously with vanillic acid. In pea particularly, the growth of the roots was suppressed by vanillic acid at high concentrations (Vaughan and Ord, 1990).
Keeping in view the effectivity of VA in improving plant metabolism and stress tolerance, it was hypothesized that seeds treated with different levesl of VA might improve the drought stress tolerance of pea plants. Thus, the primary aim of this study was to evaluate, that up to what extent exogenously applied vanillic acid as a seed treatment could improve the growth and vital physiologi-cal and biochemical processes in pea.
2 Materials and Methods
2.1 Growth conditions and treatments
An experiment using plastic pots (diameter 28.5 cm2) containing 7.5 kg soil/pot sandy-clay-loam (45:25:30) was performed from October to December 2021 to determine the effectivity of different levels of vanillic acid (VA) in the regulation of drought stress tolerance. The soil had organic matter, 0.79 %; pH 7.9, EC 3.01 dS m−1; P, 5.2 mg kg−1, and K, 398 mg kg−1. The experiment was designed using a completely randomized approach, involving three factor-factorial [drought (2) x cultivars (1) x levels of VA (8)] with four pots or replications of each treatment (total eight pots and 40 plants per treatment). The sample size (pots) was a total of 64 (2 x 1 x 8 x 4 = 64) experimental units. The pots were placed in the Plant Sanctuary, Government College University Faisalabad, Pakistan with a latitude of 31O- 26′ N; a longitude of 73O- 06′ E and an altitude of 184.4 m. The seed of a pea variety, Sarsabaz, was procured from the Vegetable Section at the Ayub Agricultural Research Institute in Faisalabad, Pakistan. During the experimental period, the average (day + night) temperature was 26.8 °C, the average relative humidity was 68.9 %, the average rainfall was 0.3 mm and the average light period was 8.1 h/day. The seeds were surface sterilized by washing them in 0.05 % sodium hypochlorite. Then the seeds were primed for 14 h with varied levels (0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 mM) of VA (C8H8O4; molecular weight, 168.15 g; Across Organic Chemicals, Pakistan). Various concentrations of VA were formulated using deionized water. Then, 10 seeds were planted in each pot (replicate). Seed germination was noted daily and all seeds germinated in eight days. Following 15 days of seed germination, a thinning process was carried out, and each pot/replicate contained five seedlings of uniform size. At the same time, drought stress treatments comprising a control group (maintained at 100 % field capacity) and a water deficit stress group (subjected to 60 % field capacity) were initiated, and the required levels were maintained based on the soil saturation (32 mL/100 g oven dry soil) level percentage as well as field capacity (16 mL/100 g dry soil). These levels took twenty days to attain the desired field capacities (100 % and 60 %). After thirty days of drought stress initiation, data for growth and physiological and biochemical characteristics were determined.
2.2 Morphological attributes
Two plants were gently pulled out from each pot/replicate (eight plants per treatment), followed by thorough rinsing with distilled water and keeping them on a blotting paper to remove water. The plant samples were separated into roots and shoots, and their lengths were measured. Subsequently, their fresh weights were recorded. After that, the shoot and root samples were air-dried before placing them in an oven set at 70 °C for 72 h, and finally, their dry weights were recorded.
2.2.1 Leaf relative water contents (LRWC)
A fresh leaf 2nd one from the top was sampled from the plants and placed in a water tub after determining their fresh weights. After keeping these samples in water for three hours, their turgid measurements of weights were documented. Then all samples were oven-dried for 72 h and recorded their dehydrated masses. Following Jones and Turner (1978), RWC was determined.
2.2.2 Relative membrane permeability (RMP)
A young 2nd leaf from the top (500 mg) was shredded in ten milliliters of deionized water. After two hours, EC0 was recorded. The specimens were retained for a night and documented their EC1. Then all samples were autoclaved for 30 min. The specimens were allowed to cool down to ambient temperature, and then their EC2 was measured using a formula proposed by Yang et al. (1996), and the RMP was calculated.
2.3 Chlorophyll (a and b) content
Following the procedure established by Arnon (1949), a freshly harvested 2nd leaf weighing 0.5 g was macerated in a mortar and pestle with 10 ml of 80 % (v/v) acetone un-der cold conditions. The samples were kept at 4 °C for 24 h. Subsequently, a spectrophotometer was employed to measure the absorbance at wavelengths of 645 and 663 nm.
2.4 Osmoprotectants (Proline and Glycinebetaine)
To measure the proline levels following the procedure of Bates et al. (1973), 10 mL of sulfosalicylic acid (3 %) was used to homogenize 0.5 g of fresh young leaf and then filtered. The sample extract (2 mL) was mixed with acidic ninhydrin (2 ml) and glacial acetic acid (2 ml). Every sample was subjected to boiling in a water bath, and subsequently, the mixture was placed in an ice bath. Subsequently, 4 mL of toluene was added to each sample, and the absorbance of the upper layer was observed at 520 nm.
For GB content determination, a fresh young leaf (500 mg) was mixed in 10 mL of deionized water, and the samples were prepared following the procedure as delineated by Grieve and Grattan (1983). A spectrophotometer was utilized to record the absorbance of the lower organic layer, at a wavelength of 365 nm.
2.4.1 Ascorbic acid content
The youngest 2nd leaf from top (500 mg) was homogenized in trichloroacetic acid (10 ml; 6 %). Further reactions were carried out following the procedure proposed by Mukherjee and Choudhuri (1983). A volume of 2 mL of each leaf extract was combined with 2 mL of 2 % (v/v) dinitrophenyl hydrazine. To this mixture, 1.0 mL of 10 % (w/v) thiourea was introduced, and the samples were subjected to boiling in a water bath for 15 min before being cooled to room temperature. Following this, 5 mL of 80 % (v/v) H2SO4 was added, and the absorbance was measured at 530 nm.
2.4.2 Total phenolic content
Freshly harvested top 2nd leaf (250 mg) was extracted in 5 mL (80 %) acetone according to the method developed by Julkunen-Tiitto (1985). Following the centrifugation process, 0.1 mL of the sample was blended with 2 ml dH2O. The sample extract was supplemented with 1.0 mL of Folin–Ciocalteu's phenol reagent and 5 mL of 20 % sodium carbonate. Subsequently, spectrophotometric analysis was conducted at 750 nm to determine the total phenolic content.
2.5 Activities of enzymatic antioxidants
A fresh top 2nd leaf was preserved in an ultra-low freezer for a week. Then, a 500 mg leaf sample was triturated in a K-buffer (10 ml; 50 mM; pH 7.8). After centrifugation, the mixture was stored in sterilized Eppendorf tubes for determining the activities of POD, CAT and SOD enzymes. The SOD activity was assessed according to the method described by Giannopolitis and Ries (1977), whereas those of POD and CAT enzymes were ob-served using the protocol outlined by Chance and Maehly (1955).
2.6 Statistical analysis
The data of different attributes mentioned earlier were subjected to data analysis using Co-Stat v. 306, employing analysis of variance (ANOVA) in a completely randomized design. Mean values were subsequently compared using the least significant difference at the 5 % probability level.
3 Results
Drought stress [60 % field capacity (F.C.)] significantly (P ≤ 0.001) inhibited the shoot fresh (29.9 %) and dry weights (28.6 %) of pea (Pisum sativum L.) plants. However, seed priming with varying levels (0.5, 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 mM) of vanillic acid (VA) caused a substantial (P ≤ 0.001) improvement regarding fresh and dry weights of pea plant shoots (Table 1; Fig. 1AB). In general, the levels of 4.0 mM and 6.0 mM of VA exhibited greater effectiveness in promoting both fresh and dry shoot weights of pea plants both under normal (100 % F.C.) and drought stress (60 % F.C.) conditions. Additionally, water stress led to a notable reduction in the fresh (P ≤ 0.01; 15.5 %) and dry (P ≤ 0.01; 11.23 %) weights of roots of pea plants.The influence of VA was also significantly (P ≤ 0.001) effective in enhancing root biomass. The more prominent results were found at 2.0 mM and 4.0 mM concentrations, particularly under water deficit conditions. Exogenous VA demonstrated a significant increase in the fresh and dry weights of the roots of pea plants (Fig. 1CD). ns = non-significant.
Source of Variation
df
Shoot fresh weight
Shoot dry weight
Root fresh weight
Root dry weight
Drought stress (D)
1
5.286***
0.099***
1.676**
0.063***
Vanillic acid (VA)
7
0.876***
0.057***
0.464**
0.019***
D x VA
7
0.198 ns
0.006*
0.455**
0.002 ns
Shoot length
Root length
Leaf area per plant
LRWC
Drought stress (D)
1
568.5***
497.2***
4.557**
1750***
Vanillic acid (VA)
7
105.4***
95.14***
2.719***
366.3***
D x VA
7
13.22 ns
20.42***
0.042 ns
43.63 ns
RMP
Chlorophyll a
Chlorophyll b
Total chlorophyll
Drought stress (D)
1
298.5***
0.261*
0.038 ns
0.262*
Vanillic acid (VA)
7
177.7***
0.114*
0.138***
0.114*
D x VA
7
6.659 ns
0.021 ns
0.002 ns
0.021 ns
Chlorophyll a/b ratio
Proline
Glycinebetaine
Ascorbic acid
Drought stress (D)
1
0.792 ns
0.799**
159.4***
15.36*
Vanillic acid (VA)
7
2.053 ns
2.604***
107.8***
16.39***
D x VA
7
0.160 ns
0.096 ns
3.242 ns
1.233 ns
Total phenolics
SOD
POD
CAT
Drought stress (D)
1
160.1***
1.693**
0.162*
0.042***
Vanillic acid (VA)
7
26.93***
1.815***
0.115***
0.006*
D x VA
7
2.477 ns
0.042 ns
0.005 ns
0.001 ns
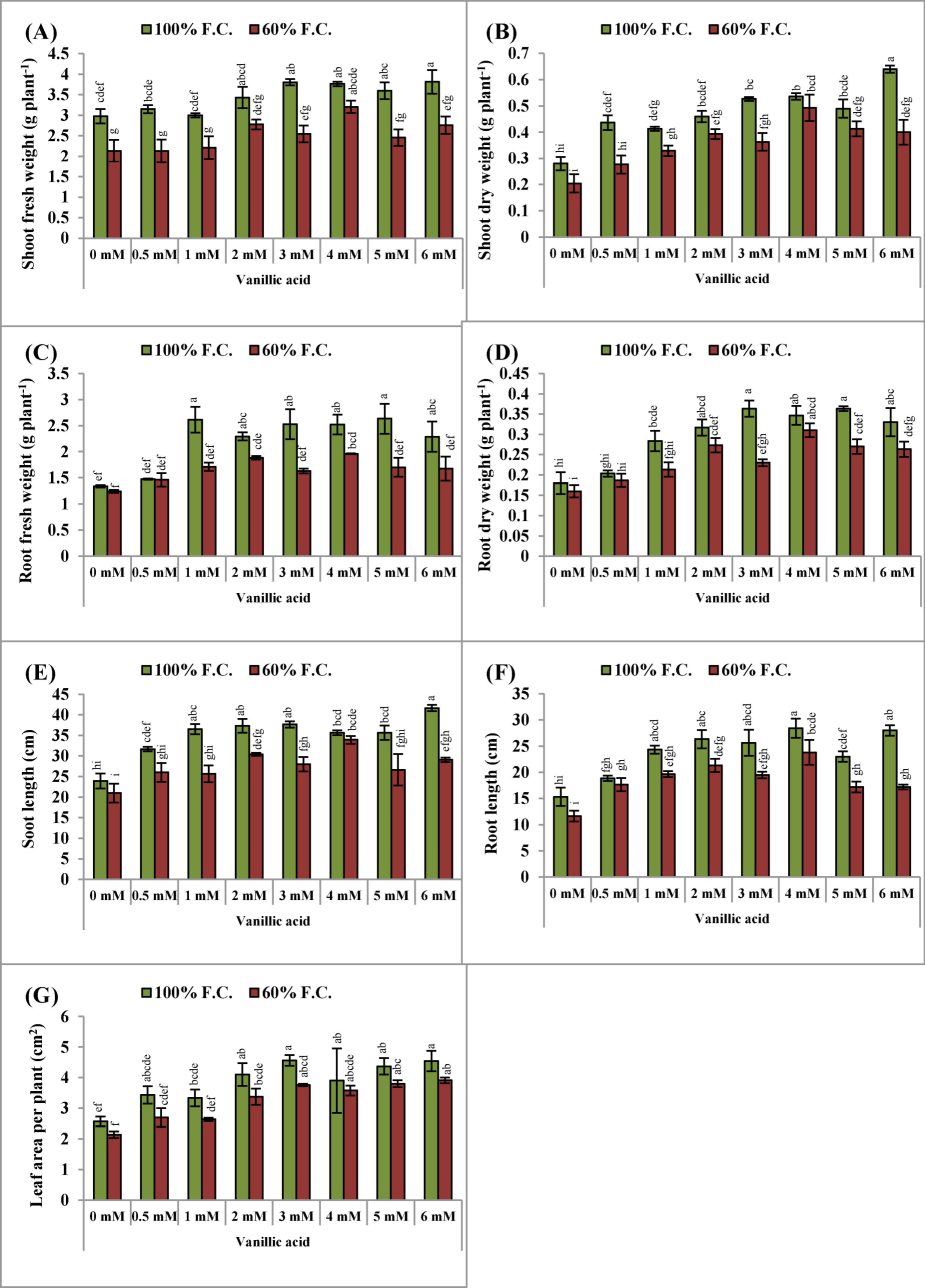
(A-G): Shoot and root fresh and dry weights, shoot and root lengths and leaf area per plant (Mean ± S.E.; n = 4) of pea (Pisum sativum L.) plants raised from seeds treated with varyings levels of vinillic acid and subjected to control (100 % field capacity) and drought stress (60 % field capacity). Different letters (a-i) showing difference among treatments.
Considerable reductions were observed in both shoot and root lengths of the pea plants (P ≤ 0.001) in dehydrated circumstances. Varying concentrations of VA significantly increased the shoot and root lengths (P ≤ 0.001) of the pea plants (Table 1). Of all levels of VA used, 2.0 mM and 4.0 mM VA were more effective in enhancing the shoot and root lengths of the pea plants in stress environments (Fig. 1EF). Similarly, a marked (P ≤ 0.01) reduction was detected in the leaf area of the pea plants (Table 1) un-der drought stress conditions (Fig. 1G). Varying levels of VA noticeably (P ≤ 0.001) enhanced leaf area per plant under both stressed and unstressed conditions. Of all VA concentrations, 4.0 mM VA was more effective in minimizing the influence of shortage of water on the pea plants.
Under water deficiency, the leaf relative water contents (LRWC) of the pea plants were recorded to be markedly declined (P ≤ 0.001). Different VA levels had noticeable effects in enhancing the LRWC, and of all VA levels, 3 and 6 mM VA were more effective than the other levels under both normal and water scarcity surroundings (Table 1; Fig. 2A).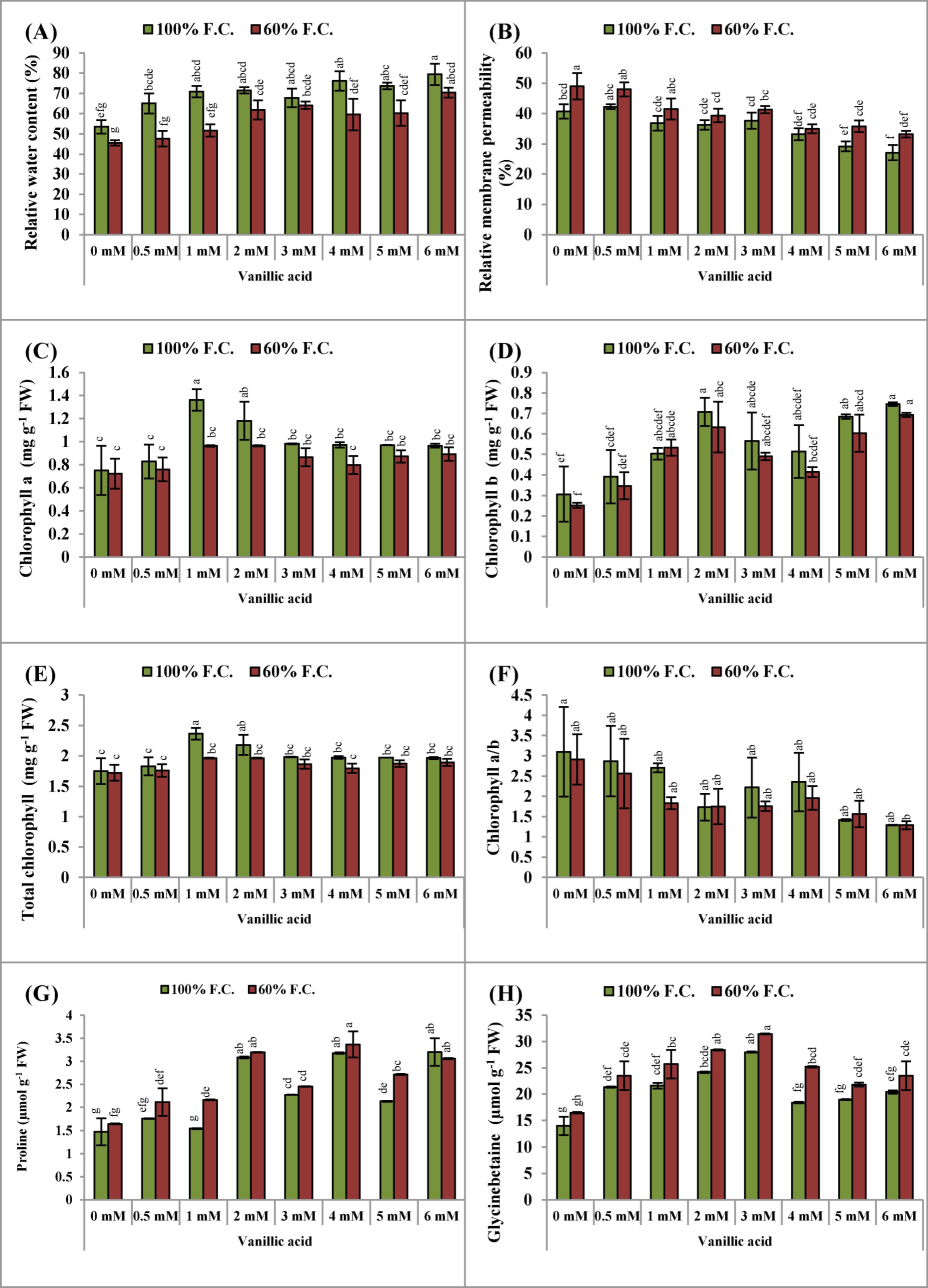
(A-H): Relative water contents, relative membrane permeability, chlorophyll pigments (a, b, total and a/b ratio), proline and glycinebetaine contents (Mean ± S.E.; n = 4) of pea (Pisum sativum L.) plants raised from seeds treated with varyings levels of vinillic acid and subjected to control (100 % field capacity) and drought stress (60 % field capacity). Different letters (a-g) showing difference among treatments.
Under water-deficit stress, relative membrane permeability (RMP) was increased noticeably (P ≤ 0.001, Table 1) in the pea plants. However, the application of VA decreased the RMP significantly (P ≤ 0.001) in the pea plants. Of all VA levels, 6 mM was more effective in dropping RMP under both water regimes (Table 1; Fig. 2B).
Chlorophyll a and total chlorophyll concentrations reduced markedly (P ≤ 0.05) in water-stressed circumstances around the pea plants. Nonetheless, there was no noticeable alteration induced by drought in the chlorophyll b levels and the chlorophyll a/b ratio of the pea plants. The pre-treatment of seeds with different concentrations of VA significantly enhanced the levels of chlorophyll a, b, and total chlorophyll (P ≤ 0.05, P ≤ 0.001, and P ≤ 0.05, respectively). Notably, among all the levels employed under both water conditions, 2 mM VA exhibited the most pronounced effectiveness (refer to Table 1 and Fig. 2CDEF). There was no notable alteration detected in the chlorophyll a/b ratio of the pea plants at both water regimes.
A promising (P ≤ 0.01) increase in proline contents was noticed in shortage of water situations (Fig. 2G). Seed priming with different levels of VA (0.5, 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 mM), some levels of VA was found to be very effective (P ≤ 0.001) in enhancing the proline accumulation in the pea plants under water scarcity circumstances (Table 1). Of all concentrations of VA, 1.0 and 5.0 mM were observed more promising for the pea plants in accumulating a substantial amount of proline under water deficit stress.
A noticeable (P ≤ 0.001) rise in glycinebetaine (GB) was perceived in drought-stressed pea plants. Exogenous supplementation of VA was found instrumental for raising GB contents of the pea plants 4.0 and 6.0 mM doses of VA were rated as the best for achieving increased GB content in the pea plants at 60 % F.C. (Table 1; Fig. 2H).
Ascorbic acid (AsA) concentration was recorded to be higher in the drought-stressed pea plants than in the control untreated plants. However, varying levels of VA considerably (P ≤ 0.001) improved the AsA contents, and 2.0 mM was more promising than the other VA levels in increasing the AsA levels in the drought-stressed pea plants (Fig. 3A).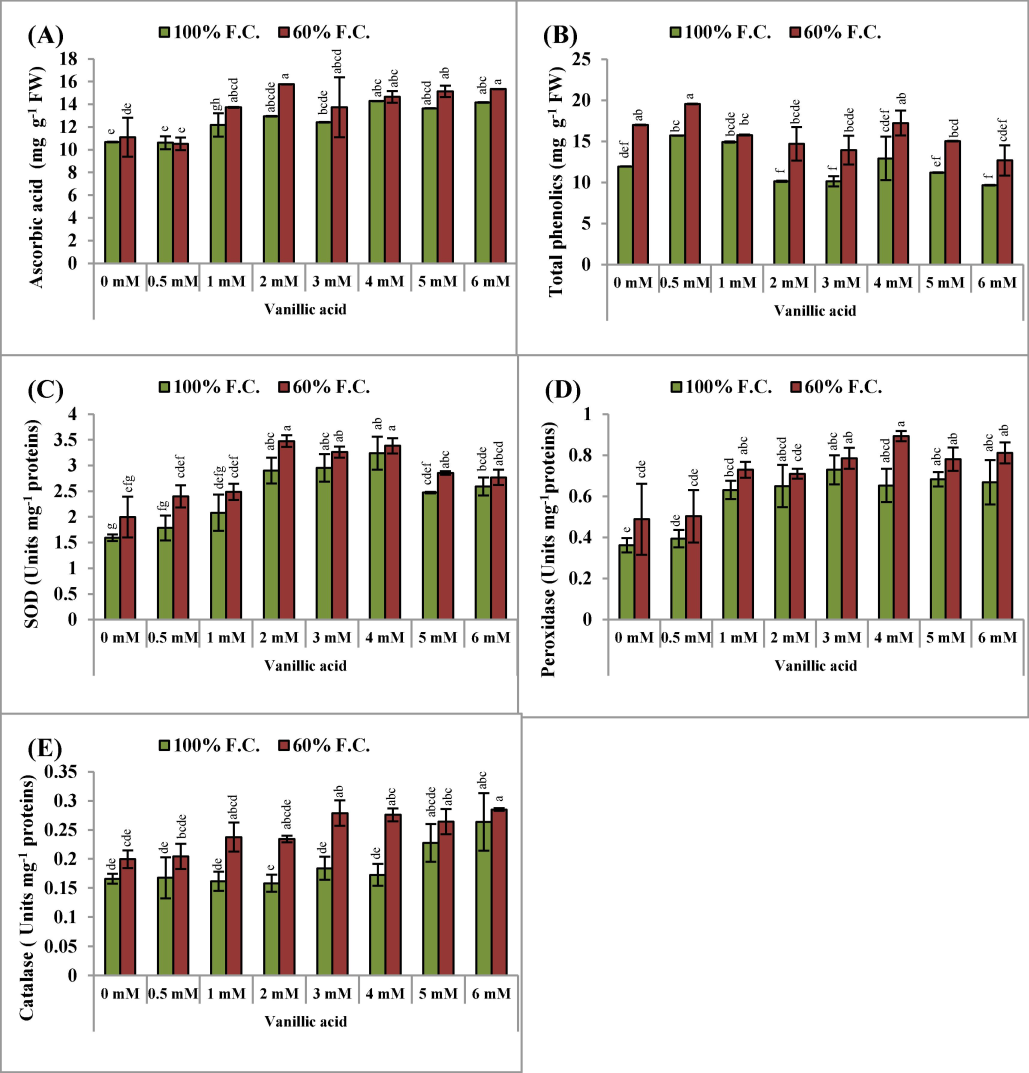
(A-E): Ascorbic acid, total phenolics as well as activities of superoxide dismutase, peroxidase and catalase enzymes (Mean ± S.E.; n = 4) of pea (Pisum sativum L.) plants raised from seeds treated with varyings levels of vinillic acid and subjected to control (100 % field capacity) and drought stress (60 % field capacity). Different letters (a-g) showing difference among treatments.
Total phenolic contents were significantly (P ≤ 0.001) higher in the water-stressed pea plants than those in the untreated plants. The supplementation of VA resulted in a marked rise in the total phenolic content of the pea plants, with the most substantial enhancement observed at 0.5 mM VA under water-deficient conditions (Table 1 and Fig. 3B).
Drought stress led to a significant increase in the activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) (P ≤ 0.01; 0.05; 0.001, respectively) enzymes in the leaves of the pea plants (Table 1). The priming of pea seeds with VA had a significant (P ≤ 0.001; 0.001; 0.05) stimulating influence in accelerating the activities of SOD, POD and CAT, particularly under drought stress conditions (Table 1; Fig. 3CDE). Under both water regimes, 2.0, 3.0 and 4.0 mM VA markedly boosted the functionality of all antioxidant enzymes.
4 Discussion
Water scarcity is recognized as a primary factor causing a major decline in crop bio-mass and yields all over the world, particularly in water-scarce and semi-arid areas (Akram et al., 2023). It is widely known that water deficit conditions profoundly affect the growth and development of almost all plant species (Shafiq et al., 2014; Ahluwalia et al., 2021; Seleiman et al., 2021) because adequate water availability is essential at each phase of a plant's life cycle. This challenging condition may lead to a reduction of more than 50 % in the average yield of major crops (Wang et al., 2003; Ashraf et al., 2011; Lamaoui et al., 2018). However, there is substantial evidence that plants can adjust themselves by altering physiological, biochemical, and anatomical features in response to both internal and external climate related factors including drought stress (Naz et al., 2023). Through the adaptive mechanisms including osmoprotection, osmotic adjustment, ionic compartmentalization, upregulation of antioxidants and accumulation of stress hormones, plants can endure and thrive well in challenging environmental conditions (Shafiq et al., 2015; Akram et al., 2016; Naz et al., 2023). Moreover, a multitude of strategies are in vogue to alter plants that could flourish well under harsh environmental indications. Of those, seed priming is considered a shotgun and efficient technique for promoting plant growth under stressful regimes (Ashraf and Foolad, 2005; Akram et al., 2020). However, for seed priming, a variety of organic and inorganic chemicals are currently under use (Akram et al., 2020; Kong et al., 2023).
In the current investigation, water stress significantly decreased both the fresh and dry weights of shoots and roots in the pea plants, whereas seed priming with different levels of vanillic acid triggered an important improvement in the fresh and dry weights of the pea plants (Table 1; Fig. 1) under varying water regimes. Overall, 4.0 mM and 6.0 mM levels of VA were more effective in enhancing the fresh and dry weights of the pea plants. Since no relevant literature is available to exhibit the role of the exogenous application of VA (a phenolic compound) to plants, the results recorded here could be treated as the first study in this regard. Nonetheless, Moran-Palacio et al. (2014) observed a positive relationship between total phenolic content and antioxidant properties in Rhizophora mangle and Krameria erecta plants. Furthermore, vanillic acid and p-hydroxy benzoic acid were identified as growth-promoting substances that mitigated leaf contraction and senescence in sorghum exposed to temperature stress conditions (Ahmad et al., 2016). Consequently, it is plausible to suggest that the foliar treatment of VA may play a vital role in triggering the production of osmoprotectants, to enhance drought resistance and improve the survival capacity of plants under water-limited conditions as found in rice (Ahmad et al., 2016).
The relationship between reduced water content and the adaptability of cell mem-branes to withstand various environmental signals, including those of drought stress has already been widely reported (Liu et al., 2002; Ahmad et al., 2016; Yang et al., 2021). Moreover, under water-deficit conditions, the sustainability and permeability of cell membranes tend to decrease plant growth (Blokhina et al., 2003). When assessing the physiological implications of cellular water scarcity, LRWC is considered a potential criterion for evaluating the water status of plants. However, consistent with earlier reported studies, the findings of the current study demonstrated that LRWC was significantly affected in the drought-stressed pea plants. However, VA exogenous application had a positive effect on LRWC, which is consistent with the findings of Hura et al. (2012). They reported a notable increase in phenolics bound to the cell wall, which were associated with enhanced water retention within the plant. This led to a delay in leaf desiccation and the development of leaf undulations. Additionally, these phenolic compounds, including derivatives of hydroxycinnamic acids (such as ferulic acid and p-coumaric acid) and flavonoids (such as kaempferol and quercetin), were localized in the cell walls and vacuoles of the epidermis. This localization potentially functions as a photoprotective mechanism for the photosynthetic apparatus, providing defense against the potential damage to leaf cell structures caused by UV radiation (Hura et al., 2012; Nichols et al., 2015).
Under water deficit conditions, chlorophyll pigments play a crucial role in energy dissipation and light absorption during photosynthesis (Akram et al., 2018). In the existing study, scarcity of water led to a decline in photosynthetic pigments in the pea plants. This reduction in pigments under water shortage is a commonly observed reaction across various crops, such as mung bean (Batra et al., 2014), potato (Arabshahi and Mobasser, 2017), chickpea (Mafakheri et al., 2010), carrot (Razzaq et al., 2017), and canola (Akram et al., 2018), suggesting a shared adaptive mechanism of plants to drought conditions. The decrease in chlorophyll levels can be attributed to excessive production of ROS, disruptions in nutrient balance, and disturbances in enzyme activities caused by cellular or plant-level water deficiency. In accordance with these findings, the current study exhibited a reduction in chlorophyll a content under water deficit situations. However, the use of VA pointedly mitigated the harmful effects of drought on the pea plants. Likewise, Xuan and Khang (2018), reported that foliar application of low concentrations of vanillic acid increased the chlorophyll contents in rice plants.
Two essential osmolytes, glycinebetaine (GB) and proline, are known to accumulate in numerous crop species under stressful conditions and they play a critical part in osmotic modification (Raza et al., 2016). Raza et al. (2014) conveyed that a high accumulation of GB enhanced plant tolerance to various abiotic stresses, together with water shortage. The accumulation of GB at a high level enhanced the drought resistance of plants by promoting the functionality of antioxidant enzymes (Ma et al., 2014) and maintaining turgor pressure (Ashraf and Foolad, 2007). In the deficient supply of water, the decrease in leaf water potential triggers an accelerated synthesis of GB, which helps maintain the osmotic potential of leaves (Ashraf and Foolad, 2007). Moreover, proline is also known for its role in protecting plants against ROS and regulating osmoregulation (Aranjuelo et al., 2010; Yaqoob et al., 2019). This study revealed a noteworthy elevation in the concentration of both proline and GB, particularly under the water stress level of 60 % F.C. Studies on radish plants (Akram et al., 2016) and rice by Galahitigama and Wathugala (2016) demonstrated that the enhanced accumulation of proline and GB under drought stress contributed to increased stress tolerance. Moreover, in the current study, the exogenous spray of vanillic acid augmented the concentration of proline and GB contents in the pea plants subjected to water stress as well as normal watering. However, VA-induced accumulation in GB or proline could not be linked with any earlier investigation, as no report is available in the literature on this aspect.
The plant's defense against oxidative stress includes both enzymatic and non-enzymatic antioxidants, which shield plant cells from damage caused by drought stress. Some investigations have demonstrated that augmentation of the antioxidative defense system could enhance drought tolerance across various plant species, e.g., radish (Shafiq et al., 2015), and rice (Nounjan et al., 2012). Among non-enzymatic compounds, ascorbic acid is widely known for its ability to protect plants against various abiotic stresses by effectively rummaging oxy-gen-free radicals (Shafiq et al., 2014). Ejaz et al. (2012) stated that the cellular level of AsA is associated with the stimulation of plants' protective system. Ascorbic acid plays a crucial role in plant growth and is implicated in various physiological processes, e.g., division of cells, cell expansion, and several others (De Gara, 2004). In this study, we observed a rise in ascorbic acid content in the pea plants subjected to drought stress, which is consistent with earlier findings in maize (Dolatabadian et al., 2010) wherein a significant elevation in AsA, particularly reported under high drought intensity. Furthermore, in our study, the use of VA enhanced the accumulation of ascorbic acid in water-deficit environments. Under drought-induced oxidative stress, phenolic compounds accumulate and protect fatty acids, as previously reported by Frary et al. (2010) and Amri et al. (2017). In the course of our investigation, we noted a rise in total phenolic content in the pea plants under water deficit conditions, which aligns with the findings recorded in maize (Moharramnejad et al., 2015), canola (Dawood and Sadak, 2014), and quinoa (Aziz et al., 2018) under drought conditions.
Superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) are enzymatic antioxidants that perform a crucial role in neutralizing reactive oxidants, thereby en-hancing stress tolerance in crops against drought. Previous studies by Ashraf (2009) and Akram et al. (2018) have reported the involvement of these enzymes in the mechanism of drought tolerance in dif-ferent plants. It has been suggested that the ability of a species to tolerate drought stress can be linked to the enhanced activity of antioxidant enzymes, as demonstrated by Lima et al. (2002) and Yadav and Sharma (2016). In our current study, we observed higher activities of SOD, CAT, and POD in the drought-stressed pea plants, similar to the findings reported earlier in canola (Akram et al., 2018) and radish (Shafiq et al., 2015) cultivars that exhibited increased enzyme activities under stress conditions. The exogenous application of VA positively influenced the ac-tions of superoxide dismutase, catalase, and peroxidase enzymes in drought-hit plants, similar to the stimulation of catalase and superoxide dismutase for nullifying the reactive oxygen species as observed in rice under flooding conditions (Xuan and Khang, 2018). Numerous scientists have described the role of phenolic compounds in increasing the activities of superoxide dismutase and catalase in water-deficit plants for ROS detoxification (Abu El-Soud et al., 2013; Singh et al., 2019).
5 Conclusions
Seed priming with vanillic acid enhanced the growth and regulated physio-biochemical parameters of drought-stressed pea plants. It also improved these attributes in non-stressed control plants. These results suggested that vanillic acid treatment can be advantageous for promoting plant growth under both stressful and non-stressful conditions. So, the capability of vanillic acid to enhance stress tolerance in crop plants can provide valuable benefits to farmers facing water deficit stress conditions.
6 Consent to participate
All authors consent to participate in the manuscript publication.
7 Consent for publication
All authors approved the manuscript to be published.
Ethics approval
Not applicable.
CRediT authorship contribution statement
Abdul Rehman: Methodology, Writing original draft. Nudrat Aisha Akram: Visualization, Supervision. Muhammad Ashraf: Conceptualization, review & editing. Abdulaziz Abdullah Alsahli: Formal analysis. Parvaiz Ahmad: Data curation, review & editing.
Funding
This research received no external funding.
Acknowledgments
The Ph.D. scholar acknowledges the support provided by Stress Biology Lab, Department of Botany, Government College University, Faisalabad, Pakistan. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R236), King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ability of ellagic acid to alleviate osmotic stress on chickpea seedlings. Plant Physiol. Biochem.. 2013;71:173-183.
- [Google Scholar]
- A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021;5:100032
- [Google Scholar]
- Improvement of sorghum crop through exogenous application of natural growth-promoting substances under a changing climate. Sustainability. 2016;8:13-30.
- [Google Scholar]
- Drought-induced anatomical changes in radish (Raphanus sativus L.) leaves supplied with trehalose through different modes. Arid Land Res. Manag.. 2016;30:412-420.
- [Google Scholar]
- Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma. 2018;255:163-174.
- [Google Scholar]
- Foliage application and seed priming with nitric oxide causes mitigation of salinity-induced metabolic adversaries in broccoli (Brassica oleracea L.) plants. Acta Physiol. Plant.. 2020;42:1-9.
- [Google Scholar]
- Exogenous α-tocopherol regulates the growth and metabolism of eggplant (Solanum melongena L.) under drought stress. Plants. 2023;12:237.
- [Google Scholar]
- Performance evaluation of gladiolus varieties under diverse climatic conditions. Plant Gene. Trait.. 2016;7:1-8.
- [Google Scholar]
- Oil characterization and lipids class composition of pomegranate seeds. Biomed. Res. Int.. 2017;8
- [Google Scholar]
- Effect of drought stress on carotenoid and chlorophyll contents and osmolyte accumulation. Med. Chem. Res. 2017;2:193-197.
- [Google Scholar]
- Plant physiology and proteomics reveals the leaf response to drought in alfalfa (Medicago sativa L.) J. Exp. Bot. 2010;62:111-123.
- [Google Scholar]
- Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949;24:1.
- [Google Scholar]
- Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv.. 2009;27:84-93.
- [Google Scholar]
- Drought tolerance: roles of organic osmolytes, growth regulators, and mineral nutrients. Adv. Agron. 2011;111:249-296.
- [Google Scholar]
- Pre-sowing seed treatment -A shotgun approach to improve germination, plant growth, and crop yield under saline and non‐saline conditions. Adv. Agron.. 2005;88:223-271.
- [Google Scholar]
- Improving plant abiotic-stress resistance by exogenous application of osmoprotectants glycine betaine and proline. Environ. Exp. Bot.. 2007;59:206-216.
- [Google Scholar]
- Estimation of genetic variability and association among different physio-logical traits related to biotic stress (Fusarium oxysporum L.) in chickpea. J. Anim. Plant. Sci.. 2013;23:1679-1685.
- [Google Scholar]
- Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem.. 2018;123:192-203.
- [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205-207.
- [Google Scholar]
- Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul.. 2007;51:119-128.
- [Google Scholar]
- Drought-induced changes in chlorophyll fluorescence, photosynthetic pigments, and thylakoid membrane proteins of Vigna radiata. J. Plant Interact. 2014;9:712-721.
- [Google Scholar]
- Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot.. 2003;91:179-194.
- [Google Scholar]
- Estimating variability in grain legume yields across Europe and the Americas. Sci. Rep.. 2015;5:11171.
- [Google Scholar]
- Quantification of terrestrial ecosystem carbon dynamics in the conterminous United States combining a process-based biogeochemical model and MODIS and Ameri Flux data. Biogeosci. Discuss. 2011;8:2665-2688.
- [Google Scholar]
- Physiological role of glycine betaine in alleviating the deleterious effects of drought stress on canola plants (Brassica napus L. Mid. East J. Agric. Res.. 2014;3:943-954.
- [Google Scholar]
- Ascorbate and plant growth: from germination to cell death. In: Vitamin C. Taylor & Francis; 2004. p. :92-105.
- [Google Scholar]
- Heat stress tolerance in peas (Pisum sativum L.): Current status and way forward. Front. Plant Sci. 2023;13:1108276
- [Google Scholar]
- Effect of ascorbic acid foliar application on yield, yield component and several morphological traits of grain corn under water deficit stress conditions. Not. Sci.biol.. 2010;2:45-50.
- [Google Scholar]
- Effects of drought stress on growth and development of wheat seedlings. Int. J. Agric. Biol.. 2017;19:1119-1124.
- [Google Scholar]
- Effect of exogenous application of ascorbic acid on antioxidant enzyme activities, proline contents, and growth parameters of Saccharum spp.; hybrid cv. HSF-240 under salt stress. Turk. J. Biol.. 2012;36:630-640.
- [Google Scholar]
- Salt tolerance in Solanum pennellii: Antioxidant response and related QTL. BMC Plant Biol.. 2010;10:58.
- [Google Scholar]
- Pre-sowing seed treatments improves the growth and drought tolerance of rice (Oryza sativa L.) Imp. J. Interdiscip. Res.. 2016;2:1074-1077.
- [Google Scholar]
- Antioxidative effects of the acetone fraction and vanillic acid from Chenopodium murale on tomato plants. Weed Biol. Manag.. 2010;10:64-72.
- [Google Scholar]
- Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol.. 1977;59:309-314.
- [Google Scholar]
- Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant Soil. 1983;70:303-307.
- [Google Scholar]
- An increase in the content of cell wallbound phenolics correlates with the productivity of triticale under soil drought. J. Plant Physiol.. 2012;169:1728-1736.
- [Google Scholar]
- Drugs, their targets and the nature and number of drug targets. Nat. Rev. Drug Discov. 2006;5:821-834.
- [Google Scholar]
- A review of the pharmacological characteristics of vanillic acid. J.drug Del. Therap.. 2021;11:200-204.
- [Google Scholar]
- Assesment of genetic diversity in wheat synthetic double haploids for yield and drought related traits through factor and cluster analyses. Plant Gene. Trait.. 2016;7:1-9.
- [Google Scholar]
- Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiol.. 1978;61:122-126.
- [Google Scholar]
- Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J. Agric. Food Chem.. 1985;33:213-217.
- [Google Scholar]
- Foliar application of humic acid or a mixture of catechol and vanil-lic acid enhanced growth and productivity of alfalfa. J. Kor. Grass. Forage. Sci.. 2017;37:248-253.
- [Google Scholar]
- The beneficial effect of vanillic acid on ulcerative colitis. Molecules. 2010;15:7208-7217.
- [Google Scholar]
- Seed priming with fullerol Improves Seed Germination, Seed-ling Growth and Antioxidant Enzyme System of Two Winter Wheat Cultivars under Drought Stress. Plants. 2023;12:1417.
- [Google Scholar]
- Understanding migration aversion using elicited counterfactual choice proba-bilities. J. Econom.. 2022;231:123-147.
- [Google Scholar]
- Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018;6:26.
- [Google Scholar]
- Photochemical responses and oxidative stress in two clones of Coffea canephora under water deficit conditions. Environ. Exp. Bot. 2002;47:239-247.
- [Google Scholar]
- Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002;80:780-787.
- [Google Scholar]
- Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014;80:60-66.
- [Google Scholar]
- Bioactive steroids and triterpenes from Melilotus messanensis and their allelopathic potential. J. Chem. Ecol. 1997;23:1781-1803.
- [Google Scholar]
- Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010;4:580.
- [Google Scholar]
- Proline, glycine betaine, total phenolics and pigment contents in response to osmotic stress in maize seedlings. J. Biosci.. 2015;Biotechnol.:4.
- [Google Scholar]
- Antioxidant capacity, radical scavenging kinetics and phenolic profile of methanol extracts of wild plants of Southern Sonora, Mexico. Trop. J. Pharm. Res.. 2014;13:1487-1493.
- [Google Scholar]
- Implications of water stress‐induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant.. 1983;58:166-170.
- [Google Scholar]
- Global changes in drought conditions under different levels of warming. Geophys. Res. Lett. 2018;45:3285-3296.
- [Google Scholar]
- Green HRM, psychological green climate and pro-environmental behaviors: An efficacious drive towards environmental performance in China. Curr. Psychol.. 2023;42:1346-1361.
- [Google Scholar]
- Morpho-anatomical and physiological attributes for salt tolerance in sewan grass (Lasiurus scindicus Henr.) from Cholistan Desert. Pakistan. Acta. Physiol. Plant. 2014;36:2959-2974.
- [Google Scholar]
- Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ. Exp. Bot.. 2015;119:40-47.
- [Google Scholar]
- Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012;169:596-604.
- [Google Scholar]
- Effect of heat and drought stress on the expression of regulatory transcription factors and the genes involved in different metabolic pathways. Plant Sci. 2018;7:1941-1945.
- [Google Scholar]
- Modulation in activities of antioxidant enzymes in salt stressed and non-stressed wheat (Triticum aestivum L.) plants raised from seed treated with triacontanol. Pak. J. Bot. 2011;43:2463-2468.
- [Google Scholar]
- Exogenous vanillic acid enhances salt tol-erance of tomato: insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020;150:109-120.
- [Google Scholar]
- Exogenous application of glycinebetaine and potassium for im-proving water relations and grain yield of wheat under drought. J. Soil Sci. Plant Nutr. 2014;14:348-364.
- [Google Scholar]
- Bio-economics of foliar applied GB and k on drought stressed wheat (Triticum aestivum L.) J. Agr. Biol. Sci. 2016;11:1.
- [Google Scholar]
- Interactive effect of drought and nitrogen on growth, some key physiological attributes and oxidative defense system in carrot (Daucus carota L.) Plants. Sci. Hort. 2017;225:373-379.
- [Google Scholar]
- Variation in pea (Pisum sativum L.) seed quality traits defined by physicochemical functional properties. Foods. 2019;8:570.
- [Google Scholar]
- Drought or/and heat-stress effects on seed filling in food crops: impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018;9:1705.
- [Google Scholar]
- Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants. 2021;10:259.
- [Google Scholar]
- Synergistic effects of drought and ascorbic acid on growth, mineral nutrients and oxidative defense system in canola (Brassica napus L.) plants. Acta Physiol. Plant. 2014;36:539-1553.
- [Google Scholar]
- Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci. Horti. 2015;185:68-75.
- [Google Scholar]
- Attenuation of vanillic acid toxicity by foliar application with indole-3-acetic acid in tomato seedlings. Int. J. Veg. Sci. 2021;00:1-22.
- [Google Scholar]
- Nitrogen alleviates salinity toxicity in Solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol. Biochem. 2019;141:466-476.
- [Google Scholar]
- Hyperaccumulation of cadmium in maize plant (Zea mays. Cellulose Chem. Technol. 2011;45:287-290.
- [Google Scholar]
- Influence of phenolic acids on morphological changes in roots of Pisum sativum. J. Sci. Food Agric. 1990;52:289-299.
- [Google Scholar]
- Plant responses to drought, salinity and extreme temperatures: towards genetic engi-neering for stress tolerance. Planta. 2003;218:1-14.
- [Google Scholar]
- Effects of exogenous application of protocatechuic acid and vanillic acid to chlorophylls, phenolics and antioxidant enzymes of rice (Oryza sativa L.) in submergence. J. Mol. 2018;620:23.
- [Google Scholar]
- Reactive oxygen species, oxidative stress and ros scavenging system in plants. J. Chem. Pharm. Res. 2016;8:595-604.
- [Google Scholar]
- Effects of high temperature on membrane stability and chlorophyll fluorescence in gly-cinebetaine-deficient and glycinebetaine-containing maize lines. Funct. Plant Biol. 1996;23:437-443.
- [Google Scholar]
- Seed pretreatment and foliar application of proline regulate morphological, physio-biochemical processes and activity of antioxidant enzymes in plants of two cul-tivars of quinoa (Chenopodium quinoa Willd. Plants. 2019;8:58.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103544.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







