Translate this page into:
Impact nano- and micro- form of CdO on barley growth and oxidative stress response
⁎Corresponding author. rvishnu@sfedu.ru (Vishnu D. Rajput),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The objective was investigated the effects of CdO and nano-CdO as potential toxic pollutants on growth and redox response of barley. CdO and nano-CdO have been found to cause significant phytotoxicity in barley seedlings, with nano-CdO increasing plant tissue cadmium accumulation. This accumulation is linked to growth retardation and oxidative stress. Low molecular weight antioxidants like restored glutathione and ascorbate have been found to increase the activity of glutathione peroxidase (GPX), glutathione-S-transferase (GSTs), and ascorbate peroxidase (APX) in green tissues. Catalase (CAT) activity increased from 50 % with 100 mg/l CdO to 70 % with 1000 mg/l and nano-CdO. The observed disturbance in redox balance signals the upregulation of corresponding genes. Antioxidant enzyme isoform gene transcripts increased for SODB, CAT2, and APX. Cadmium buildup in root cells causes oxidative stress, leading to upregulation of SOD, CAT, GR, and GSTs isoform genes as well as protein carbonylation, sulfhydryl group degradation, and MDA accumulation. CdO and nano-CdO have similar phytotoxic effects, but bioavailability affects biochemical and molecular responses.
Keywords
Antioxidant enzymes
Gene expression
Phytotoxic
NPs
HMs
1 Introduction
Heavy metal (HMs) contamination, a significant social and ecological issue, is exacerbated by the extreme toxicity of certain metals, such as cadmium, which are not essential for life. Naturally, cadmium concentrations in soils range from 0.1 to 1.5 mg/kg (Smolders and Mertens, 2013), but levels can significantly increase near human pollution sources. Studies indicate that cadmium content in surface soil can exceed 750 mg/kg near steelmaking and non-ferrous metal mining sites (Alloway and Steinnes, 1999). Environmental concentrations of this metal are increasing due to its widespread presence in dyes, plastics, hydrocarbons, fertilizers, and metallurgical waste (Annu et al., 2016). Moreover, cadmium compounds exhibit high bioavailability and mobility in soil, leading to significant accumulation in plants (An et al., 2022). Cadmium adversely affects plant physiology and biochemical activities (Kaznina and Titov, 2014). Upon entering the cell, cadmium ions disrupt the ETS in chloroplasts and mitochondria, displace vital metals from protein complexes, and generate reactive oxygen species (ROS) (Chen et al., 2020). Increased ROS levels deactivate enzymes, damage DNA, and disrupt membranes (Dobrikova et al., 2021). In the past decade, the environment has seen an increase in heavy metals (HMs) in nanoparticulate form, largely due to widespread nanomaterial use and fossil fuel combustion (Mitra et al., 2022). Nanoparticles (NPs) represent a new type of chemical pollutant with unique size, shape, surface area, and reactivity, posing novel challenges. Cadmium oxide (CdO) NPs are widely utilized in gas catalysts, chemical sensors, and microelectronics (Skheel et al., 2021). Various industrial activities, such as steel mills, release substantial quantities of CdO NPs into the environment (Večeřová et al., 2019). Research indicates their impact often results in increased reactive oxygen species (ROS) and disruption of oxidative-reductive balance (Rizwan et al., 2021). However, there is limited research on the phytotoxicity of CdO NPs. This study, therefore, examines the effects of CdO and nano-CdO on barley growth and redox status as a primary nonspecific stress response mechanism.
2 Materials and methods
2.1 Nanoparticle characteristic and growth preparation
The synthesis and analysis of nano-CdO have been done with follow previously conducted experiment (Shuvaeva et al., 2024). The experiment used hydroponic conditions with pre-germinated barley (Hordeum vulgare cv. Medikum 157OS) seeds to ensure uniformity. Seeds were germinated for 36 h at 25 ± 1 °C and placed in containers with CdO and nano-CdO at 0, 100, and 1000 mg/l. The plants were maintained at 25 ± 1 °C, 4 ± 0.5 klux light intensity, and a 16/8-hour photoperiod for 7 days. Morphometric parameters, biochemical, and gene activities were then assessed.
2.2 Cadmium content analysis
Cd concentration was determined using atomic absorption spectrophotometry (KVANT2AT, KortecLtd.) at λ = 228.8 nm. Samples were dried at 90 °C for 48 h, incinerated at 450 °C, and 1 g of the combusted sample was diluted in 5 ml of 20 % HCl, filtered through 0.45 mm Whatman filter paper, and analyzed for metal content (Azarin et al., 2022).
2.3 Determination of MDA content
The biomaterial (100 mg) was mixed with 20 % trichloroacetic acid (1.5 ml), centrifuged at 10,000g (4 °C), and 0.3 ml of the supernatant was added to 1.2 ml of 0.5 % thiobarbituric acid in trichloroacetic acid. Following incubation (30 min, 95 °C) and centrifugation, absorbance was measured at λ = 532 nm (Heath and Packer, 2022).
2.4 Sulfhydryl and carbonyl content
High molecular weight thiols were quantified by measuring the dinitrophenyl anion at 412 nm (Ivanov and Kerchev, 2007). Carbonyl compounds were determined spectrophotometrically at 370 nm using 2,4-dinitrophenylhydrazine (DNPH) to form carbonyl-DNPH hydrazone derivatives (Georgiou et al., 2018).
2.5 Antioxidant enzyme activity
2.5.1 Determination of CAT antioxidant enzyme
CAT activity was spectrophotometrically assessed by creating a stable colored complex with molybdenum salts and hydrogen peroxide. The homogenate (0.1 ml) was mixed with 9 mM hydrogen peroxide (2 ml), and after 10 min, 0.2 M ammonium molybdate (1 ml) was added to stop the reaction. Absorbance was measured at 410 nm.
2.5.2 Determination of GR antioxidant enzyme
GR activity was measured based on the degree of NADPH oxidation, which was accompanied by a decrease in the optical density of the solution. In a solution containing 0.2 M sodium phosphate buffer (pH 7.4), 0.1 M KCl, 8 mM EDTA, 2 mM NADPH, and 8 mM glutathione disulfide, 0.1 ml of extract was added, and the absorbance was measured at a wavelength of λ = 340 nm.
2.5.3 Glutathione peroxidase (GPx)
The activity of the GPx enzyme was analyzed by measuring the decrease in optical density at 340 nm, which occurs when the enzyme oxidizes NADPH (Navrot et al., 2006). The reaction mixture included 30 mM Tris-HCl buffer (pH 8), 1 mM EDTA, 2 mM glutathione, 0.5 U glutathione reductase, and 200 μM NADPH.
2.5.4 APX
APX activity was measured by reducing the ascorbate content at 290 nm for 3 min and with the 3 ml of reaction mixture containing 0.1 ml of plant extract, 50 mM sodium phosphate buffer (pH 7.0), 0.5 mM ascorbic acid, 0.1 mM H2O2, 0.1 mM EDTA.
2.5.5 GSTs
GST activity was quantified by the production rate between 1-chloro-2,4-dinitrobenzene and reduced glutathione. The assay began with 0.1 ml of supernatant added to 2.5 ml of 0.1 M potassium phosphate buffer (pH 6.5) and 0.2 ml of 0.015 M reduced glutathione. Subsequently, 0.2 ml of 0.015 M 1-chloro-2,4-dinitrobenzene was added. GST concentration was measured spectrophotometrically at 340 nm over 1.5 min.
2.6 Low-molecular-weight antioxidants
2.6.1 Glutathione (GSH)
A 300 mg tissue sample was homogenized in sodium phosphate buffer, centrifuged, and the supernatant was treated with trichloroacetic acid. The resulting supernatant was combined with Tris-HCl and 5,5′-dithiobis (2-nitrobenzoic acid) (Ivanov and Kerchev, 2007). Absorbance was measured at 412 nm after incubation.
2.6.2 Ascorbic acid (AsA)
The spectrophotometric calculation of AsA content was performed by analyzing the stable coloring with the Folin reagent (Olgun et al., 2014). After combining 0.8 ml of homogenate with 0.2 ml of 50 % trichloroacetic acid, the mixture was centrifuged at 1000g for 5 min. The supernatant was then added to the Folin reagent (0.2 ml) and readings were taken at λ = 410 nm after 10 min.
2.6.3 Proline
Homogenization of 100 mg of tissue in 1 ml of 3 % sulfosalicylic acid, followed by centrifugation at 18,000g for 10 min, was performed to determine the proline content. The supernatant was then mixed with a reaction mixture containing 10 ml of glacial acetic acid, 25 mg of ninhydrin, and 85 % o-phosphoric acid in a 6:3:1 v/v/v ratio. Optical density was measured at 520 nm after incubation at 90 °C for one hour.
2.7 Gene expression analysis
Barley roots and leaves were used to isolate total RNA using the ExtractRNA kit (Eurogen, Russia) (Azarin et al., 2020). Reverse transcription and amplification were performed according to (Azarin et al., 2023) using specific primers (Supplementary 1). The expression levels of target genes were normalized to actin using the 2−ΔΔCt technique.
2.8 Statistical analysis
The analyses were conducted using R and Microsoft Excel. Data are presented as mean values ± SD (standard deviation) and were obtained from 3 replicates. The t-test was employed to evaluate significant differences between the experimental and control groups. Statistical significance was defined as p < 0.05.
3 Result
3.1 Determination of seedlings growth and developments indices of barley and bioaccumulation of Cd
The study on the effects of CdO and nano-CdO on barley seedlings showed a dose-dependent decline in fresh and dry weight, and root and shoot length (Table 1). At 100 mg/l CdO, root and shoot lengths decreased by 13.6 % and 19.6 %, respectively, while 1000 mg/l caused reductions of 39.4 % and 37.8 %. Fresh and dry root weights decreased by about 10 % and 21 % at 100 mg/l, and by about 35 % and 44 % at 1000 mg/l. Notably, changes in morphometric parameters under CdO and nano-CdO were similar, except for root and shoot length at 100 mg/l, where nano-CdO had a more pronounced suppression. Significant cadmium accumulation was observed in barley plants, with CdO resulting in less cadmium accumulation in tissues compared to nano-CdO (Table S1).
3.2 Sulfhydryl (−SH), carbonyl (C⚌O) and MDA content
The increase in the pool of −SH groups was observed in the green tissue under the influence of low concentrations of CdO and nano-CdO Fig. 1. In the roots, CdO at a concentration of 100 mg/l led to an increase in −SH groups, while under the influence of 1000 mg/l CdO and nano-CdO, their content decreased by 34 % and 31 %, respectively (Fig. 1 a, b). High pollutant doses increased the carbonyl compounds' content in barley seedlings (Fig. 1 c, d). The greatest, twofold, increase was recorded in the roots under the influence of nano-CdO at a dose of 1000 mg/l.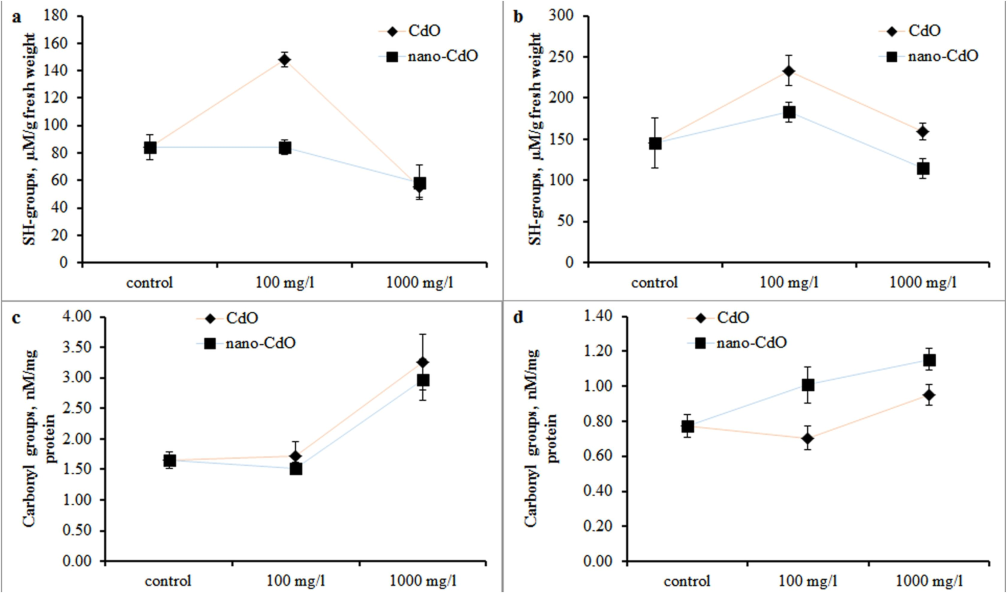
Sulfhydryl (−SH) concentration in the (a) root and (b) shoot and carbonyl (C⚌O) concentration in the (c) root and (d) shoot in barley after one week of cultivation at a dosage of 0 mg/L, 100 mg/L and 1000 mg/L CdO and nano-CdO.
The investigation of MDA revealed a significant increase in its content in root tissues, while in the shoots of treated barley, its content did not differ from the control values (Fig. 2 a, b).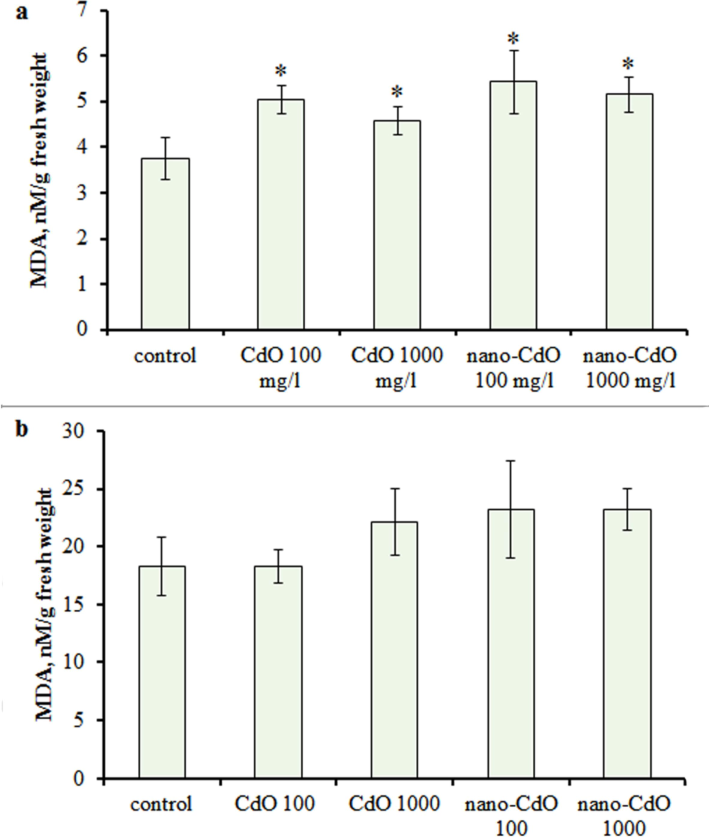
MDA concentration in the (a) root and (b) shoot in one-week-old barley seedlings under CdO and nano-CdO treatment at 100 mg/L and 1000 mg/L. Asterix indicates statistically significant differences from the control at p < 0.05.
3.3 Antioxidant enzyme activity
In the control, CAT activity was 0.047 ± 0.002 µM/min*g fresh weight in the roots and 0.88 ± 0.075 µM/min*g fresh weight in shoots. Exposure to 100 and 1000 mg/l nano-CdO increased CAT activity in roots by 51 % and 74 %, respectively. A dose of 1000 mg/l CdO raised CAT by 38 %, while 100 mg/l CdO did not affect enzyme activity (Fig. 3a). In shoots, CAT activity increased by 50 % with 100 mg/l CdO and by 70 % with 1000 mg/l CdO (Fig. 3b).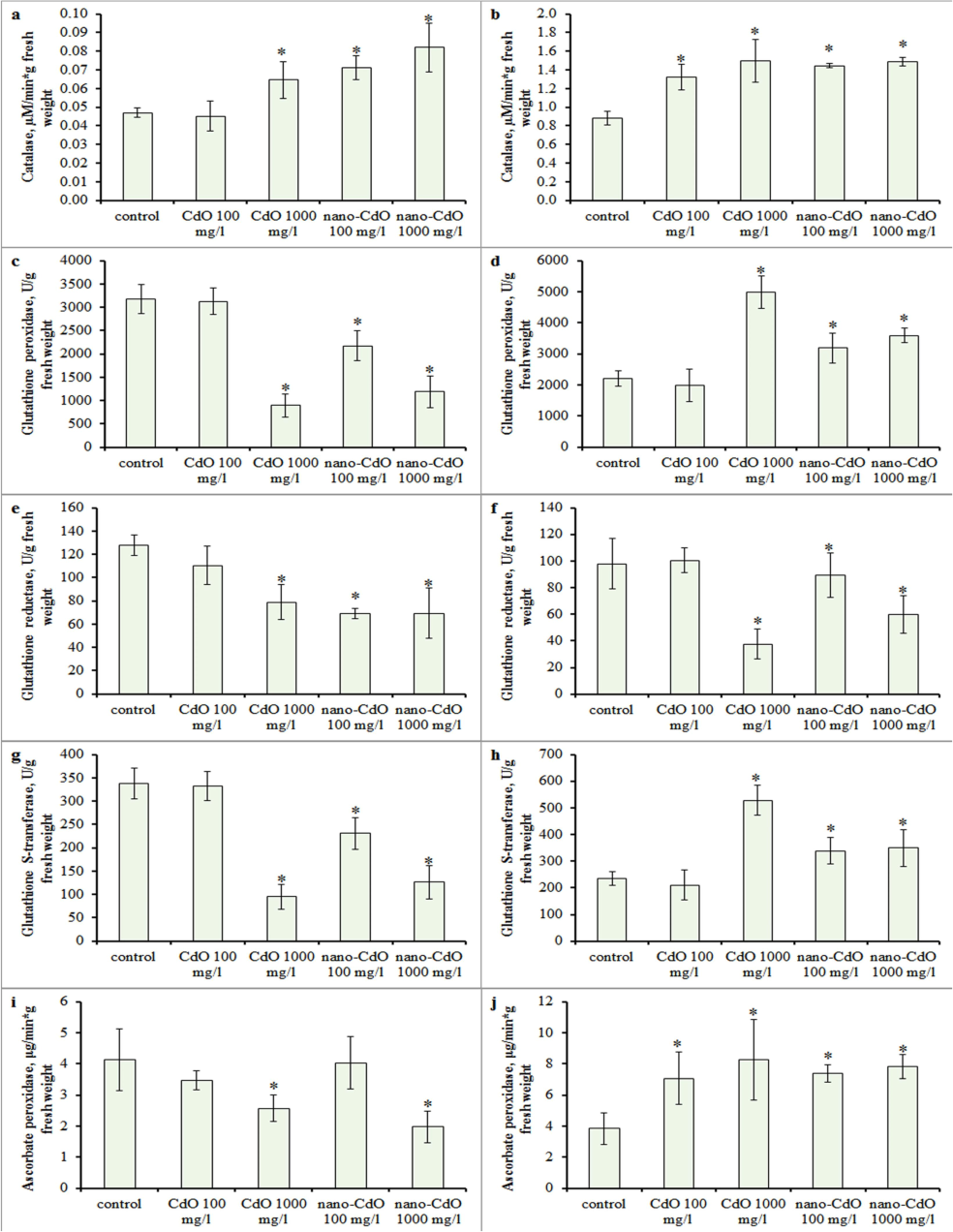
Effect of CdO and nano-CdO treatment at a dose of 100 mg/l and 1000 mg/l on the antioxidant enzyme activities in barley seedling. Catalase (CAT) in the (a) root and (b) shoot; glutathione peroxidase (GPX) in the (c) root and (d) shoot; glutathione reductase (GR) in the (e) root and (f) shoot; glutathione S-transferase (GSTs) in the (g) root and (h) shoot; ascorbate peroxidase (APX) in the (i) root and (j) shoot. Asterix indicates statistically significant differences from the control at p < 0.05.
GPX activity in control roots and shoots was 3174 ± 310 U/g and 2215 ± 243 U/g fresh weight, respectively, with no alteration at 100 mg/l CdO (Fig. 3c, d). Higher doses increased shoot GPX activity and decreased root activity (Fig. 3c, d). Root GR activity decreased by 49 % with 1000 mg/l CdO and by 66 % with 100 and 1000 mg/l nano-CdO (Fig. 3e, f), while leaf GR activity declined only at 1000 mg/l CdO and nano-CdO (Fig. 3e). Control GSTs activity was 337 ± 32 μM/min*g in roots and 235 ± 25 μM/min*g in shoots, remaining unchanged at 100 mg/l CdO. However, 100 mg/l nano-CdO decreased root GSTs activity by 32 %, and 1000 mg/l CdO and nano-CdO suppressed it by 72 % and 63 %, respectively (Fig. 3g, h). Conversely, shoot GSTs activity increased by 44 % with 100 mg/l nano-CdO and by 125 % with 1000 mg/l CdO (Fig. 3g, h). Control APX activity was 4.12 ± 0.99 μg/min*g in roots and 3.84 ± 1.01 U/g in shoots, increasing in shoots (Fig. 3j) but decreasing in roots by 37 % and 62 % at 1000 mg/l CdO and nano-CdO, respectively (Fig. 3i).
3.4 Low-molecular-weight antioxidants
Quantitative analysis of reduced glutathione demonstrated an upregulated in its content in root tissues only under the influence of a high dose of CdO and nano-CdO (Fig. 4 a). In shoot the level of glutathione exceeded control values under all treatment conditions (Fig. 4 b). An increase in ascorbate concentration in root and shoot was observed along with both root and aerial parts of the plant (Fig. 4 c, d).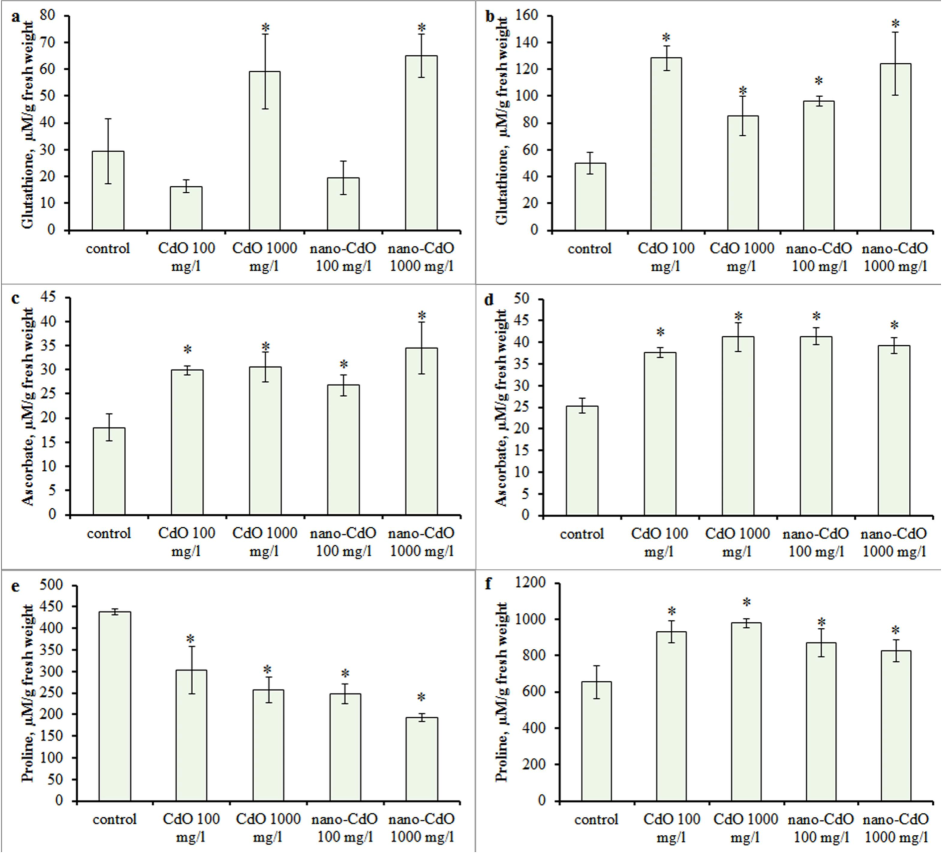
Effect of CdO and nano-CdO treatment at a dose of 100 mg/l and 1000 mg/l on the low-molecular-weight antioxidant content in barley seedling. Glutathione in the (a) root and (b) shoot; ascorbate in the (c) root and (d) shoot; proline in the (e) root and (f) shoot. Error bars express SD. Asterix indicates statistically significant differences from the control at p < 0.05.
Analysis of the proline content in roots revealed a significant decrease in all treatments (Fig. 4 e). The most significant effect (a decrease in proline by 64 %) was observed with 1000 mg/l nano-CdO. In contrast, an increase in proline content from 27 % to 50 % relative to control values was demonstrated in shoot (Fig. 4 f).
3.5 Transcriptional expression analysis
Gene expression analysis in roots showed increased transcription levels of CAT isoforms CAT1 and CAT2 under CdO and nano-CdO exposure at 1000 mg/l (Fig. 5a). In green tissue, CAT2 transcriptional activity rose across all experimental conditions, while CAT1 activity surpassed control values only with low CdO levels (Fig. 5b). A dose-dependent rise in SODA transcripts was observed in root cells across all treatments (Fig. 5a). SODB gene expression decreased by 27 % at 100 mg/l CdO but increased by 67 % and 93 % under 1000 mg/l CdO and nano-CdO, respectively (Fig. 5b). In shoots, high-dose pollutants suppressed SODA expression by 40–53 %, whereas low concentrations had no effect (Fig. 5a). Except for 100 mg/l CdO, SODB expression increased in shoot tissue (Fig. 5b). APX gene expression was higher in roots with 100 mg/l CdO and nano-CdO but unchanged at 1000 mg/l (Fig. 5a). In green tissue, pollutant treatment increased APX transcripts (Fig. 5b). GR gene transcriptional activity rose in root tissue across all treatments (Fig. 5a). In shoots, GR expression decreased at higher CdO and nano-CdO concentrations but remained unchanged at lower levels (Fig. 5b). GST1 and GST6 gene activities were mostly suppressed in shoots (Fig. 5b). In roots, GST1 transcription decreased at 100 mg/l and increased at 1000 mg/l CdO and nano-CdO (Fig. 5a). GST6 expression remained unchanged compared to control (Fig. 5a).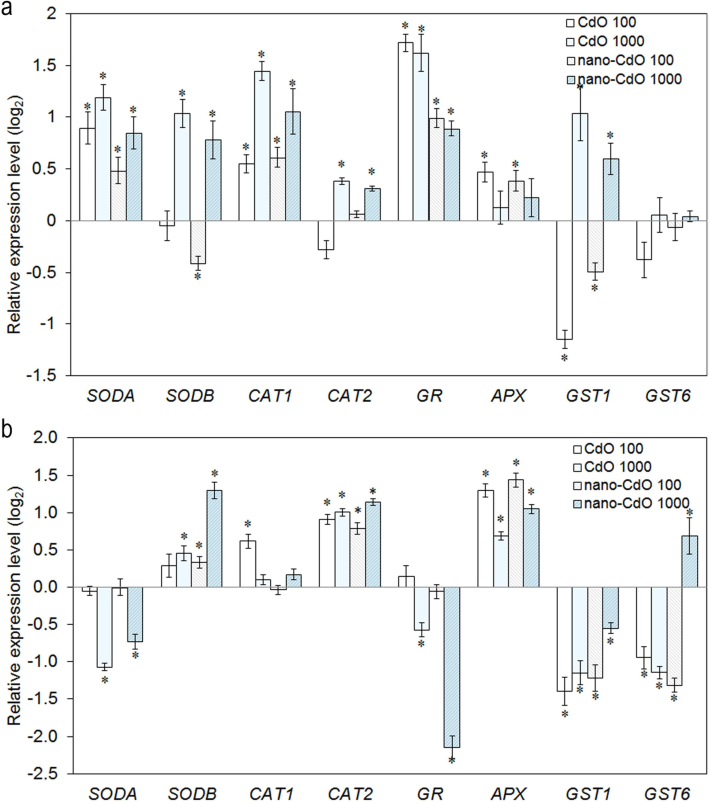
Expression analysis of oxidative stress related genes in the (a) root and (b) shoot in barley. The genes examined included superoxide dismutase (SODA and SODB), catalase (CAT1 and CAT2), glutathione reductase (GR), ascorbate peroxidase (APX), and glutathione S-transferase (GST1 and GST6). Transcript levels are expressed relative to the control and are presented on a logarithmic (log2) scale. Asterix indicates statistically significant differences from the control at p < 0.05.
4 Discussion
This study demonstrated a significant phytotoxic effect of CdO and nano-CdO on barley seedlings, manifested by a dose-dependent reduction in their growth parameters (Fig. 6). Simultaneously, considerable accumulation of cadmium in plant organs was observed.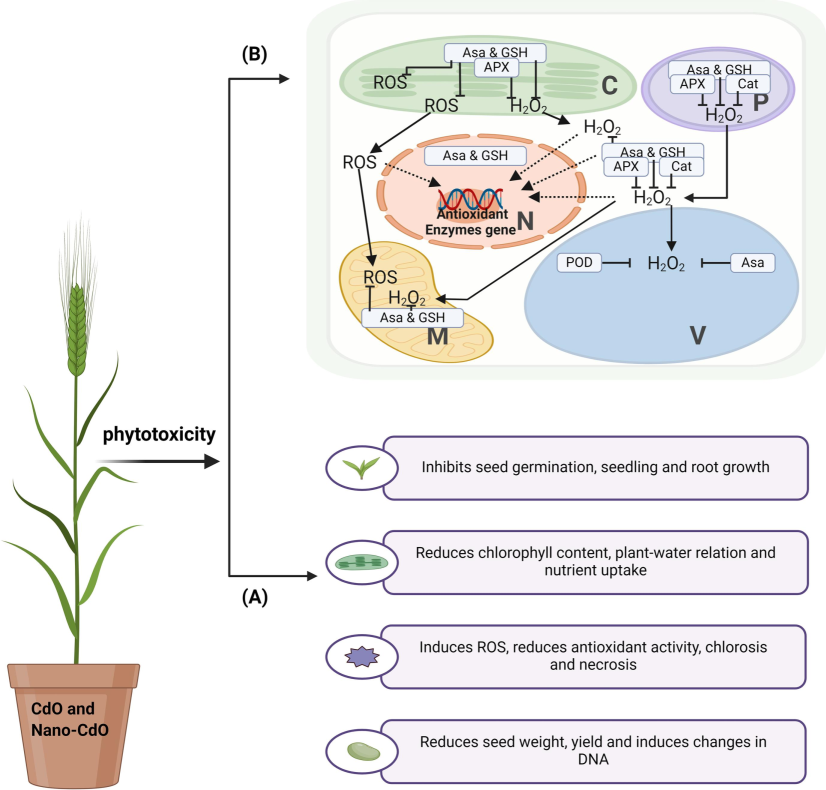
Mode of action of Cdo and Nano-Cdo may create the (A) risk related to phytotoxicity and reduce the growth and development of barley plants, and (B) antioxidant enzyme-based mitigation effect of ROS, which helps improve plant growth and development.
Interestingly, treatment with nano-CdO, compared to CdO, led to greater cadmium accumulation in plant tissues, especially evident under the influence of low pollutant concentrations. In this regard, it is worth noting that at a low dose (100 mg/l), nano-CdO demonstrated more significant suppression of root and shoot length compared to CdO, while such patterns were not observed at high pollutant doses (1000 mg/l). Additionally, many parameters of redox homeostasis, such as the amount of carbonyl compounds, CAT, GPX, GSTs, and GR activity, unlike treatment with 100 mg/l nano-CdO, did not significantly differ from the control when exposed to 100 mg/l CdO. Thus, in this case, the cadmium accumulation indicator reflects the degree of growth suppression and oxidative stress in barley seedlings. Similar patterns between the level of metal accumulation in tissues, dependent on the size of introduced particles, and physiological effects have also been demonstrated for chromium (Kumari et al., 2023) copper (Rajput et al., 2021) and zinc (Nemček et al., 2020). Overall, the lower accumulation of cadmium in the green part of the plant is associated with lower oxidative stress in these tissues. Shoot cells contained a high pool of low-molecular-weight antioxidants such as proline, reduced glutathione, and ascorbate compared to controls. The high level of reduced glutathione contributes to increased GPX activity, which uses glutathione as a cofactor in reducing fatty acid peroxides and H2O2 (Bela et al., 2015). In addition, increased activity of GSTs, involved in the reduction of membrane phospholipids (Katsuhara et al., 2005) and detoxification by conjugation with glutathione of the end products of lipid peroxidation and various xenobiotics, including HMs (Estévez and Hernández, 2020), was observed. Ascorbate, besides its non-enzymatic antioxidant capacity, acts as an electron donor for APX, one of the main antioxidant enzymes neutralizing H2O2 in the glutathione-ascorbate cycle of plants (Li, 2023). Increased activity of APX at the transcriptional and biochemical levels was demonstrated in the green tissues.
High levels of ROS induced by HMs such as cadmium have an extremely negative effect on plants, causing damage to proteins, lipids, and DNA (Gupta et al., 2018). To mitigate such consequences, antiradical protection is activated. Gene expression analysis revealed an increase in the number of CAT2 transcripts in shoots. The exact functional significance of the CAT2 isoform is still debated, but it was previously suggested that the upregulation of CAT2 could be a key regulator of CAT activity during stress (Luan et al., 2023). Simultaneously, the overexpression of the SODB gene, encoding the chloroplast-localized isoform of SOD, was observed. Notably, the increase in CAT transcription and catalytic activity occurs only in the presence of a relatively high concentration of H2O2, which is formed as a mode of action of SOD antioxidant enzyme (Wang et al., 2018). Thus, increasing the transcription of genes such as SODB, CAT2, and APX leads to an increase in the corresponding isoforms of antioxidant enzymes in leaf cells. This helps to neutralize the effects of pollutant-mediated ROS and improves the growth and development of the above-ground part of the plant.
In contrast to the shoot, excessive cadmium accumulation in root cells led to significant oxidative stress, manifested by lipid peroxidation and MDA accumulation. Additionally, there was a dose-dependent shift in the ratio of sulfhydryl and carbonyl groups towards the latter, reflecting the oxidation of protein molecules. At the transcriptional level, an increase in the expression of genes encoding all investigated isoforms of CAT and SOD was observed. A higher reduction of glutathione and ascorbate was noted in plants. Still, the investigated enzymes of the glutathione-ascorbate cycle demonstrated dose-dependent decreases in activity. This suppression of enzyme activity may be associated with the direct ability of cadmium ions to cause protein denaturation and interact with their catalytic centres (Vestena et al., 2011). Enzymatic function inhibition also occurs under severe oxidative stress due to the ability of MDA to form cross-links between proteins and ROS-mediated protein oxidation (Ahmed et al., 2024).
Interestingly, the expression level of genes encoding enzymes of the glutathione-ascorbate cycle was increased. This should promote the growth of antioxidant enzymes, but as shown above, their activity decreased. Thus, the observed disturbance of the redox balance signals the upregulation of the corresponding genes. At the same time, the decrease in the function of antioxidant enzymes likely occurs at the post-translational level due to disorganization processes induced by cadmium compounds and ROS.
It has been previously shown that toxic levels of nano-CdO can cause an oxidative burst. Generation of ROS by cadmium has been confirmed in HeLa cells (Zahera et al., 2020). In plants, a study of the long-term effect of nano-CdO in a wide range of concentrations (2.5–20 mg/l) recorded an increase in the total antioxidant activity in Dodonaea viscosa (Gul et al., 2020). In this study, we have for the first time conducted a comprehensive molecular genetic and biochemical comparative study of the effect of CdO and nano-CdO on growth and oxidative stress response. It was found that nano-CdO had a greater effect due to its greater bioavailability. In the long term, this may lead to a decrease in the productivity of agricultural crops in contaminated areas. This circumstance may have broader ecological implications for both microorganisms and soil conditions, as well as for human health, which needful be taken into account when predicting the ecological consequences of the spread of NPs. However, the research has limitations. Future studies should be aimed at replicating the results in the field.
5 Conclusion
The study revealed a significant phytotoxic effect of CdO and nano-CdO on barley seedlings due to cadmium accumulation in plant organs. Nano-CdO enhanced cadmium absorption and translocation in plants. Lower oxidative stress in green tissues correlated with high levels of low molecular weight antioxidants (proline, reduced glutathione, and ascorbate) and increased activity of antioxidant enzymes, especially CAT, glutathione peroxidase, glutathione-S-transferase, and ascorbate peroxidase. Excessive cadmium accumulation in root cells caused notable oxidative stress, evident in protein oxidation and lipid peroxidation. This redox imbalance led to the upregulation of genes encoding glutathione-ascorbate cycle enzymes, though at the biochemical level, inhibition of these enzymes was observed. The suppression of these enzymes in root tissues, despite higher gene expression, indicates post-translational regulatory mechanisms triggered by cadmium and ROS. These findings are crucial for evaluating the phytotoxicity of nanoparticles and their potential environmental impact.
6 Declarations
6.1 Ethics approval and consent to participate
Not applicable.
6.2 Consent for publication
The article contains no such material that may be unlawful, defamatory, or which would, if published, in any way whatsoever, violate the terms and conditions as laid down in the agreement.
6.3 Availability of data and materials
Not applicable.
CRediT authorship contribution statement
Kirill Azarin: . Alexander Usatov: . Tatiana Minkina: . Ilya Alliluev: . Nadezhda Duplii: . Saglara Mandzhieva: . Abhishek Singh: . Vishnu D. Rajput: . Sandeep Kumar: . Marwa A. Fakhr: Mohamed S. Elshikh: . M. Ajmal Ali: Writing – review & editing, Writing – original draft, Visualization, Data curation, Conceptualization, Funding. Karen Ghazaryan: Writing – review & editing, Writing – original draft, Visualization, Data curation, Conceptualization.
Acknowledgment
The study was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation (grant number: FENW-2023-0008). Karen Ghazaryan is supported by internal grant of Yerevan State University Republic of Armenia. Abhishek Singh is supported by the 23PostDoc-4D007 grant provided by the Higher education and science committee MESCS Republic of Armenia. The authors also extend their appreciation to the Researchers Supporting Project number (RSP2024R306), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmed, A.R., Farris, F.F., Ray, S.D., 2024. Lipid peroxidation. In: Encyclopedia of Toxicology. Elsevier, pp. 861–870. https://doi.org/10.1016/B978-0-12-824315-2.00624-2.
- Effect of silicon on morpho-physiological attributes, yield and cadmium accumulation in two maize genotypes with contrasting root system size and health risk assessment. Plant and Soil. 2022;477:117-134.
- [CrossRef] [Google Scholar]
- Annu, Garg, A., Urmila, 2016. Level of Cd in different types of soil of Rohtak district and its bioremediation. Journal of Environmental Chemical Engineering 4, 3797–3802. https://doi.org/10.1016/j.jece.2016.08.023.
- A point mutation in the photosystem I P700 chlorophyll a apoprotein A1 gene confers variegation in Helianthus annuus L. Plant Mol. Biol.. 2020;103:373-389.
- [CrossRef] [Google Scholar]
- Effects of ZnO nanoparticles and its bulk form on growth, antioxidant defense system and expression of oxidative stress related genes in Hordeum vulgare L. Chemosphere. 2022;287:132167
- [CrossRef] [Google Scholar]
- Effects of bulk and nano-ZnO particles on functioning of photosynthetic apparatus in barley (Hordeum vulgare L.) Environ. Res.. 2023;216:114748
- [CrossRef] [Google Scholar]
- Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol.. 2015;176:192-201.
- [CrossRef] [Google Scholar]
- The effects of exogenous organic acids on the growth, photosynthesis and cellular ultrastructure of Salix variegata Franch. Under Cd stress. Ecotoxicol. Environ. Saf.. 2020;187:109790
- [CrossRef] [Google Scholar]
- Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol. Environ. Saf.. 2021;209:111851
- [CrossRef] [Google Scholar]
- Plant Glutathione S-transferases: An overview. Plant Gene. 2020;23:100233
- [CrossRef] [Google Scholar]
- Protein and cell wall polysaccharide carbonyl determination by a neutral pH 2,4-dinitrophenylhydrazine-based photometric assay. Redox Biol.. 2018;17:128-142.
- [CrossRef] [Google Scholar]
- Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf.. 2018;161:624-633.
- [CrossRef] [Google Scholar]
- Reprint of: Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.. 2022;726:109248
- [CrossRef] [Google Scholar]
- Separation and quantification of the cellular thiol pool of pea plants treated with heat, salt and atrazine. Phytochem. Anal. 2007;18:283-290.
- [CrossRef] [Google Scholar]
- Salt stress-induced lipid peroxidation is reduced by glutathione S-transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in Arabidopsis. Plant Sci.. 2005;169:369-373.
- [CrossRef] [Google Scholar]
- The influence of cadmium on physiological processes and productivity of Poaceae plants. Biol.. Bull. Rev.. 2014;4:335-348.
- [CrossRef] [Google Scholar]
- Speciation of macro- and nanoparticles of Cr2O3 in Hordeum vulgare L. and subsequent toxicity: A comparative study. Environ. Res.. 2023;223:115485
- [CrossRef] [Google Scholar]
- Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol.. 2023;64:102789
- [CrossRef] [Google Scholar]
- Transcriptome analysis of barley (Hordeum vulgare L.) under waterlogging stress, and overexpression of the HvADH4 gene confers waterlogging tolerance in transgenic Arabidopsis. BMC Plant Biol.. 2023;23:62.
- [CrossRef] [Google Scholar]
- Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Uni. – Sci.. 2022;34:101865
- [CrossRef] [Google Scholar]
- Plant glutathione peroxidases are functional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol.. 2006;142:1364-1379.
- [CrossRef] [Google Scholar]
- Impact of bulk ZnO, ZnO nanoparticles and dissolved Zn on early growth stages of Barley—a pot experiment. Plants. 2020;9:1365.
- [CrossRef] [Google Scholar]
- Folin-Ciocalteu spectrophotometric assay of ascorbic acid in pharmaceutical tablets and orange juice with pH adjustment and pre-extraction of lanthanum(III)–flavonoid complexes. J. Sci. Food Agric.. 2014;94:2401-2408.
- [CrossRef] [Google Scholar]
- Assessing the toxicity and accumulation of bulk- and nano-CuO in Hordeum sativum L. Environ. Geochem. Health. 2021;43:2443-2454.
- [CrossRef] [Google Scholar]
- Effects of nanoparticles on trace element uptake and toxicity in plants: A review. Ecotoxicol. Environ. Saf.. 2021;221:112437
- [CrossRef] [Google Scholar]
- Synthesis and properties of nano-cadmium oxide and its size-dependent responses by barley plant. Environ. Res.. 2024;246:118045
- [CrossRef] [Google Scholar]
- Green synthesis of cadmium oxide nanoparticles for biomedical applications (antibacterial, and anticancer activities) Mater. Today:. Proc.. 2021;45:5793-5799.
- [CrossRef] [Google Scholar]
- Smolders, E., Mertens, J., 2013. Cadmium, in: Alloway, B.J. (Ed.), Heavy Metals in Soils. Springer Netherlands, Dordrecht, pp. 283–311. https://doi.org/10.1007/978-94-007-4470-7_10.
- Temperature alters susceptibility of Picea abies seedlings to airborne pollutants: The case of CdO nanoparticles. Environ. Pollut.. 2019;253:646-654.
- [CrossRef] [Google Scholar]
- Cadmium-induced oxidative stress and antioxidative enzyme response in water hyacinth and salvinia. Braz. J. Plant Physiol.. 2011;23:131-139.
- [CrossRef] [Google Scholar]
- Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol.. 2018;217:1915-1928.
- [CrossRef] [Google Scholar]
- Cadmium oxide nanoparticles: An attractive candidate for novel therapeutic approaches. Colloids Surf. A Physicochem. Eng. Asp.. 2020;585:124017
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103493.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







