Translate this page into:
Mechanistic insights into the hepatoprotective properties of Mallotus phillipensis fruit: Targeting proinflammatory cytokines and free radicals using isolated compound
⁎Corresponding authors. waseemnakhat@gmail.com (Waseem Rizvi), quahmed@iium.edu.my (Qamar Uddin Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
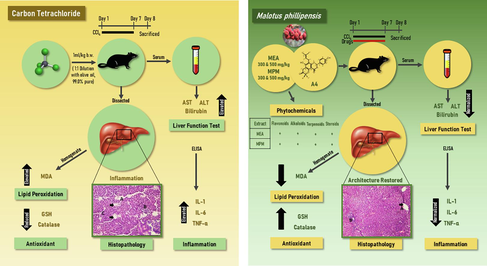
Ethnopharmacological relevance: Mallotus phillipensis (MP) belongs to the endangered plants of great medicinal value. Widely used in the Ayurvedic healthcare system, parts of the plant which are rich in secondary metabolites are used in the treatment of several diseases like eczema, bronchitis, worm infestation, diabetes, kidney stones, cancer, and malaria. In ethnomedicine, MP has been reported to be traditionally used in the treatment and healing of jaundice and inflammatory conditions in the Ayurveda system. This plant was thus selected to explore its hepatoprotective activity.
Objective
This study was undertaken to investigate the protective property of MP and a flavanone i.e. 7,4′-Dihydroxy-3′′,3′′-dimethyl-(5,6-pyrano-2′′-one)-8′′′-(3′′′,3′′′-dimethylallyl)-flavanone (A4) previously identified and obtained from the same plant on CCl4-induced liver injury in animal models to validate the folkloric claims. Traditional medicinal plants have the potential to provide phytoconstituents capable of healing liver cells. In this regard, MP is used in various traditional medicinal systems to treat several diseases. Though it has been studied for its hepatoprotective potential, activity on the isolated compounds is yet to be reported to confirm the active principle. Therefore, the study assessed the hepatoprotective effect of ethyl acetate and methanol extracts and a flavanone isolated from MP fruit extract.
Methods
Methanol and ethyl acetate fractions of MP fruits at two different doses (300 mg/kg and 500 mg/kg) and a flavanone (A4-50 mg/kg) isolated from MP were tested for hepatoprotective potential in rats. At 50 mg/kg dosage/ day orally, silymarin was used as a standard drug. The effect on liver enzymes and serum cytokines was also verified in animal models. Histopathology and antioxidant tests were performed on liver tissue. Elemental analysis, 1H- & 13C NMR, MS, IR and UV spectral data were used to characterize the structure of isolated flavanone (A4).
Results
The ethyl acetate and methanol fractions of MP fruit and the isolated compound A4 i.e. flavanone showed strong hepatoprotective effects, as evidenced by their ability to impede the rise in serum transaminases. They also decreased the serum IL-1, IL-6 and TNF-α levels and showed significant antioxidant potential. The effect was found to be in decreasing order as MEA 500 > MPM 500 > MEA 300 > A4 > MPM 300.
Conclusions
The results indicate that MP fruit fractions with methanol, ethyl acetate and A4 can lessen CCL4-induced liver damage. This may be because of their antioxidant and cytokine-inhibitory properties.
Keywords
Hepatoprotection
Mallotus phillipensis
Cytokine
Isolated compound
Flavanone
- MP
-
Mallotus phillipensis L.
- DILI
-
Drug-induced liver injury
- CCl4
-
Carbon tetrachloride
- TLC
-
Thin layer chromatography
- MPM
-
Methanol extract of MP
- MEA
-
Ethyl acetate fraction of MP
- A4
-
Isolated flavonone compound (A4)
- MDA
-
Malondialdehyde
- TBARS
-
Thiobarbituric acid reacting substance
- GPx
-
Glutathione peroxidase
- Catalase
-
Catalase
- GSH
-
Glutathione Reduced
- GSSH
-
Oxidized glutathione
- SEM
-
Standard Error of Mean
- ANOVA
-
One way Analysis of Variance
Abbreviations
1 Introduction
Chronic liver diseases significantly contribute to morbidity and mortality in human populations. As a source of morbidity and mortality, they rank 15th and 11th, respectively. Globally in 2016, these diseases were responsible for 2.2 % of deaths and accounted for 1.5 % of disability-adjusted life years (Global Health Estimates, WHO, 2016). Treatment options for chronic liver diseases are limited in modern medicine. Therefore, there is a pressing need to explore alternative therapeutic sources. Plants have been utilized as medicine since time immemorial to treat various illnesses, including liver diseases.
Mallotus philippensis L. (MP) also known as “Kamala” in northern parts of India. This plant belongs to the family known as Euphorbiaceae. It is found in South China, Tibet, Nepal to North India and Southeast Asian countries (Gangwar et al., 2014b). It has been reported to exert antibacterial effects and generally used as folkloric medicine to treat meningitis and typhoid using a decoction of the stem (Cheenpracha et al., 2019; Kumar et al., 2020). The medicinal values of MP are revealed in several Indian and Chinese texts, such as the Sushruta Samhita, Charaka Samhita, Indian Materia Medica, and Indosyunic Medicine (Khare, 2008; Kunwar et al., 2021; Ye et al., 2021). Ayurveda and Yunani, two of the oldest medical traditions, also recommend its use to treat various disorders as an alexiteric, anthelmintic, appetiser, purgative, styptic (Khare, 2019; Quattrocchi, 2016). Commercially available drugs like Roghan Kameela and Zimad Jarb prescribed for dermatological conditions and Krikuthar Rasa prescribed for removing intestinal worms already contain MP as one of the active ingredients possessing therapeutic properties along with other active herbs. It has been documented that many people from different ethnic groups and geographical areas continue to use this plant traditionally as an ethnomedicine to treat various diseases. Various formulations made from this traditional medicinal plant have been used to treat a variety of health related issues, including diarrhoea, dysentery, bronchitis, jaundice, urinary complications, and parasitic infections (Barkatullah et al., 2015; Kunwar et al., 2021). It is frequently used to treat worm infestations and other gastrointestinal tract illnesses. Its hepatoprotective potential has been demonstrated in vitro on hepatoma cells using a derived compound Bergenin (Sriset et al., 2020), while its antioxidant activity of fruits and rottlerin (a derived polyphenolic compound) was analysed using in-vitro tests (Gangwar et al., 2014a; Lee et al., 2020). Despite folkloric claims of MP in jaundice, proper scientific validation is still lacking. Therefore, the present work was undertaken to provide a scientific basis for traditional use in hepatic disease.
2 Material and methods
2.1 Chemicals
Propylene glycol (BDH, Mumbai) and rat ELISA kits were bought from Koma Biotech, Korea. Carbon tetrachloride (CCl4) with 99.0 % purity was purchased from the Central Drug House (CDH), New Delhi 110002, India. All organic solvents and other important organic and inorganic chemicals/reagents used in this investigation were directly purchased from the Merk. All solvents were initially purified by steam distillation and then dried over calcium chloride and sodium wire.
2.2 Experimental animals
Adult Charles Foster strain albino rats (both male and female) of 200–250 g were carefully kept under standard conditions and were given a pellet diet and water throughout the experiment. Before the start of the experiment, the adult Charles Foster strain albino rats were acclimatized to the laboratory condition for seven days. Ethical clearance was obtained from the Institutional Animal Ethical Committee, J.N.M.C., A.M.U., Aligarh (U.P., India), No. 401/CPCSEA and Research was conducted in accordance with the protocols outlined by the New Delhi, India-based Committee for Control and Supervision of Animal Experiments (CPCSEA).
2.2.1 Preparation of model
CCl4 was given orally to the rats to cause hepatic injury. Every day at 9:00 am for seven days, a 1:1 (v/v) suspension of 99.0 % pure CCl4 was given in liquid olive oil at a dose of 1 ml per kilogramme of body weight. Silymarin was the standard medication, administered orally at a dose of 50 mg/kg/day (Li et al., 2017).
2.3 Plant material
Dr Athar Ali Khan (Taxonomist) from the Department of Botany and Dr Rifat Afridi (Research Officer (Pharmacognosy) from the Regional Research Institute Unani Medicine, Aligarh Muslim University (A.M.U.) Aligarh identified the plant after it was obtained from a local market (5 kg of fresh fruit). A voucher specimen having reference number Rump-433 was placed in the herbarium of the Department of Botany at AMU.
The MP fruits were initially dried under shade and ground into a powder approximately of 3 kg. Subsequently, non-polar and polar organic solvents namely petroleum ether, benzene, chloroform, ethyl acetate, and methanol were used for extraction using a Soxhlet extractor (in the increasing order of polarity). Distillation was used to get rid of the solvent. Under reduced pressure, the petroleum ether and benzene extracts were concentrated to produce a greenish-sticky substance. An analysis using thin-layer chromatography (TLC) was conducted to determine the number of chemicals. Since the petroleum ether and benzene extracts behaved similarly during TLC examination, they were combined. Further silica gel column chromatography was performed on the fraction of the combined petroleum ether-benzene-and-ethyl acetate. Petroleum ether, petroleum ether-benzene (9:1, 8:2, 7:3, 1:1), benzene-ethyl acetate (9:1, 8:2, 7:3, 1:1), ethyl acetate, ethyl acetate–methanol (9:1, 8:2, 7:3, 6:4, 1:1), and finally, methanol was used to elute the column. Repeated column chromatography separated these fractions which upon recrystallisation produced four different compounds (A1 to A4; Isorotlerin, 5,7-Dihydroxy-8-methyl-6-prenylflavanone, 6″,6″-Dimethylepyrano (2″,3,“7,6)-5-hydroxyl-8-methylflavanone and 7,4′-Dihydroxy-3′′,3′′-dimethyl-(5,6-pyrano-2′′-one)-8-(3′′′,3′′′-dimethylallyl)-flavanone) (Supplementary file 1). A flavonoid compound in the form flavanone i.e., 7,4′-Dihydroxy-3′′,3′′-dimethyl-(5,6-pyrano-2′′-one)-8-(3′′′,3′′′-dimethyl allyl)-flavanone was isolated from ethyl acetate and methanol (1:1) fraction of MP, (Compound- A4, Fig. 1) (Rizvi et al., 2016). For additional analysis, a methanol extract in its raw form was also produced. Different solvents upon sequential extraction method afforded 11.70 % (Petroleum ether), 6.99 % (Benzene), 17.71 % (Chloroform), 39.88 % (Ethyl Acetate) and 79.13 % (Methanol) extracts, respectively.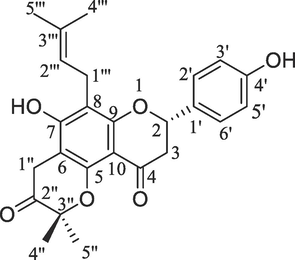
Structure of 7,4′-Dihydroxy-3′′,3′′-dimethyl-(5,6-pyrano-2′′-one)-8-(3′′′,3′′′-dimethyl allyl)-flavanone isolated from M. philippensis.
7,4′-Dihydroxy-3′′,3′′-dimethyl-(5,6-pyrano-2′′-one)-8-(3′′′,3′′′-dimethylallyl)-flavanone (compound A4).
Ethyl acetate–methanol (1:1) fraction yielded compound A4. Crystallization from the binary solvent system namely chloroform–methanol gave light yellowish solid (35 mg), melting point: 260–261 °C. Anal. Calc. for C25H26O6; C, 71.07; H, 6.20; found: C, 71.09; H, 6.23. IR (KBr) ν cm−1: 3411, 3326, 2916, 2848, 1681, 1623, 1586, 1443, 1365, 1298, 1107, 980, 801. Proton (1H) NMR (400 MHz, CDCl3, δ, ppm): 5.58 (dd, 1H, H-2, J = 13.6, 2.6 Hz), 2.83–2.88 (dd, 2H, H-3, J = 14.8, 2.6; J = 13.6, 17.2 Hz), 7.54 (dd, 1H, H-2′, J = 2.4, 10.0 Hz), 6.67 (d, 1H, H-3′, J = 10.0 Hz), 7.52 (d, 1H, H-5′, J = 10.8 Hz), 7.50 (dd, 1H, H-6′, J = 2.4, 10.8 Hz), 3.63 (brs, 2H, H- 1′′), 2.50 (brs, 3H, H-4′′), 1.98 (brs, 3H, H-5′′), 3.30–3.26 (d, 2H, H-1′′′, J = 12.6, 2.6 Hz), 5.54 (dd, 1H, H-2′′′, J = 2.6, 12.6 Hz), 1.57 (brs, 3H, H-4′′′), 1.59 (brs, 3H, H-5′′′). Carbon-13 (13C NMR) (100 MHz, CDCl3, δ, ppm): 81.66 (C-2), 42.94 (C-3), 195.90 (C-4), 159.52 (C-5), 103.12 (C-6), 162.49 (C-7), 104.54 (C-8), 157.91 (C-9), 106.27 (C-10), 136.16 (C-1′), 115.61 (C-2′), 129.29 (C-3′), 157.52 (C-4′), 127.13 (C-5′), 126.50 (C-6′), 16.61 (C-1′′), 203.84 (C-2′′), 81.52 (C-3′′), 33.20 (C-4′′), 27.85 (C-5′′), 29.13 (C-1′′′), 130.16 (C-2′′′), 136.16 (C-3′′′), 27.60 (C-4′′′), 27.91 (C-5′′′). ESI-MS (m/z): 422.47 [M + H+·] (Supplementary file 1).
2.3.1 Phytochemical analysis
Qualitative phytochemical analysis of ethyl acetate and methanol extracts of M. philippensis fruits was done to confirm the existence of reducing sugars, anthraquinones, terpenoids, alkaloids, flavonoids, saponins, tannins, phlorotannins, cardiac glycosides and steroids (Balamurugan, 2019) using double beam spectrophotometer.
2.4 Experimental design
Methanol and ethyl acetate extracts along with A4 were used for in vivo studies. A4 was taken forward as it showed a potential to possess hepatoprotective property.
2.4.1 Acute toxicity study
The acute toxicity experiment was performed using limit toxicity testing as described by OECD 423 guidelines. The animals were carefully monitored for the first half an hour during the first whole day (24 h) and then daily for 14 days.
Three non-pregnant female rats were given 2 g/kg b.w. of the ethyl acetate and methanol extracts and observed for any mortality and the same test was repeated in the 2 g/kg b.w. group to confirm the findings. Apart from that any sign of toxicity including any change in behaviour, activity, food and water intake, any abnormal movements, or any abnormal discharge from the orifices of animals was not seen in the animals. So, a lower dose of 300 & 500 mg/kg were used in the study.
For the isolated compound (A-4), which was in a limited amount, the dose was selected based on the pilot toxicity study, where a maximum dose of 500 mg/kg was given, hence 1/10 dose of 50 mg/kg was selected.
2.4.2 In-vivo procedure
Eight groups of six rats each (n = 6) were divided as follows. Treatment was given for seven days.
S.No
Groups
Treatment given
I
Normal control
Propylene glycol 1 ml/kg
II
Negative control
CCl4
III
Positive control
Silymarin (50 mg/kg orally) with CCl4.
IV & V
Methanol extract of MP (MPM)
MPM 300 mg/kg and 500 mg/kg with CCl4
VI & VII
Ethyl acetate extract (MEA)
Ethyl acetate extract (MEA 300 mg/kg and 500 mg/kg) with CCl4
VIII
A4 compound
A4: 50 mg/kg with CCl4
For seven days, the test medications were administered orally at 3:00 pm in addition to CCl4 treatment. The animals were sacrificed using an overdose of pentobarbitone at 8th day. Before administration, all medicines were dissolved in propylene glycol. The blood and liver tissues were collected. The serum was isolated after centrifuging the blood. The liver enzymes (AST, ALT, ALP), total bilirubin and cytokines (TNFα-, IL-6, IL-1) were measured using the serum. One gram of liver tissue was homogenised with 10 ml of phosphate buffer, and the resulting homogenate was utilised for in vivo antioxidant testing (GPx, catalase and MDA). Additionally, some liver tissue was kept in 10 % formalin for histological analysis.
2.4.3 Tissue lipid peroxidation
In summary, tissue homogenate (0.2 ml), 1.5 ml of 0.8 % thiobarbituric acid, 20 % acetic acid (1.5 ml) and 8.1 % sodium dodecyl sulphate (0.2 ml) were combined and heated for 60 min at 95 °C. After cooling, this was combined with 1:15 mixture of pyridine and n-butanol and centrifuged for ten minutes at 4000 RMP. Absorbance of the organic layer was measured at 532 nm using a double beam UV spectrophotometer (Janero, 1990). The results are shown as nmol/mg of protein.
2.4.4 Antioxidant enzyme activity
The in vivo activity of two enzymes, catalase (CAT) and glutathione peroxidase (GPx), was evaluated. When H2O2 is present, the GPx converts it to H2O while also catalysing the reaction that changes GSH (reduced form of glutathione) into oxidised glutathione (GSSG). The glutathione reductase reaction uses NADPH as the reducing substrate and reduces the GSSG back to GSH. To calculate the GPx activity, a spectrophotometer was used to quantify the drop in absorbance that occurs during the oxidation of NADPH to NADP + at 340 nm. 19 U/mg protein was used to represent the results. The CAT activity was estimated by reducing H2O2 (10 mM) to phosphate buffer − 20 mM (pH 7), a free radical in serum. The absorbance at 240 nm was then measured to track the process. The enzyme activity was determined using a molar absorption coefficient and was measured in nanomoles of hydrogen peroxide, dissipating per milligram of protein per 60 s. Using a standard of bovine serum, the Lowry technique was employed to quantify the protein concentration (Parimelazhagan, 2015). The units of expression for the enzyme activity were shown as per mg of protein.
2.4.5 Estimation of serum cytokines
After centrifugation, the serum was collected and kept at −40 °C for future experiment. Following the instructions provided with the kit, TNF-, IL-6, and IL-1 levels from each sample were tested twice using highly sensitive rat Elisa Kits. Pg/mL of serum was used to express the results.
2.4.6 Hepatoprotection percentage
The hepatoprotection was calculated using the continuous variables ALT, AST and ALP. The formula provided by Syed et al. (2017) was used to calculate the extract's percentage of hepatic protection. H% = 1 − ((T − V)/(C − V)) × 100 where H% stands for hepatoprotection percentage, T for the mean value of the group received test drug + CCl4, C for the mean value of the group received CCl4, and V for mean value for the Animals in the Control Group.
2.4.7 Histopathology
All rats were sacrificed using overdose of pentobarbitone. The liver was dissected after opening the abdomen; specimens were preserved in 10 % formalin and processed for histopathology.
2.4.8 Statistical analysis
The data were observed to be normally distributed after the Shapiro-Wilk test was taken into consideration to verify normality. A one-way analysis of variance (ANOVA) was employed to compare the groups, and statistical significance was assessed using “Dunnett's Multiple Comparison Test” post hoc analysis. p < 0.05 was considered to be a statistically significant value. The data was compiled in Microsoft Excel Sheet, and the analysis was done using Statistical Software - Social Science Software program (SPSS) version 23.
3 Results
3.1 Results of phytochemical analysis
The qualitative analysis revealed the presence of flavonoids in methanol extract while flavonoids, terpenoids, alkaloids and steroids were found to exist in the ethyl acetate extract of M. phillipensis (Table 1). “+” indicates for the presence of the compound screened and “-” indicates for the absence of the compound screened in methanol ethyl acetate fractions of M. phillipensis.
Phytochemicals
M. phillipensis
Methanol Extract
M. phillipensis
Ethyl Acetate Extract
Flavonoids
+
+
Terpenoids
–
–
Anthraquinone
–
–
Saponins
–
+
Alkaloids
–
+
Cardiac glycosides
–
–
Sugar
–
–
Steroids
–
+
3.2 Results of acute toxicity study
As no mortality was observed at a 2 g/kg dose, the LD50 was found to be less than 2 g/kg. Hence, lower doses viz., 300 mg/kg and 500 mg/kg were then selected for the study. For the isolated compound, 1/10th of 500 mg/kg (i.e., 50 mg/kg) was chosen.
3.3 Results of physical parameters
The variation in the weight of rat and rat liver was not statistically significant in normal control compared to CCl4 treated group. Similarly, the administration of silymarin and the test compounds produced no significant findings (Table 2). The negative control group was compared with the Normal control group, and all other groups were compared to the Negative control group, * p < 0.05 deemed significant. MPM- Methanol fraction of M. phillipensis, MEA-Ethyl acetate fraction of M. phillipensis, A 4 Active compound.
Group
Weight of Rat
(g)
Weight of Liver
(g)
Volume of Liver
(ml)
Weight per 100 g of Rat
(g/g)
Volume per 100 g of Rat
(ml/g)
NORMAL CONTROL
(Propylene Glycol)
245 ± 5.5
8.22 ± 0.78
8.85 ± 0.45
3.41 ± 0.19
3.67 ± 0.06
NEGATIVE CONTORL (CCl4)
240 ± 8.2
9.2 ± 0.88
8.39 ± 0.89
3.83 ± 0.27
3.50 ± 0.43
POSITIVE CONTROL (SILYMARIN)
244 ± 5.7
8.44 ± 0.43
8.58 ± 0.22
3.46 ± 0.45
3.52 ± 0.18
MPM
(300 mg/kg)
239 ± 5.5
8.68 ± 0.12
8.80 ± 0.72
3.63 ± 0.87
3.68 ± 0.21
MPM
(500 mg/kg)
240 ± 6.9
8.56 ± 0.23
7.92 ± 0.18
3.57 ± 0.09
3.30 ± 0.24
MEA
(300 mg/kg)
246 ± 8.1
8.70 ± 0.98
8.28 ± 0.87
3.54 ± 0.05
3.37 ± 0.19
MEA
(500 mg/kg)
238 ± 7.8
8.93 ± 0.38
8.49 ± 0.09
3.75 ± 0.23
3.57 ± 0.65
A-4
(50 mg/kg)
232 ± 8.9
8.54 ± 0.13
8.30 ± 0.54
3.68 ± 0.34
3.58 ± 0.32
3.4 Results of liver function tests
The ability of the MP fruit's methanol and ethyl acetate extracts to prevent the rise of serum transaminases was evidence of their strong hepatoprotective efficacy. The methanol and ethyl acetate extracts had a greater impact at 500 mg/kg (p < 0.001), although the liver-specific enzyme ALT did not significantly decrease at 300 mg/kg. Additionally, the active ingredient A4 considerably (p < 0.05) reduced ALT. When compared to the corresponding control groups, no discernible difference in the bilirubin levels of the test groups or the negative control groups was observed. (Fig. 2: a-d). Hepatoprotection was calculated for all test groups as a function of AST, ALT, and ALP as indicated in Table 3. MPM- Methanol fraction of M. phillipensis, MEA-Ethyl acetate fraction of M. phillipensis, A 4 Active compound (Effects in decreasing order MEA 500 > MPM 500 > MEA 300 > A4 > MPM 300).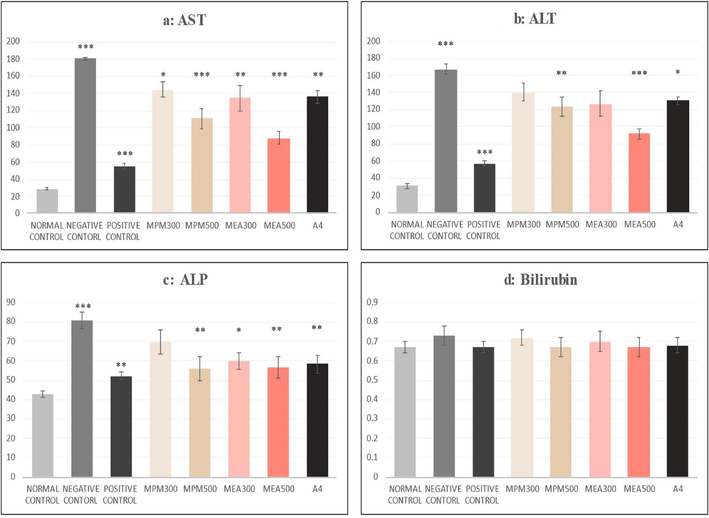
(a-d): Effect of M. philippensis fractions on liver function tests.
Groups
AST (%)
ALT (%)
ALP (%)
POSITIVE CONTROL (SILYMARIN)
82.6
81.5
74.7
MPM
(300 mg/kg)
24.0
19.6
28.7
MPM
(500 mg/kg)
45.9
32.1
65.0
MEA
(300 mg/kg)
30.3
29.2
54.5
MEA
(500 mg/kg)
61.1
55.4
63.2
A4
(50 mg/kg)
29.3
26.9
58.9
3.5 Results of antioxidants
The ethyl acetate fraction produced significant changes in all variables (Catalase, GSH and MDA) at both doses. When compared to the CCl4 group, the catalase levels were elevated (p < 0.01) by the MEA fraction at 500 mg and the A4 compound. Similarly, the MDA levels of the MEA 500 mg group were raised (p < 0.01). In contrast, the A4 compound group was raised to 3.5 ± 0.30 μmoL/mg, which was also higher (p < 0.05) than the negative control group. On the other hand, MDA levels were significantly lowered in the MEA 500 and the A4 compound group, implying a substantial decrease in hepatocyte membrane damage due to fatty acid oxidation. At 300 mg/kg, methanol extract produced a significant (p < 0.01) effect for GSH only i.e.0.3.8 ± 0.23 μmoL/mg (Fig. 3: a-c).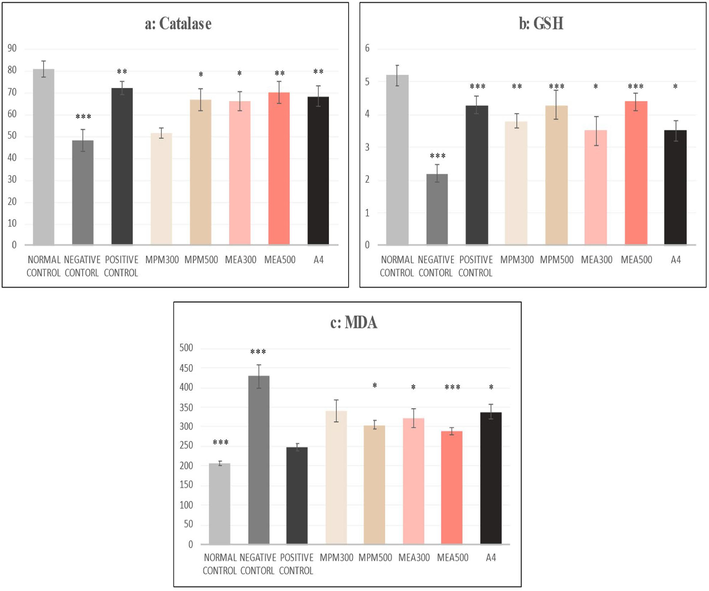
(a-c): Effect of M. philippensis fractions on antioxidants. The negative control group was compared with the normal control group, and all other groups were compared with the Negative control group; *p < 0.05, **p < 0.01 and ***p < 0.001 were considered significant. MPM- Methanol fraction of M. phillipensis, MEA-Ethyl acetate fraction of M. phillipensis, A 4 Active compound.
3.6 Results of serum cytokines
As shown in Fig. 4 (a-c), MPM 500 mg/kg and MEA 300 mg/kg produced a significant (p < 0.05) change in TNF α only. At the same time, the MEA fraction at 500 mg/kg decreased all the cytokines TNF α (p < 0.05), IL-6 (p < 0.05) and IL-1 (p < 0.05). The active compound produced a positive effect on TNF alpha (p < 0.05) and Interleukin-1 (p < 0.05).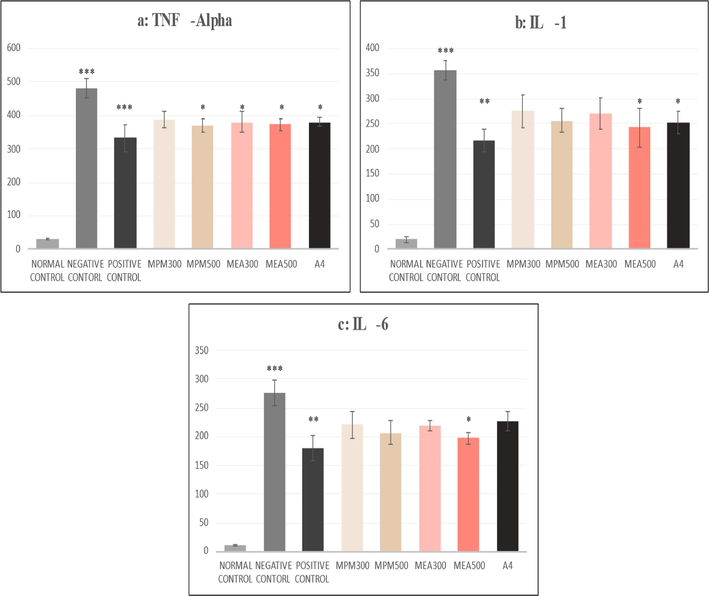
(a-c): Effect of M.philippensis fractions on serum cytokines. The negative control group was compared with the normal control group, and all other groups were compared with the Negative control group;* p < 0.05, **p < 0.01 and ***p < 0.001 were considered significant. MPM- Methanol fraction of M. phillipensis, MEA-Ethyl acetate fraction of M. phillipensis, A 4 Active compound.
3.7 Results of histopathological analysis
Histopathological examination of liver tissue sections was done on different control and treated groups. CCl4-induced changes like sinusoidal integrity, hepatocytic degeneration, centrilobular changes, and inflammatory infiltrates were considered parameters for microscopic examination; these changes were reduced by pre-treatment with silymarin, fractions and isolated compound of M. phillipensis (Fig. 5: A-H). Table 4 shows the histopathological scoring of liver sections (Kamisan et al., 2014). The severity of hepatic injury was evaluated based on the following scoring pattern: - Absent, + Mild effect, ++ Moderate effect, +++ Severe effect.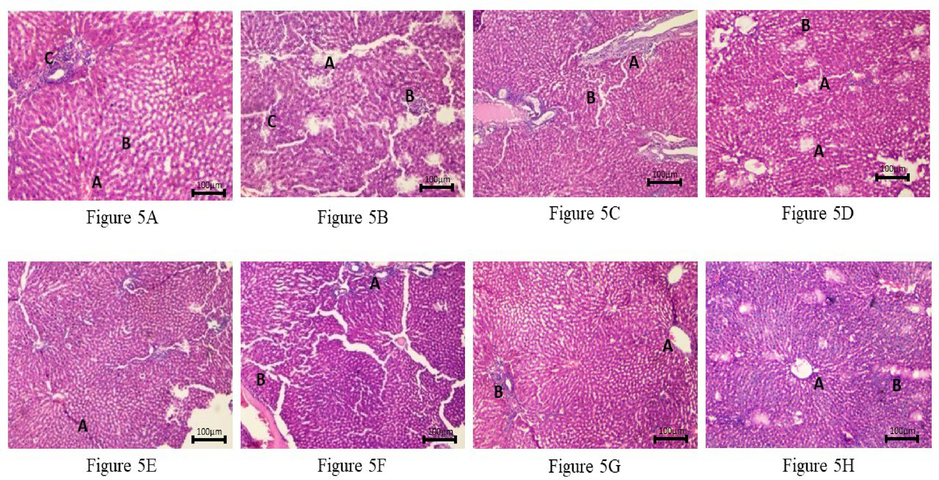
Histopathology of rat liver. (10X, H & E stain), Fig. 5 A: Photomicrograph of liver from Normal Control Group (Propylene glycol) showing normal liver microstructure central vein (A) with parallel hepatic cords (B) with normal foci of the portal triad (C). Fig. 5 B: Photomicrograph of rat liver from Negative Control Group (CCI4) showing spotty necrosis with periportal fibrosis (A) with diffuse mild acute parenchymal inflammation (B) with diffuse ballooning degeneration(C). Fig. 5 C: Photomicrograph of rat liver from Positive Control Group (Silymarin) showing mild sinusoidal dilatation with mild portal fibrosis (A) and mild lymphocytic infiltration (B). Fig. 5 D: Photomicrograph of rat liver from Test Group (CCI4 + MP Methanol 300 mg/kg) showing focal hepatocytic (A) necrosis with dilated central vein and mild lymphocytic infiltration (B) in the parenchyma. Fig. 5 E: Photomicrograph of rat liver from Test Group (CCl4 + MP Methanol 500 mg/kg) showing a decrease in necrosis of hepatocytes with irregular and thickened hepatocytes with mitosis and moderate interface hepatitis (A). Fig. 5 F: Photomicrograph of rat liver from Test Group (CCI4 + MP Ethyl acetate 300 mg/kg) showing mild periportal lymphocytic infiltrate (A) with sinusoidal congestion (B). Fig. 5 G: Photomicrograph of rat liver from Test Group (CCl4 + MP Ethyl acetate 500 mg/kg) showing mildly dilated central vein (A) with mild periportal fibrosis (B). Fig. 5 H: Photomicrograph of rat liver from Test Group (CC14 + A-450 mg/kg) showing irregular and thickened hepatocyte plate (A) with bi-nucleated forms and mild fibrosis (B) and focal chronic inflammation.
Groups
Dose
Inflammation
Necrosis
Degeneration
Fibrosis
Congestion
1
Normal Control
1 ml/Kg
–
–
–
–
–
2
Negative control
1 ml/Kg
+++
+++
++
+
+
3
Positive Control
50 mg/Kg
+
+
–
+
++
4
Methanol Extract
300 mg/Kg
++
++
–
–
+
5
Methanol Extract
500 mg/Kg
+
+
–
+
+
6
Ethyl acetate extract
300 mg/Kg
++
+
–
+
++
7
Ethyl acetate extract
500 mg/Kg
+
–
–
++
+
8
A4
50 mg/Kg
+
+
+
+
+
4 Discussion
Traditional medicinal plants have been used as a source of medicine for all forms of diseases since time immemorial. Plants have shown remarkable activity in treating ailments which has a definite rationale in modern pharmacology and therapeutics (Sen and Samanta, 2015; Colalto, 2018; Alhassan et al., 2019; Benchoula et al., 2019). It has been discovered that a number of plant products guard against liver damage. A milk thistle bioflavonoid called silymarin was found to be useful in preventing liver damage brought on by CCl4 (Gillessen and Schmidt, 2020), as was colchicine, the toxin from autumn-blooming flowering plant crocus i.e., Colchicum autumnale L. (Awad et al., 2020). Both have antioxidant properties. Since the liver has great potential to regenerate, therefore, the aim should be to come up with a molecule that could reduce the injurious effect or preserve the architecture and the physiological function of the liver disturbed by the hepatotoxin (Dezső et al., 2017).
A substantial community from many ethnic groups and geographical areas continues to use M. phillipensis in the traditional way as an ethnomedicine to cure medical ailments. Different formulations made from this tree have been used for the treatment of worm infestations and other gastrointestinal tract ailments, as well as bronchitis, jaundice, urinary complications (Barkatullah et al., 2015; Kunwar et al., 2021).
Among several mechanisms, oxidative stress i.e., the generation of free radicals, is one important explanation of liver injury (Li et al., 2015). CCl4 is often used for screening hepatoprotective drugs, it is a potent hepatotoxin that results in centrilobular liver inflammation and necrosis (Dong et al., 2016; El Rabey et al., 2021). The stages of liver damage include fat accumulation, CCl3-OO* radical generation, lipid peroxidation, membrane damage, loss of Ca2+ sequestration, apoptosis, fibrosis, dehalogenation, covalent binding of the resulting radicals, protein synthesis inhibition (especially of apolipoprotein synthesis), assembly, packaging, and release of VLDL and HDL (Lackner and Tiniakos, 2019; Weber et al., 2003, Fareed et al., 2023). In our study, the administration of CCl4 enhanced the serum markers of hepatotoxicity in the negative control group (Fig. 2). Additionally, it depleted tissue GSH. It increased lipid peroxidation (Fig. 3). Significant hepatoprotective activity was seen in the M. phillipensis methanol and ethyl acetate fractions, as evidenced by their capacity to restrain the increase in serum transaminases. The active compound A4 also significantly decreased ALT, although its effect was less as compared to the ethyl acetate extract. The reason might be due to the presence of some important bioactive molecules more active than the A4 in it. It also produced a significant antioxidant effect and reduced lipid peroxidation. Our results indicate that the two extracts and the active compound possess antioxidant activity, decreased lipid peroxidation, and protect the liver cells exposed to CCl4 from oxidative stress, possibly through the membrane stabilizing effect of extracts and compound. A4 compound has shown lesser activity as compared to the high dose of ethyl acetate extract. It may be due to the fact that apart from the flavonoid (including A4) ethyl acetate fraction also contains other phytochemicals like steroids, alkaloids and saponins.
Besides free radical injury, inflammation is an important cause of hepatitis. The liver injury induced by either infection or free radicals is mediated by inflammation as well as other innate immune cells (Tacke and Zimmermann, 2014, Rani et al., 2024), during which pro-inflammatory cytokines like TNF-α are produced by liver cells (Brenner et al., 2013; Niederreiter and Tilg, 2018). Proliferation, inflammation, and the control of immunological responses that result in apoptosis and necrosis are only a few of the physiological and pathological processes that the pro-inflammatory cytokine TNF- α is involved in (Chu, 2013). According to a study, alcoholic liver disease is initiated and promoted in part by TNF-α. and has been hypothesised to cause acute liver failure (Chastre et al., 2012). TNF-α is typically overexpressed in fatty liver disease and alcohol-related liver disease; cytokines that contribute to liver protection include IL-6 and IL-10 (Tiegs and Horst, 2022). TNF- α is another possible therapeutic target in individuals with the condition, as evidenced by its significant role in animals with steatohepatitis (Sehrawat et al., 2020).
In our research, we observed reduced concentrations of proinflammatory cytokines supporting the protective role of MP in CCl4 induced hepatotoxicity (Fig. 4). In our previous studies, we also have reported that MP attenuates proinflammatory cytokines (Rizvi, 2016).
The pro-inflammatory cytokines like IL-6 and IL-1 modulates the inflammatory process in the hepatocytes through JAK- STAT3 activation (Scheller et al., 2011) which regulates the gene transcription of anti-apoptosis and proliferative regulatory proteins (Tiegs and Horst, 2022). The levels of IL-6 decrease in the presence of IL-10 (Braun et al., 2013). Apart from IL to 6, IL-10 an anti-inflammatory cytokine produced by Kupffer cells which targets immune cells also activates STAT3 signalling pathway (Wang et al., 2011). Although we did not measure the levels of IL-10, it seems that decrease in IL-6 could possibly be due to the presence of IL-10.
The histological investigation, which revealed repair of architecture and decreased inflammation and necrosis of hepatocytes, supports, and confirms the findings of the biochemical tests. This could be attributed to flavonoids, terpenoids, alkaloids and steroids in the two fractions of M. phillipensis.
5 Conclusion
We conclude that the ethyl acetate and methanol fractions of MP has good hepatoprotective activity. It was also found that a flavanone (A4; 7,4′-Dihydroxy-3′′,3′′-dimethyl-(5,6-pyrano-2′′-one)-8-(3′′′,3′′′-dimethylallyl)-flavanone) has potential hepatoprotective activity. The hepatoprotective activity might be due to the compound's antioxidant properties and cytokine-lowering effect. Further research is essential to ascertain the exact mechanism of action of the compound.
CRediT authorship contribution statement
Waseem Rizvi: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft. Syed Shariq Naeem: Investigation. Ompal Singh: Data curation, Investigation. Shagufta Moin: Data curation, Formal analysis. Kafil Akhtar: Formal analysis, Investigation. Syed Najmul Hejaz Azmi: . Zubair Ahmed: Funding acquisition, Methodology, Visualization, Writing – review & editing. Monowarul Mobin Siddique: Formal analysis. Zainul Amiruddin Zakaria: Data curation, Writing – review & editing. Qamar Uddin Ahmed: Formal analysis, Methodology, Resources, Writing – review & editing.
Acknowledgements
Grateful acknowledgements are made to all the technical staff who helped us in the research laboratory during the conduction of the study. This work was supported by Researchers Supporting Project number (RSPD2024R1113), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A new sulphated flavone and other phytoconstituents from the leaves of Tetracera indica merr. and their alpha-glucosidase inhibitory activity. Nat. Prod. Res.. 2019;33(1):1-8.
- [Google Scholar]
- The possible protective effect of colchicine against liver damage induced by renal ischemia-reperfusion injury: role of Nrf2 and NLRP3 inflammasome. Can. J. Physiol. Pharmacol.. 2020;98:849-854.
- [Google Scholar]
- Balamurugan, V., 2019. Fundamentals of Phytochemical Analysis. Independently Published. ISBN: 107304436X, 9781073044368.
- Barkatullah, Ibrar, M., Rauf, A., Ben Hadda, T., Mubarak, M.S., Patel, S., 2015. Quantitative ethnobotanical survey of medicinal flora thriving in Malakand Pass Hills, Khyber Pakhtunkhwa, Pakistan. J. Ethnopharmacol. 169, 335-346.
- Optimization of hyperglycemic induction in zebrafish and evaluation of its blood glucose level and metabolite fingerprint treated with Psychotria malayana Jack leaf extract. Molecules. 2019;24(8):1506.
- [CrossRef] [Google Scholar]
- Cytokine response is determined by duration of receptor and signal transducers and activators of transcription 3 (STAT3) activation. J. Biol. Chem.. 2013;288:2986-2993.
- [Google Scholar]
- Inflammatory cascades driven by tumor necrosis factor-alpha play a major role in the progression of acute liver failure and its neurological complications. PloS One. 2012;7:e49670.
- [Google Scholar]
- Mallopenins A-E, antibacterial phenolic derivatives from the fruits of Mallotus philippensis. J. Nat. Prod.. 2019;82:2174-2180.
- [Google Scholar]
- What phytotherapy needs: evidence-based guidelines for better clinical practice. Phytother. Res. PTR. 2018;32:413-425.
- [Google Scholar]
- Human liver regeneration in advanced cirrhosis is organized by the portal tree. J. Hepatol.. 2017;66:778-786.
- [Google Scholar]
- Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J. Toxicol. Sci.. 2016;41:561-572.
- [Google Scholar]
- Green coffee methanolic extract and silymarin protect against CCl4-induced hepatotoxicity in albino male rats. BMC Complement. Med. Ther.. 2021;21:19.
- [CrossRef] [Google Scholar]
- Fareed MM, Khalid H, Khalid S, Shityakov S. 2023. Deciphering molecular mechanisms of carbon tetrachloride induced hepatotoxicity (Fibrosis): A brief systematic review. Curr Mol Med. 2023 Oct 5. doi: 10.2174/0115665240257603230919103539.
- Gangwar, M., Goel, R.K., Nath, G., 2014b. Mallotus philippinensis Muell. Arg (Euphorbiaceae): Ethnopharmacology and phytochemistry review. BioMed Res. Int. 2014, 213973. doi: 10.1155/2014/213973.
- Antioxidant capacity and radical scavenging effect of polyphenol rich Mallotus philippenensis fruit extract on human erythrocytes: an in vitro study. Scientific World Journal. 2014;2014:279451
- [CrossRef] [Google Scholar]
- Silymarin as supportive treatment in liver diseases: a narrative review. Adv. Ther.. 2020;37:1279-1301.
- [Google Scholar]
- Global Health Estimates. Geneva: World Health Organization; 2016. Available at: https://www.who.int/healthinfo/global_burden_disease/estimates/en/. Accessed January 12, 2024.
- Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med.. 1990;9:515-540.
- [Google Scholar]
- Effect of methanol extract of Dicranopteris linearis against carbon tetrachloride-induced acute liver injury in rats. BMC Complement Altern Med.. 2014;14:123.
- [CrossRef] [Google Scholar]
- Indian medicinal plants: an illustrated Dictionary. Springer Science & Business Media; 2008.
- Evidence-based ayurveda: defining a new scientific path. Routledge; 2019.
- Mallotus philippensis (lam.) müll. arg.: a review on its pharmacology and phytochemistry. J. Herbmed Pharmacol.. 2020;10:31-50.
- [Google Scholar]
- Ethnobotany of the Himalayas. Springer Nature; 2021.
- Rottlerin, a natural polyphenol compound, inhibits upregulation of matrix metalloproteinase-9 and brain astrocytic migration by reducing PKC-δ-dependent ROS signal. J. Neuroinflammation. 2020;17:177.
- [CrossRef] [Google Scholar]
- Hepatoprotective activities of antrodia camphorata and its triterpenoid compounds against CCl4-induced liver injury in mice. J. Ethnopharmacol.. 2017;206:31-39.
- [Google Scholar]
- The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci.. 2015;16:26087-26124.
- [Google Scholar]
- Pharmacological assays of plant-based natural products. Springer; 2015.
- Quattrocchi, U., 2016. CRC world dictionary of medicinal and poisonous plants: Common names, scientific names, eponyms, synonyms, and etymology (5 Volume Set). CRC Press.
- Drug-induced liver injury and anti-hepatotoxic effect of herbal compounds: a metabolic mechanism perspective. Phytomedicine. 2024;122:155142
- [CrossRef] [Google Scholar]
- Cytokine attenuation and free radical scavenging activity of a new flavanone 7,4’-Dihydroxy-3″,3″-dimethyl -(5,6-Pyrano-2″-one)- 8- (3‴,3‴-dimethyl allyl)- isolated from Mallotus philippensis: possible mechanism for its anti-inflammatory activity. PloS One. 2016;11:e0167294.
- [Google Scholar]
- The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011;1813:878-888.
- [CrossRef] [Google Scholar]
- The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol. Hepatol.. 2020;5:494-506.
- [Google Scholar]
- Medicinal plants, human health and biodiversity: a broad review. Adv. Biochem. Eng. Biotechnol.. 2015;147:59-110.
- [Google Scholar]
- Hepatoprotective activity of bergenin against xenobiotics-induced oxidative stress in human hepatoma (HepG2) cells. Chiang Mai Univ. J. Nat. Sci.. 2020;20
- [CrossRef] [Google Scholar]
- A study to evaluate the antioxidant and hepatoprotective activity of aqueous extract of roots of Valeriana wallichii in CCl4 induced hepatotoxicity in rats. Int. J. Basic Clin. Pharmacol.. 2017;3:354-358.
- [Google Scholar]
- Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol.. 2014;60:1090-1096.
- [Google Scholar]
- TNF in the liver: targeting a central player in inflammation. Semin. Immunopathol.. 2022;44:445-459.
- [Google Scholar]
- Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int. J. Biol. Sci.. 2011;7:536-550.
- [Google Scholar]
- Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol.. 2003;33:105-136.
- [Google Scholar]
- Ye, H., Li, C., Ye, W., Zeng, F., 2021. Common Chinese Materia Medica: Volume 3. Springer Nature.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103192.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







