Translate this page into:
Water extract of onion peel for the synthesis of bisindolylmethanes
⁎Corresponding author at: School of Marine Science and Environmental Sciences, Universiti Malaysia Terengganu, Terengganu, Malaysia. pohwai@umt.edu.my (Poh Wai Chia)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
An efficient catalytic system for the synthesis of bisindolylmethanes (BIMs) using Water Extract of Onion Peel (WEOP) is described in this study. The advantages of this protocol include avoid the use of acids and bases, reusability of the WEOP catalytic system, good to excellent yield and reduce the use of toxic reagents. This is the first report of using WEOP catalytic system in the preparation of BIMs. We anticipate the method presented here will find great utility in the field of synthetic chemistry and the synthesis of other heterocyclic compounds in the near future.
Keywords
Water extract of onion peel
Bisindolylmethanes
Green and recyclable protocol
Heterocyclic compounds
1 Introduction
Lately there has been an increasing trend towards substituting toxic, polluting and expensive chemical reagents and catalysts with more eco-friendly ones. The organic reactions performed in water have exhibited many desirable characteristics such as environmental friendly, benign, inexpensive and a readily-available medium (Kiyani and Ghorbani, 2017, Heravi et al., 2015). In addition, numerous studies reported that chemical reaction rates were enhanced by using water as solvent (Bhowmick et al., 2015, Eymur et al., 2014, He et al., 2013, Jimeno, 2016, Miklós and Fülöp, 2016). In the development of new synthetic and catalytic protocols, the use of water as a reaction medium is worthwhile to be explored and is still an active field of research devoted to accomplish greener chemical processes.
In parallel to the use of clean water as solvent, continuous efforts were also made by using waste water, and water extracts of fruits and vegetables juices as biocatalysts and solvents in catalyzing organic reactions. Current examples of using such system to accomplish organic reactions include the Suzuki–Miyaura reaction (Boruah et al., 2015), synthesis of coumarin-3-carboxylic acid and cinnamic acids (Fiorito et al., 2016), Sonogashira reaction (Dewan et al., 2016), Henry reaction (Surneni et al., 2016), peptide coupling (Konwar et al., 2016), Dakin reaction (Saikia and Borah, 2015) and several other examples. Water extract of bio-wastes is a better alternative to pure water and has become an emerging field of research in organic synthesis (Sarmah et al., 2017). Bio-wastes are waste disposed from household, food stalls, restaurants and food processing factories and mankind generates million tons of bio-wastes every month (Choi et al., 2015). With the increasing demand on agricultural products as a result of the booming human population, the generation of bio-wastes has greatly escalated in recent years (Marshall and Farahbakhsh, 2013). In the past, most of the bio-wastes are destined for disposal in landfills and this has a considerable effect towards our ecosystem, wildlife and human health (Gao et al., 2015). In contrast, if used wisely, such bio-wastes may in turn act as an effective catalytic system to mediate organic reactions that was hard to be achieved conventionally or mostly achieved by using hazardous chemical reagents. One of the notable examples of bio-wastes produced is the waste generated from onion (Allium cepa L.) processing. Onion is considered as one of the valuable crops grown globally for its pharmaceutical, nutritional and other functional properties (Nile and Park, 2013). Human consumption and food processing industries have generated huge amount of onion waste. Statistics showed that the US and European countries alone dispose of 100,000 to 500,000 tonnes of onion waste annually (Sharma et al., 2016).
Indoles are important chemical scaffold and are found in abundance in biologically active natural products, pharmaceuticals and also embedded in biological systems (Ruiz-Sanchis et al., 2011, Vicente, 2011). The bisindolylmethanes (BIMs) which consist of indoles as its core units, were mainly isolated from both terrestrial and marine sources (Shiri et al., 2010). BIMs are an appealing class of heterocyclic compounds which exhibit a wide range of biological activities which include anti-cancer (Safe et al., 2008), anti-leishmanial (Bharate et al., 2013), anti-viral (Liu et al., 2015), anti-inflammatory (Qu et al., 2013) activities and others. Among the synthetic protocols that had been employed in the synthesis of BIMs include ionic liquid (Kalantari, 2012), NaNO TiO2/SiO2 (Haghighi and Nikoofar, 2016), ammonium niobium oxalate (Mendes et al., 2015), tetrabutylammonium hydrogen sulphate (Siadatifard et al., 2016) or ZnCl2/Urea (Seyedi et al., 2015) and others. In view of the wide scope of biological activities of BIMs and the growing demand to develop a simple and environmental benign method of synthesizing diverse heterocyclic privileged compounds, herein, we disclosed the synthesis of BIMs via the Water Extract of Onion Peel (WEOP) catalytic system (Scheme 1). The significant advantages of this protocol are free of external acids and bases, reusability of the WEOP catalytic system, good to excellent product yield, reduction in the use of toxic reagents and a simple and effective catalytic system for the synthesis of BIMs.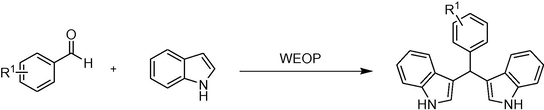
Synthetic route towards the preparation of bisindolylmethanes (BIMs).
2 Experimental
2.1 Materials and methods
In this experiment, the chemicals and solvents utilized were of technical grade and were used without further purification unless stated. The fine chemicals used including ethyl acetate, benzaldehyde, 2-chlorobenzaldehyde, 3-chlorobenzldehyde, 4-chlorobenzaldehyde, 2-bromobenzaldehyde, 3-bromobenzaldehyde, 3-fluorobenzaldehyde, 4-isopropylbenzaldehyde, 4-methoxybenzaldehyde and silica gel 60 (0.063–0.200 mm). The 1H and 13C NMR spectra were recorded using CDCl3 as solvent on Bruker Avance III 400 spectrometer. The CHNS analysis was performed using CHNS Analyzer Flashea 1112 series.
2.2 Preparation of the Water Extract of Onion Peel (WEOP)
The onion peel waste was collected from a local restaurant. The outer layer of the onion was separated from bulb and washed thoroughly with distilled water. The onion peel was then left for air drying for three days. The WEOP was prepared according to a known literature (Bhuyan and Saikia, 2005). Firstly, the dried onion peels (1.0 g) were cut into small pieces and were put into a 100 mL conical flask. Subsequently, 20 mL distilled water was added into the same conical flask and the mixture was heated at 70 °C for 3 h. The aqueous medium was then filtered using Whatman filter paper No.1 and the filtrate was termed as WEOP (Fig. 1). The filtrate was then stored at 4 °C in a sample vial.
Preparation of WEOP for the synthesis of Bisindolylmethanes.
2.3 Synthesis procedure for bisindole 3a-3 k
To a 25 mL round-bottom flask containing indole (2.00 mmol) and benzaldehydes (1.00 mmol) was added with the WEOP (1 mL). The reaction mixture was stirred magnetically at 50 °C. The formation of bisindole was monitored using Think-Layer Chromatography (TLC). After the completion of reaction, the reaction mixture was extracted with ethyl acetate (3 × 10 mL). The organic layer was then separated from the aqueous layer and concentrated under reduced pressure. The crude product was subjected over silica gel column purification and ethyl acetate/hexane (9:1) as eluent. All the pure products were characterized by NMR (1H and 13C) and were further supported by CHNS analysis.
2.4 Spectroscopic data of bisindoles
2.4.1 3,3′-(Phenylmethylene)bis(1H-indole) (3a)
1H NMR (CDCl3, 400 MHz) δ: 5.85 (s, 1H), 6.63 (s, 2H), 7.01 (t, J = 6.7 Hz, 2H), 7.12–7.21 (m, 3H), 7.24–7.31 (m, 2H), 7.32–7.41 (m, 6H), 7.92 (brs, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 40.2, 111.1, 119.2, 119.4, 119.7, 121.7, 123.5, 126.0, 126.9, 128.0, 128.6, 135.6, 143.9 ppm; GC–MS: C23H18N2, m/z 322.15 (M)+. Anal. calcd for C23H18N2: C 85.68, H 5.63, N, 8.69; found C 85.54, H 5.57, N 8.76.
2.4.2 3,3′-(2-Chlorophenylmethylene)bis(1H-indole) (3b)
1H NMR (CDCl3, 400 MHz) δ: 6.33 (s, 1H), 6.64 (s, 2H), 7.03 (t, J = 8.0 Hz, 2H), 7.12–7.22 (m, 6H), 7.35–7.42 (m, 4H), 7.95 (brs, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 36.6, 112.0, 117.0, 118.4, 119.8, 122.0, 123.8, 127.0, 127.3, 128.0, 129.4, 130.5, 133.2, 136.7, 141.3 ppm; GC–MS: C23H17ClN2, m/z 356.11 (M)+. Anal. calcd for C23H17ClN2: C 77.41, H 4.80, N, 7.85; found C 77.39, H 4.77, N 7.81.
2.4.3 3,3′-(3-Chlorophenylmethylene)bis(1H-indole) (3c)
1H NMR (CDCl3, 400 MHz) δ: 5.87 (s, 1H), 6.55 (d, J = 7.5 Hz, 2H), 7.07 (t, J = 7.5 Hz, 2H), 7.23 (m, 5H), 7.35 (t, 3H), 7.43 (d, J = 8.0 Hz, 2H), 7.80 (brs, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 39.8, 111.3, 118.7, 119.3, 119.7, 122.2, 123.7, 126.8, 127.1, 128.7, 129.3, 134.1, 136.5, 146.1 ppm; GC–MS: C23H17ClN2, m/z 356.11 (M)+. Anal. calcd for C23H17ClN2: C 77.41, H 4.80, N, 7.85; found C 77.38, H 4.79, N 7.83.
2.4.4 3,3′-((4-Chlorophenyl)methylene)bis(1H-indole) (3d)
1H NMR (CDCl3, 400 MHz) δ: 5.85 (s, 1H), 6.63 (s, 2H), 7.01 (t, J = 8.1 Hz, 2H), 7.19 (t, J = 7.8 Hz, 2H), 7.21–7.27 (m, 4H), 7.33 (d, J = 7.1 Hz, 4H), 7.95 (br s, 2H) ppm. Anal. calcd for C23H17ClN2: C 77.41, H 4.80, N 7.85; found C 77.37, H 4.79, N 7.80 ppm; 13C NMR (100 MHz, CDCl3) δ: 39.7, 111.8, 118.3, 119.4, 121.3, 124.0, 126.7, 128.5, 130.3, 130.6, 137.3, 144.4 ppm; GC–MS: C23H17ClN2, m/z 356.11 (M)+. Anal. calcd for C23H17ClN2: C 77.41, H 4.80, N, 7.85; found C 77.37, H 4.78, N 7.82.
2.4.5 3,3′-((2-Fluorophenyl)methylene)bis(1H-indole) (3e)
1H NMR (CDCl3, 400 MHz) δ: 6.16 (s, 1H), 6.63 (s, 2H), 6.90 – 7.34 (12H), 7.88 (brs, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 29.8, 111.1, 118.3, 119.3, 119.8, 123.6, 123.9, 126.9, 127.8, 127.9, 130.7, 130.4 136.7 ppm; GC–MS: C23H17FN2, m/z 340.14 (M)+. Anal. calcd for C23H17FN2: C 81.16, H 5.03, N 8.23; found C 81.14, H 4.99, N 8.18.
2.4.6 3,3′-((3-Fluorophenyl)methylene)bis(1H-indole) (3f)
1H NMR (CDCl3, 400 MHz) δ: 5.80 (s, 1H), 6.58 (s, 2H), 6.80 – 7.31 (12H), 7.86 (brs, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 29.7, 111.1, 119.0, 119.3, 119.7, 122.1, 124.4, 126.9, 129.5, 129.6, 136.7, 144.6 ppm; GC–MS: C23H17FN2, m/z 340.14 (M)+. Anal. calcd for C23H17FN2: C 81.16, H 5.03, N 8.23; found C 81.15, H 5.01, N 8.19.
2.4.7 3,3′-((4-Fluorophenyl)methylene)bis(1H-indole) (3g)
1H NMR (CDCl3, 400 MHz) δ: 5.86 (s, 1H), 6.61 (s, 2H), 6.95 (t, J = 7.9 Hz, 2H), 7.01 (t, J = 8.0 Hz, 2H), 7.18 (t, J = 7.3 Hz, 2H), 7.26–7.30 (m, 2H), 7.35 (t, J = 7.3 Hz, 4H), 7.88 (br s, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 29.7, 111.1, 115.1, 119.3, 119.6, 119.9, 122.0, 123.6, 126.9, 130.0, 130.1, 136.7, 139.7 ppm; GC–MS: C23H17FN2, m/z 340.14 (M)+. Anal. calcd for C23H17FN2: C 81.16, H 5.03, N 8.23; found C 81.13, H 4.98, N 8.19.
2.4.8 3,3′-((2-Bromophenyl)methylene)bis(1H-indole) (3h)
1H NMR(CDCl3, 400 MHz): δ: 6.35 (s, 1H), 6.60 (s, 2H), 6.95–7.46 (m, 12H), 7.97 (s, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 36.7, 111.7, 118.4, 119.3, 120.2, 122.1, 123.7, 126.7, 127.6, 129.2, 130.2, 133.4, 136.3, 141.6 ppm; GC–MS: C23H17BrN2, m/z 400.06 (M)+. Anal. calcd for C23H17BrN2: C 68.84, H 4.27, N, 6.98; found C 68.79, H 4.22, N 6.95.
2.4.9 3,3′-((3-Bromophenyl)methylene)bis(1H-indole) (3i)
1H NMR(CDCl3, 400 MHz): δ: 5.95 (s, 1H), 6.55 (s, 2H), 7.94–6.98 (m, 14H) ppm; 13C NMR (100 MHz, CDCl3) δ: 39.7, 111.6, 118.5, 119.5, 120.1, 122.2, 123.5, 126.7, 127.8, 129.1, 131.2, 136.4, 138.3, 146.6 ppm; GC–MS: C23H17BrN2, m/z 400.06 (M)+. Anal. calcd for C23H17BrN2: C 68.84, H 4.27, N, 6.98; found C 68.80, H 4.23, N 6.94.
2.4.10 3,3′-[(4-Methoxyphenyl)methylene]bis(1H-indole) (3j)
1H NMR (CDCl3, 400 MHz) δ: 3.67 (s, 3H), 5.85 (s, 1H), 6.63 (s, 2H), 6.81 (d, J = 8.7 Hz, 2H), 6.97 (t, J = 7.2 Hz, 2H), 7.15 (t, J = 7.2 Hz, 2H), 7.26 (d, J = 8.7 Hz, 2H), 7.35 (d, J = 7.6 Hz, 2H), 7.39 (d, J = 7.6 Hz, 2H), 7.90 (br s, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 39.6, 55.6, 111.4, 113.5, 119.4, 120.4, 121.8, 123.4, 126.6, 129.8, 132.2, 135.8, 137.3, 158.5 ppm; GC–MS: C24H20N2O, m/z 352.16 (M)+. Anal. calcd for C24H20N2O: C 81.79, H 5.72, N 7.95; found C 81.76, H 5.68, N 7.91.
2.4.11 3,3′-[(4-Isopropylyphenyl)methylene]bis(1H-indole) (3k)
1H NMR (CDCl3, 400 MHz) δ: 1.28 (d, J = 7.0 Hz, 6H), 2.84 (sept, J = 7.00 Hz, 1H), 5.74 (s, 1H), 6.71 (s, 2H), 7.05 (t, J = 7.5 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 7.34 (d, J = 7.6 Hz, 2H), 7.39 (d, J = 8.0 Hz, 2H), 7.45 (d, J = 8.0 Hz, 2H), 7.90 (br s, 2H) ppm; 13C NMR (100 MHz, CDCl3) δ: 24.6, 34.2, 39.8, 111.4, 113.5, 119.5, 120.4, 120.5, 122.4, 124.1, 126.7, 127.5, 128.9, 137.2, 141.6, 146.9 ppm; GC–MS: C24H24N2, m/z 364.19 (M)+. Anal. calcd for C24H20N2: C 85.68, H 6.64, N 7.69; found C 85.62, H 6.60, N 7.67.
3 Results and discussion
For the reaction optimization process, the optimized reaction condition was determined by using benzaldehyde (1 mmol) and indole (2 mmol) as the model starting materials. Both compounds were added into a 25 mL round bottom flask suspended with WEOP and the reaction mixture was stirred under magnetic stirring at 50 °C. The progress of the reaction was monitored by TLC analysis using hexane/ethyl acetate (8:2) solvent mixture. The influence of WEOP and temperature were studied (Table 1, entries 1–9). Initially, a 0.2 mL of WEOP was used as solvent for the model reaction and was heated at 50 °C. The yield of the required product was found to be at 50% (Table 1, entry 2). Further increment of the amount of WEOP used increased the yield to 65% (Table 1, entry 3). The complete conversion of the 3a (≥90% yield) was observed with the use of 1.0 mL of WEOP and the reaction mixture was heated at 50 °C for 5 h (Table 1, entry 5). In contrast, we observed no change in the yield of the desired product, even in a prolonged reaction time (Table 1, entry 7) and increment of reaction temperature (Table 1, entry 8). The temperature that rendered the best yield of 3a was found to be at 50 °C, whereas at lower temperature (Table 1, entry 9) the reaction yield was decreased. With these results in mind, subsequent study was proceeded with 1.0 mL of WEOP as reaction medium at 50 °C for 5 h (Table 1, entry 5) as the optimized reaction condition. A controlled experiment was also carried out by subjecting the model starting materials to pure water as the solvent. Under the identical temperature and reaction time, the conversion of model starting materials to 3a was found to be in poor yield (20%) as compared to that of WEOP as solvent (Table 1, entry 5).
Entry
Solvent (mL)
Time (h)
Temperature (°C)
Yielda (%)
1
H2O (1.0)
5
50
20
2
WEOP (0.2)
1
50
50
3
WEOP (0.5)
2
50
65
4
WEOP (0.5)
3
50
75
5
WEOP (1.0)
5
50
90
6
WEOP (2.0)
5
50
89
7
WEOP (1.0)
12
50
86
8
WEOP (1.0)
5
80
85
9
WEOP (1.0)
5
40
76
To widen the scope of our study on the WEOP, commercially available aldehydes were selected to synthesize a variety of different substituted 3. In all of the cases, the pure BIMs were purified over a short silica column chromatography (62–90%). As shown in Table 2, high yields were achieved for the synthesis of different substituted bisindoles 3 within a reasonable time (Table 2, entries 1–11). Benzaldehydes substituted with electron-withdrawing groups (Table 2, entries 1–9) afforded the bisindoles of 3 in good to excellent yields in 5 h. In contrast, the benzaldehydes substituted with electron-donating groups (Table 2, entries 10–11) required a longer reaction time to accomplish good conversion.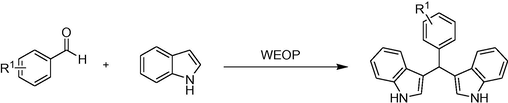
Entry
R1
Product
Reaction Time (h)
Isolated yield (%)
1
H
3a
5
90
2
2-Cl
3b
5
84
3
3-Cl
3c
5
88
4
4-Cl
3d
5
87
5
2-F
3e
5
89
6
3-F
3f
5
78
7
4-F
3 g
5
75
8
2-Br
3 h
5
76
9
3-Br
3i
5
72
10
4-OCH3
3j
12
62
11
4-(CH3)2CH
3 k
12
65
The chemoselectivity of the WEOP catalytic system was also investigated (Scheme 2). In order to explore this, acetophenone 3 l (1 mmol) and benzaldehyde 3a (1 mmol) were added into a 100 mL round bottom flask containing indole (4 mmol) and WEOP (1.0 mL). The reaction mixture was magnetically stirred at 50 °C for 5 h. It was found that 3a was the sole isolated product after heating for 5 h resulting in a 90% yield, while 3 l remained unchanged. This result indicated the high chemoselectivity of the WEOP catalytic system.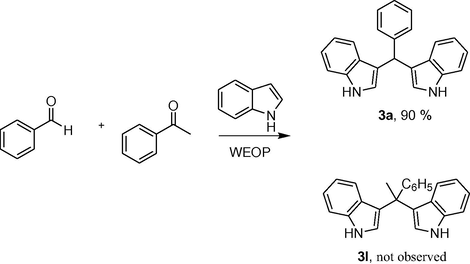
The chemoselectivity study using the WEOP catalytic system.
Prior to the reusability study of the WEOP catalyst system, a LC-MS analysis was carried out on the WEOP catalytic system before condensation of indole and benzaldehyde and compared to that of the WEOP which was recovered after five successive usages. The LC-MS revealed that most of the phytochemicals in the WEOP are water-soluble compounds, such as caffeic acid, ferullic acid, sinapinic acid, cyanidin, tannic acid and other organic acids (Fig. 2). These organic acids were still detectable in the aqueous system of WEOP after being reused for five successive cycles for the synthesis of 3a with good yields (85–88%) (Fig. 3). These organic acids were believed to serve as catalysts in the formation of 3a. Though there was a slight decrease in terms of product yield that might be due to the use of ethyl acetate in extraction of crude product after each subsequent cycle of using reused WEOP, the reusability test showed that the overall yield of 3a and other bisindoles were still satisfactory, as shown in Table 3.![Selected spectra of LC-MS analysis on the WEOP. a) [M-H]− of caffeic acid, b) [M]+ of ferullic acid, and c) [M]+ of sinapinic acid.](/content/185/2019/31/4/img/10.1016_j.jksus.2018.05.029-fig5.png)
Selected spectra of LC-MS analysis on the WEOP. a) [M-H]− of caffeic acid, b) [M]+ of ferullic acid, and c) [M]+ of sinapinic acid.
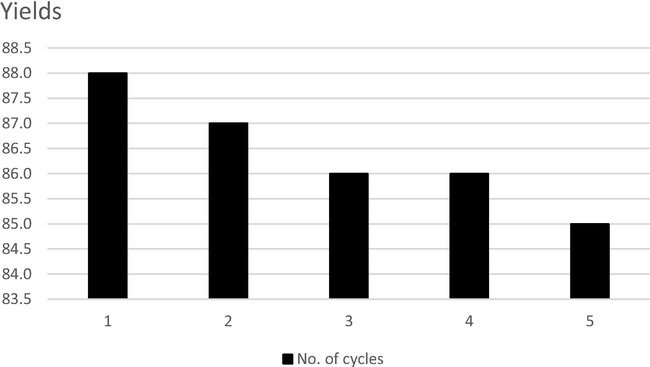
Recycling of WEOP for synthesis of 3a. Reaction condition: 1 mmol of benzaldehyde, 2 mmol of indole, 1 mL of WEOP, 50 °C.
Entry
R1
Product
Isolated yield (%)
1
H
3a
90
88
87
86
86
85
2
2-Cl
3b
84
83
82
81
80
78
3
3-Cl
3c
88
85
84
83
82
80
4
4-Cl
3d
87
86
85
83
82
79
5
2-F
3e
89
85
84
83
81
78
6
3-F
3f
78
76
75
72
71
71
7
4-F
3 g
75
74
73
70
69
68
8
2-Br
3 h
76
73
72
70
68
67
9
3-Br
3i
72
71
70
69
65
64
10
4-OCH3
3j
62
60
58
58
57
55
11
4-(CH3)2CH
3 k
65
63
61
59
58
54
The dramatic promotion of the synthesis of BIMs by using the WEOP catalytic system is currently not well understood. Literature report revealed that onion peels contain phenolic acids as major constituent, along with other minor chemical constituents such as flavanols, flavones and anthocyanidines (Benkeblia, 2007). pH measurement for the WEOP media revealed a pH value of 3.68 that are due to the presence of water soluble phytochemicals- caffeic acid, ferullic acid, sinapinic acid, cyanidin, tannic acid and other organic acids. These organic acids were still detectable in the recovered WEOP after five successive usages. Therefore, we propose that the organic acids present in the WEOP served to protonate the oxygen atom on the carbonyl group of aldehydes, thereby facilitating the nucleophilic attack on indole and promoting the synthesis of BIMs, in a way similar way to that reported in a previous literature (Fiorito et al., 2016). In order to show the merit of the WEOP protocol, the previous methods and their yields for synthesising 3a were summarised in Table 4. To date, many efficient catalytic system have been reported for this reaction, however, most of the previous methods encompassed the use of harsh condition, high temperature, expensive and non-recyclable catalysts for the synthesis of bisindolylmethanes.
Entry
Catalyst
Yield (%)
Reference
1
WEOP
90
2
Ammonium niobium oxalate
99
(Mendes et al., 2015)
3
Tetrabutylammonium hydrogen sulphate
91
(Siadatifard et al., 2016)
4
ZnCl2/Urea
92
(Seyedi et al., 2015)
5
Squaric acid/water
90
(Azizi et al., 2012)
6
[bnmim][HSO4]/microwave irradiation
93
(Sadaphal et al., 2008)
7
ZrOCl2.8H2O
89
(Mishra and Ghosh, 2011)
8
Glacial acetic acid
90
(El-Sayed et al., 2014)
9
Oleic acid
98
(Ganesan et al., 2015)
4 Conclusions
In summary, we have demonstrated the synthesis of BIMs in the presence of WEOP. The current protocol offers many advantages including a simple and effective catalytic system, simple work-up, benign reagents, cheap but good to excellent yields and the reusability of the WEOP catalytic system. Further application of the WEOP in the synthesis of other bioactive heterocyclic compounds is currently ongoing in our laboratory and the results will be reported in due course.
Acknowledgement
We are thankful to the Universiti Malaysia Terengganu for providing the Talent and Publication-Enhancement Research Grant (TAPE-RG), Vot. No. 55111.
References
- Green procedure for the synthesis of bis (indolyl) methanes in water. Scientia Iranica. 2012;19:574-578.
- [Google Scholar]
- Discovery of 3,3′-diindolylmethanes as potent antileishmanial agents. Eur. J. Med. Chem.. 2013;63:435-443.
- [Google Scholar]
- Water: the most versatile and nature’s friendly media in asymmetric organocatalyzed direct aldol reactions. Tetrahedron: Asymmetry. 2015;26:1215-1244.
- [Google Scholar]
- Isolation of colour components from native dye-bearing plants in northeastern India. Bioresour. Technol.. 2005;96:363-372.
- [Google Scholar]
- Pd(OAc)2 in WERSA: a novel green catalytic system for Suzuki-Miyaura cross-coupling reactions at room temperature. Chem. Commun.. 2015;51:11489-11492.
- [Google Scholar]
- Onion skin waste as a valorization resource for the by-products quercetin and biosugar. Food Chem.. 2015;188:537-542.
- [Google Scholar]
- A green protocol for ligand, copper and base free Sonogashira cross-coupling reaction. Tetrahedron. Lett.. 2016;57:3760-3763.
- [Google Scholar]
- Glacial acetic acid as an efficient catalyst for simple synthesis of dindolylmethanes. Curr. Chem. Lett.. 2014;3:7-14.
- [Google Scholar]
- Direct enantioselective aldol reactions catalyzed by calix[4]arene-based l-proline derivatives in the water. Tetrahedron. 2014;70:4471-4477.
- [Google Scholar]
- A green chemical synthesis of coumarin-3-carboxylic and cinnamic acids using crop-derived products and waste waters as solvents. Tetrahedron. Lett.. 2016;57:4795-4798.
- [Google Scholar]
- Oleic acid: a benign Brønsted acidic catalyst for densely substituted indole derivative synthesis. RSC Adv.. 2015;5:28597-28600.
- [Google Scholar]
- Recycling the biowaste to produce nitrogen and sulfur self-doped porous carbon as an efficient catalyst for oxygen reduction reaction. Nano Energy. 2015;16:408-418.
- [Google Scholar]
- Nano TiO2/SiO2: An efficient and reusable catalyst for the synthesis of oxindole derivatives. J. Saudi Chem. Soc.. 2016;20:101-106.
- [Google Scholar]
- Water promoted enantioselective aldol Reaction by proline-cholesterol and -diosgenin based amphiphilic organocatalysts. Tetrahedron. 2013;69:5136-5143.
- [Google Scholar]
- Water in asymmetric organocatalytic systems: a global perspective. Org. Biomol. Chem.. 2016;14:6147-6164.
- [Google Scholar]
- Synthesis of 1,8-dioxo-octahydroxanthenes and bis(indolyl)methanes catalyzed by [Et3NH][H2PO4] as a cheap and mild acidic ionic liquid. Arabian J. Chem.. 2012;5:319-323.
- [Google Scholar]
- Potassium phthalimide as efficient basic organocatalyst for the synthesis of 3,4-disubstituted isoxazol-5(4H)-ones in aqueous medium. J. Saudi Chem. Soc.. 2017;21:S112-S119.
- [Google Scholar]
- A green protocol for peptide bond formation in WEB. Tetrahedron Lett.. 2016;57:2283-2285.
- [Google Scholar]
- Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica. J. Asian Nat. Prod. Res.. 2015;17:689-704.
- [Google Scholar]
- Recent advances in stereoselective organic reactions in aqueous media. Curr. Org. Chem.. 2015;19:813-868.
- [Google Scholar]
- Systems approaches to integrated solid waste management in developing countries. Waste Manage.. 2013;33:988-1003.
- [Google Scholar]
- Synthesis of bis(indolyl)methanes using ammonium niobium oxalate (ANO) as an efficient and recyclable catalyst. Green Chem.. 2015;17:4334-4339.
- [Google Scholar]
- A simple green protocol for the condensation of anthranilic hydrazide with cyclohexanone and N-benzylpiperidinone in water. J. Heterocyclic Chem.. 2016;53:32-37.
- [Google Scholar]
- Mishra, S., Ghosh, R. 2011. Ecofriendly and sustainable efficient synthesis of bis (indolyl) methanes based on recyclable Brønsted (CSA) or Lewis (ZrOCl 2. 8H 2 O) acid catalysts.
- Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.) Front. Life Sci.. 2013;7:224-228.
- [Google Scholar]
- Bisindole Alkaloids with Neural Anti-inflammatory Activity from Gelsemium elegans. J. Nat. Prod.. 2013;76:2203-2209.
- [Google Scholar]
- Structure, bioactivity and synthesis of natural products with hexahydropyrrolo[2,3-b]indole. Chem. Eur. J.. 2011;17:1388-1408.
- [Google Scholar]
- Ionic liquid promoted synthesis of bis (indolyl) methanes. Open Chemistry. 2008;6:622-626.
- [Google Scholar]
- Cancer chemotherapy with indole-3-carbinol, bis(3′-indolyl)methane and synthetic analogs. Cancer Lett.. 2008;269:326-338.
- [Google Scholar]
- Agro-waste extract based solvents: emergence of novel green solvent for the design of sustainable processes in catalysis and organic chemistry. ChemistrySelect. 2017;2:5180-5188.
- [Google Scholar]
- ZnCl2/Urea as a deep eutectic solvent for the preparation of bis(indolyl)methanes under ultrasonic conditions. Synth. React. Inorg. Met.-Org. Nano-Metal Chem.. 2015;45:1501-1505.
- [Google Scholar]
- Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) waste. Food Funct.. 2016;7:3354-3369.
- [Google Scholar]
- An efficient method for synthesis of bis(indolyl)methane and di-bis(indolyl)methane derivatives in environmentally benign conditions using TBAHS. Cogent Chem.. 2016;2:1188435.
- [Google Scholar]
- Application of natural feedstock extract: the Henry reaction. Tetrahedron Lett.. 2016;57:2814-2817.
- [Google Scholar]
- Recent advances in indole syntheses: New routes for a classic target. Org. Biomol. Chem.. 2011;9:6469-6480.
- [Google Scholar]







