Translate this page into:
Utilization of triethylammonium hydrogen sulphate-mediated solvent for optimization of asiaticoside extraction and antioxidant capacity of Centella asiatica (L.)
⁎Corresponding author at: Department of Chemical Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia. shahrul.zahari@usim.edu.my (Shikh Mohd Shahrul Nizan Shikh Zahari), syareeda@ukm.edu.my (Nur Hasyareeda Hassan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
Abstract
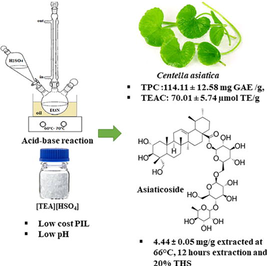
Asiaticoside, a pentacyclic triterpene of Centella asiatica with broad pharmacological actions. Higher asiaticoside content of Centella extracts in food products increases their nutritional and medicinal values. Protic ionic liquids (PIL) were utilized as bioactive extraction additives. The research focuses on obtaining the optimum extraction parameters for higher asiaticoside yield, and Centella extract antioxidant capacity. Optimization of all responses (asiaticoside yield, TPC, TEAC) achieved through faced-centered composite design (FCCD) involving three factors (temperature, extraction time, and triethylammonium hydrogen sulfate, [TEA][HSO4] %). The optimal conditions were 66 °C, 12 h duration, and 20% [TEA][HSO4], which resulted in 4.44 ± 0.05% (w/w) asiaticoside, TPC of 114.11 ± 12.58 mg GAE /g and TEAC of 70.01 ± 5.74 µmol TE/g respectively. All responses fit the quadratic model with proximity between predicted and experimented values. Procurement of higher asiaticoside yield, TPC, and TEAC verified the pertinent of the optimal conditions. In addition, the outcomes give an overview of PILs potential for higher bioactive extractions. Research expansion by utilizing other PILs for plant extraction in addition to solute–solvent interaction study will be beneficial for the designation of an efficient plant extraction process that maximizes the plant-based product market.
Keywords
Triethylammonium hydrogen sulfate
Asiaticoside
Phenolic compounds
Antioxidant
Optimization
1 Introduction
Centella asiatica (L.) or locally known as “Pegaga” in Malaysia, is a prominent therapeutic herb with multiple pharmacological actions (Jhansi and Kola, 2019; Wong and Ramli, 2021) such as anti-inflammatory, antioxidant, wound ameliorating, neuroprotective (Yadav, 2021), antimicrobial, anti-diabetic, antifungal, and anticancer properties (Tripathy and Srivastav, 2023; Tripathy et al., 2022). These benefits contributed by flavonoids and terpenoids content such as asiatic acid, madecassoside, and asiaticoside (Fig. 1). Commercially, there were more than 100 Centella-based formulations in the market with at least 2% asiaticoside and madecoside content required for the product benefits (Idris and Mohd Nadzir, 2021; Prasad et al., 2019).![Chemical structure of [TEA][HSO4] and asiaticoside.](/content/185/2023/35/8/img/10.1016_j.jksus.2023.102863-fig2.png)
Chemical structure of [TEA][HSO4] and asiaticoside.
Therefore, the extraction processes parameters such as solvent concentration, pH, temperature, and extraction duration are crucial in obtaining the bioactive compounds (Kumar et al., 2021; Sridhar et al., 2021). Ethanolic extraction has been widely employed for C. asiatica leaves extraction (Idris and Mohd Nadzir, 2021; Monton et al., 2019; Thong-On et al., 2021; Yingngam et al., 2020). The asiaticoside yield through ethanol-based extraction reportedly ranged from 0.09% to 0.193% (Monton et al., 2019). Other studies reported the optimum conditions for polyphenols extraction from Centella to be 37% ethanol concentration at 70.2 °C and 110.5 min (Mohapatra et al., 2021). Even at optimum conditions reported, the asiaticoside yield was still low than the minimum requirement.
Ionic liquids (ILs) efficiency as green solvents for bioactive extraction attracts attention (Choi and Verpoorte, 2019; Ferreira et al. 2022; Lim et al., 2022; Yansheng et al., 2011). Protic ionic liquids (PILs) are a subgroup of IL that are non-volatile, non-flammable, and more stable at higher temperatures than conventional organic molecular solvents (Clough et al., 2015; Greaves and Drummond, 2015; Nasirpour et al., 2020). Triethylammonium hydrogen sulfate, [TEA][HSO4] (Fig. 1) is one of the PILs that has received increasing attention due to its ultra-low-cost that can be made at bulk scale for $1.24 kg−1, favorably comparable to acetone (Chen et al. 2014). [TEA][HSO4] effectively deconstruct various types of biomass by providing dual functions: (1) a Brønsted acid catalyst that disrupts chemical linkages in biomass complex structure and; (2) a delignifier that dissolves lignin (Khan et al., 2020; Welton, 2013; Zahari et al., 2018). Due to this, it is plausible to destruct the plant tissues and cell walls of C. asiatica, increasing their permeability and consequent molecular diffusion, playing a crucial role in higher extraction yield (Zhao et al., 2014). Moreover, asiaticoside was reportedly more stable in acidic pH (Puttarak et al., 2016).
There has yet to be the utilization of [TEA][HSO4] reported for Centella extraction, and using previous research optimum parameters at different operational conditions is not plausible. Hence, this study investigated the optimum extraction condition for asiaticoside yield and antioxidant capacity by utilizing [TEA][HSO4] as a co-solvent in the C. asiatica leaves extraction, aiding by response surface methodology (RSM).
2 Methodology
2.1 Preparation of Triethylammonium hydrogen Sulfate, [TEA][HSO4] IL
The synthesis followed a method published elsewhere (Salahi et al. 2016; Wang et al., 2006; Zahari et al., 2018). 2.5 M of H2SO4 (98 g, 1 mol) was added dropwise to triethylamine, N222 (101 g, 1 mol) over 1 h at 60 °C. The mixture continued to be stirred at 70 °C for 1 h. Water traces were removed under pressure at 80 °C. The as-synthesized [TEA][HSO4], obtained as a colorless solid, was characterized by 1D-NMR (see Fig. S1 in ESI† for 1H and 13C NMR spectra) (ppm): 1H NMR (DMSO‑d6): 1.16 (t, 9H, 7.4 Hz), 3.06 (q, 6H, 7.4 Hz), 9.26 (s, 1H); 13C NMR (DMSO‑d6): 9.02 (CH3) and 46.05 (CH2).
2.2 Asiaticoside extraction from Centella asiatica
Centella asiatica leaves were collected in Negeri Sembilan, Tampin, Malaysia. They were cleaned, dried in an oven for 24 h at 30 °C, and ground into a particle size of 0.5 mm. A binary solvent system was first prepared by mixing [TEA][HSO4] and EtOH-40% according to the desired ratio Table 1. The mixture at 10 ml/g ratio was incubated at a specific period. The asiaticoside yield in the collected extract was quantified using reverse phase-high performance liquid chromatography (RP-HPLC).
[TEA][HSO4] /EtOH ratio (g/ml)
%[TEA] [HSO4]
Viscosity (Pa.s)
pH
0.25
20
0.0037 ± 0.0001
1.20 ± 0.012
0.66
40
0.0081 ± 0.0006
1.27 ± 0.006
1.0
50
0.0116 ± 0.0007
1.37 ± 0.042
1.5
60
0.0189 ± 0.0000
1.39 ± 0.010
2.3 Quantification of asiaticoside yield (AY)
The extract was diluted with deionized water at a 1:1 ratio, filtered using a 0.22 mm nylon syringe filter, and analyzed on a Shimadzu LC-20 with a photodiode array detector (PDA) at 220 nm and a C-18 column. The following conditions were used: 0.8 ml/min flow rate, 20 µl injection volume, methanol, and deionized water (70/30 (vol/vol)) as mobile phase and column temperature of 30 °C. Standards asiaticoside solution prepared in deionized water at concentrations ranging from 10 to 100 ppm (see Fig. S2 in ESI† for the calibration curve).
2.4 Measurement of total phenolic content (TPC)
Diluted extracts (1 mg/ml, 20 µl of each sample) were placed in microplate wells. Subsequently, the wells were left for 10 min in the dark at room temperature after the addition of 10 v/v%, 100 µl Folin Ciocalteu reagent, followed by Na2CO3 (7.5%, 80 µl) addition to each sample. After 2 h left in the dark, the mixture absorbance was read at 765 nm. Gallic acid calibration curve plot (see Fig S2 in ESI†) aids TPC quantification in mg gallic acid equivalents (GAE) unit per g of dried extract.
2.5 Trolox equivalent antioxidant capacity (TEAC) quantification
DPPH scavenging ability measures the DPPH radicals quantity scavenged by phenolic compounds (ArOH) in the extract. Neutralization of DPPH radical in the assay occurred by accepting hydrogen atom or electron from antioxidant species, resulting in reduced DPPH (DPPH-H) (Bibi Sadeer et al., 2020), as shown in Eq. (1). The extracts (100 μl) and 0.2 mM of DPPH solution (100 μl) were pipetted into a 96-well plate. The absorbance was read at 517 nm after 30 min dark incubation. Equation (2) was used to determine DPPH radical scavenging %. TEAC represented the antioxidant activity in µmol TE/g and was calculated using DPPH scavenging activity of trolox (%) against the log series concentration calibration curve (see Fig. S2 in ESI†).
2.6 Optimization through response surface methodology (RSM)
RSM allowed the analysis of multiple factor effects and their interactions towards response variables (Pais-Chanfrau et al., 2021). Thus, more information can be obtained from a limited number of experiments (Goren et al. 2022). Twenty experimental trials were performed per Face-centered composite design (FCCD) with temperature, X1 (30 °C,55 °C, 80 °C), extraction time, X2 (12 h,18 h, 24 h), and [TEA][HSO4] %, X3 (20%, 40%, 60%) as variables, while, AY, TPC, and TEAC as responses. Design Expert 13 was used as a statistical tool for the experimental design and analysis. The FCCD consists of six axial points, eight factorial points, and one center with six replications.
3 Discussion
3.1 Time course extraction and experimental output
We monitored the asiaticoside yield (AY) in designing the conditions for RSM. As a control, the ground leaves were soaked in EtOH-40% at 65 °C for 1 h, yielding 0.28 ± 0.02%w/w of asiaticoside. [TEA][HSO4]/EtOH (1 g/ml) addition significantly enhanced the AY by 9 times (2.5 ± 0.27%w/w). Hence, time course extraction under the same conditions was performed. Fig. 2 shows that the AY increases significantly up to 12 h and then plateaus thereafter, which can be explained by Fick’s second law of diffusion (Benchikh et al., 2021).![Asiaticoside yields extracted from Centella asiatica (L.) by [TEA][HSO4] /EtOH = 1 g/ml at 65 °C. (NS (non-significant): p greater than 0.05) and *p < 0.05(significant).](/content/185/2023/35/8/img/10.1016_j.jksus.2023.102863-fig3.png)
Asiaticoside yields extracted from Centella asiatica (L.) by [TEA][HSO4] /EtOH = 1 g/ml at 65 °C. (NS (non-significant): p greater than 0.05) and *p < 0.05(significant).
Overall, the twenty experimental trials gave the following responses: AY ranging from 2.75.% to 4.75% (w/w); TPC of 18.98 to 112.58 mg GAE/g, and; TEAC of 29.28 to 72.05 µmol TE/g. All responses adequately fitted quadratic polynomial equations, as indicated by a significant model, non-significant Lack-of-fit test, and R2 values higher than 0.75 (Table 3).
RUN
Independent variables
Dependent variables
X1:
X2:
X3:
Y1:
Y2:
Y3:
Temperature (°C)
Extraction time (Hours)
[TEA][HSO4] %
AY (%,w/w)
TPC (mg GAE /g)
TEAC (µmolTE/g)
1
55
24
40
3.55 ± 0.030
60.84 ± 0.00
40.04 ± 1.69
2
30
24
20
3.52 ± 0.040
66.57 ± 16.24
61.88 ± 2.28
3
55
12
40
4.44 ± 0.030
68.98 ± 0.00
56.36 ± 0.00
4
30
24
60
2.75 ± 0.015
32.47 ± 0.00
29.28 ± 0.00
5
80
12
60
4.27 ± 0.017
50.60 ± 1.64
48.67 ± 13.90
6
30
18
40
3.96 ± 0.013
57.00 ± 2.47
50.07 ± 2.63
7
55
18
40
4.43 ± 0.005
75.95 ± 0.00
63.76 ± 3.02
8
55
18
40
4.29 ± 0.009
71.88 ± 6.74
53.50 ± 0.00
9
55
18
40
4.27 ± 0.002
88.28 ± 0.99
53.50 ± 0.00
10
80
24
60
3.57 ± 0.032
34.56 ± 0.00
36.60 ± 0.00
11
30
12
20
3.87 ± 0.010
111.30 ± 21.05
66.64 ± 0.35
12
55
18
20
4.00 ± 0.033
112.58 ± 26.80
72.05 ± 0.00
13
80
24
20
3.92 ± 0.001
18.98 ± 1.97
50.41 ± 0.00
14
55
18
40
4.34 ± 0.046
91.19 ± 0.16
59.38 ± 0.00
15
55
18
40
4.04 ± 0.011
82.35 ± 1.81
65.41 ± 0.00
16
80
12
20
4.75 ± 0.030
89.44 ± 0.00
66.97 ± 9.49
17
30
12
60
3.01 ± 0.019
25.72 ± 0.00
31.28 ± 0.00
18
55
18
40
4.32 ± 0.020
77.23 ± 3.12
58.43 ± 5.83
19
80
18
40
4.54 ± 0.003
55.02 ± 0.00
50.26 ± 2.91
20
55
18
60
2.97 ± 0.023
57.58 ± 8.22
36.33 ± 0.38
Responses
Model Equation
Model Significant
Lack-of-fit Test
R2
AY
+4.20 + 0.3931 X1 −0.3031 X2 −0.3486 X3 + 0.1676 X12 −0.0901 X22 – 0.5981 X32 −0.1155 X1X2 + 0.0999 X1X3 + 0.0276X2 X3
<0.0001 (Significant)
0.1118(not significant)
0.9384
Adjusted
0.8830
Predicted
0.6940
TPC
+79.69––4.45 X1 −13.26 X2 −19.80 X3 −21.50 X12 – 12.61 X22 + 7.57 X32 −6.06 X1 X2 + 12.05 X1 X3 + 13.25 X2 X3
< 0.0001
(Significant)0.3229 (not significant)
0.9447
Adjusted
0.8948
Predicted
0.7221
TEAC
+56.90 + 1.38 X1 −5.17 X2 −13.58 X3 −3.59 X12 −5.56X22 + 0.43 X32 −2.73 X1 X2 + 4.48 X1 X3 + 0.91X2 X3
0.0006
(Significant)0.3876 (not significant)
0.9028
Adjusted
0.8153
Predicted
0.6258
3.2 Independent variable effects on responses
According to the analysis of variance (ANOVA) in Table 4 (Entry 1–3), all independent variables were significant towards AY. For TPC and TEAC, extraction temperature had a non-significant individual effect, while the other variables were significant.
AY (%, w/w)
TPC (mg GAE/g)
TEAC (µmol TE/g)
Variables
Coefficient
F
Prob > F
Coefficient
F
Prob > F
Coefficient
F
Prob > F
X1-temperature
0.3931
43.03
< 0.0001
−4.45
2.77
0.1269
1.38
0.66
0.4361
X2-extraction time
−0.3031
25.58
0.0005
−13.26
24.58
0.0006
−5.17
9.29
0.0123
X3-[TEA][HSO4] %
−0.3486
33.83
0.0002
−19.80
54.83
< 0.0001
−13.58
64.05
< 0.0001
Interaction
X1X2
−0.1155
2.97
0.1155
−6.06
4.10
0.0703
−2.73
2.08
0.1801
X1X3
0.0999
2.22
0.1668
12.05
16.23
0.0024
4.48
5.58
0.0398
X2X3
0.0276
0.17
0.6885
13.25
19.63
0.0013
0.91
0.23
0.6431
Temperature showed a positive coefficient towards AY (Table 4, Entry 1), as depicted by Run 4 versus Run 10 (Table 2). Generally, secondary metabolites are secluded within the cell wall. Higher temperature aid in cell wall destruction, releasing abundant bioactive compounds (Gómez-Maqueo et al., 2020). Meanwhile, extraction time and [TEA][HSO4]% exhibited a negative coefficient toward all responses (Table 4, Entry 2, Entry 3). This indicates that longer extraction time (Table 2, Run 1 versus Run 7) and an increase in [TEA][HSO4] % (Table 2, Run 2 versus Run 4) led to a decrement in responses. Polyphenols degradation at extended extraction duration at high temperatures (Kim et al., 2018) and high viscosity of the binary solvent (Table 1) leading to mass transfer limitations (Fuad and Nadzir, 2023) explained this occurrence.
3.3 Independent variables interactions
AY does not significantly influence by all independent variables interactions (Table 4, Entry 4–6). In contrast, TPC was positively impacted by the interaction of [TEA][HSO4]% with temperature and extraction time (X1X3; X2X3) (Table 4, Entry 5 and 6). Extended extraction time aid in leaching the bioactive compound out into the solvent system, resulting in higher TPC (More and Arya, 2021; Sharma and Dash, 2021). Meanwhile, only positive interaction of temperature-[TEA][HSO4] % (X1X3) was observed for TEAC (Table 4, Entry 5). This occurrence is plausibly due to lower solvent viscosity with temperature increment, enhancing the bioactive mass transfer into the solvent system (Yusoff et al., 2022).
The variable interactions on TPC and TEAC were further visualized in 3D-surface plots, as shown in Fig. 3. In Fig. 3 (a), at 55 °C, the highest TPC was given when variables X2 and X3 had the smallest value. This suggests that lower [TEA][HSO4] % and shorter extraction time favor higher TPC value.![Three-dimensional surface of (a) TPC: extraction time - [TEA][HSO4]%; (b) TPC: temperature - [TEA][HSO4]%, and; (c) TEAC: temperature -[TEA][HSO4]%.](/content/185/2023/35/8/img/10.1016_j.jksus.2023.102863-fig4.png)
Three-dimensional surface of (a) TPC: extraction time - [TEA][HSO4]%; (b) TPC: temperature - [TEA][HSO4]%, and; (c) TEAC: temperature -[TEA][HSO4]%.
Fig. 3 (b) shows a TPC response of X1 versus X3 at 18 h. The plot proposes that lower [TEA][HSO4] % and moderate temperature leads to higher TPC value. Similar patterns were observed for the TEAC response of X1 versus X3 at 18 h (Fig. 3(c)).
3.4 Role of [TEA][HSO4]
Generally, bioactive compounds are secluded in rigid, thick cell walls containing polysaccharides as the major components. Hence, any extraction techniques should be able to make the cell walls permeable, permitting the bioactive emission from the cells.
We obtained low AY when we first performed ethanolic extraction (EtOH-40%) at 65 °C for 1 h. These suggest minimal destruction of cell walls by ethanolic extraction. Interestingly, adding [TEA][HSO4] as the co-solvent enhanced asiaticoside yield by nine times. We associate this with the intensified destruction of cell walls caused by [H3O]+ ions, which arose from H2O molecules protonation by the acidic protons of [HSO4]- ions during the extraction process (Roy et al., 2020).
Regarding TPC and subsequent TEAC, the release of phenolic compounds appeared to increase with the temperature at a fixed [TEA][HSO4]%. Higher temperatures lead to H2O molecules protonation increment, generating more [H3O]+ ions that further intensify the destruction of cell walls.
3.5 Validation of the predictive model
The optimal C. asiatica extraction was 66 °C, 12 h, and 20% [TEA][HSO4] at predicted AY of 4.39% (w/w), TPC of 112.58 mg GAE /g, and TEAC of 70.62 µmol TE/g respectively. At the same time, the experimental data at optimum conditions were 4.44 ± 0.05% (w/w), 114.11 ± 12.58 mg GAE/g, and 70.01 ± 5.74 µmol TE/g. The experimental and predicted value proximity confirms the practicability of optimum conditions.
Interestingly, the results above were far higher than those reported in previous studies. The asiaticoside yield obtained was ca. 3.40%, 3.29%, and 4.27% higher than reported (Table 5). Similarly, TPC and TEAC were markedly increased by 89% and 72%, respectively, compared to previous studies (Table 5).
Extraction conditions
Asiaticoside yield (%,w/w)
TPC
(mg GAE/g)
TEAC
(µmol TE/g)
MAE:40% EtOH, 153 W, 10 min (Thong-On et al., 2021)
1.031
–
–
UAE: 40% EtOH, 55 °C, 90 min(Thong-On et al., 2021)
1.155
–
–
95% EtOH, 60 °C, 120 min (Monton et al., 2019)
0.174
–
–
40% EtOH, 65 °C, 60 min (Chew et al., 2011)
–
12.03
19.48
4 Conclusion
Triethylammonium hydrogen sulfate, [TEA]HSO4] mediated co-solvent able to enhance asiaticoside extraction. At optimal conditions of 66 °C, 12 h, and 20% [TEA]HSO4], the yield of asiaticoside, TPC, and TEAC were 4.44 ± 0.05% (w/w), 114.11 ± 12.58 mg GAE /g, and 70.01 ± 5.74 µmol TE/g respectively. All responses fit the quadratic model, and the optimal conditions can be applied practically for efficient C. asiatica extraction. The outcomes of this research, give an overview of PILs potential as bioactive extractants, besides widening the application of other PILs toward plant extraction. The extract's high antioxidant capacity will be beneficial in plant-based product development. Research expansion comprising the solute–solvent interaction will be beneficial in designing more efficient plant extraction and broadening the plant-based product market.
Acknowledgments
The authors appreciate the financial support under grant FRGS/1/2019/STG01/UKM/02/12 and GUP-2017-014. Shikh Zahari also extends appreciation to the Ministry of Higher Education of Malaysia (MOHE) for scholarship awards (KPT(BS)860407335591) as a postdoctoral research fellow at Imperial College London, UK.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Optimising anthocyanin extraction from strawberry fruits using response surface methodology and application in yoghurt as natural colorants and antioxidants. J. Food Sci. Technol.. 2021;58:1987-1995.
- [Google Scholar]
- The versatility of antioxidant assays in food science and safety—chemistry, applications, strengths, and limitations. Antioxidants. 2020;9(8):709.
- [Google Scholar]
- Inexpensive ionic liquids:[Hso 4]−-based solvent production at bulk scale. Green Chem.. 2014;16(6):3098-3106.
- [Google Scholar]
- Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Centella asiatica extracts. Int. Food Res. J.. 2011;18(2):1472-11435.
- [Google Scholar]
- Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci.. 2019;26:87-93.
- [Google Scholar]
- Ionic liquids: not always innocent solvents for cellulose. Green Chem.. 2015;17(1):231-243.
- [Google Scholar]
- Uses of ionic liquids to obtain bioactive compounds: insights from the main international regulations for technological applications. Crit. Rev. Food Sci. Nutr. 2022:1-16.
- [Google Scholar]
- Ultrasound-assisted extraction of asiaticoside from Centella asiatica using betaine-based natural deep eutectic solvent. Ind. Crop. Prod.. 2023;192:116069
- [Google Scholar]
- Release mechanisms of bioactive compounds in fruits submitted to high hydrostatic pressure: A dynamic microstructural analysis based on prickly pear cells. Food Res. Int.. 2020;130:108909
- [Google Scholar]
- Language of response surface methodology as an experimental strategy for electrochemical wastewater treatment process optimization. In: Artificial Intelligence and Data Science in Environmental Sensing. Academic Press; 2022. p. :57-92.
- [Google Scholar]
- Protic ionic liquids: evolving structure-property relationships and expanding applications. Chem. Rev.. 2015;115(20):11379-11448.
- [Google Scholar]
- Comparative studies on different extraction methods of Centella asiatica and extracts bioactive compounds effects on antimicrobial activities. Antibiotics. 2021;10(4):457.
- [Google Scholar]
- The antioxidant potential of Centella asiatica: A review. J. Med. Plants Stud. 2019;7:18-20.
- [Google Scholar]
- Conversion of biomass to chemicals using ionic liquids. In: Green Sustainable Process for Chemical and Environmental Engineering and Science. Elsevier; 2020. p. :1-30.
- [Google Scholar]
- Degradation kinetics of phenolic content and antioxidant activity of hardy kiwifruit (actinidia arguta) puree at different storage temperatures. LWT. 2018;89:535-541.
- [Google Scholar]
- Ultrasound assisted extraction (Uae) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem.. 2021;70:105325
- [Google Scholar]
- Ionic liquids as green solvent and their applications in bioactive compounds extraction from plants. Process Biochem.. 2022;122:292-306.
- [Google Scholar]
- Influence of extraction methods and solvent system on the chemical composition and antioxidant activity of Centella asiatica L. Leaves. Biocata. Agric. Biotechnol.. 2021;33:101971
- [Google Scholar]
- An optimization approach of dynamic maceration of Centella asiatica to obtain the highest content of four centelloids by response surface methodology. Rev. Bras. 2019;29:254-261.
- [Google Scholar]
- Intensification of bio-actives extraction from pomegranate peel using pulsed ultrasound: effect of factors, correlation, optimization and antioxidant bioactivities. Ultrason. Sonochem.. 2021;72:105423
- [Google Scholar]
- Ionic liquids: promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des.. 2020;160:264-300.
- [Google Scholar]
- Uses of the response surface methodology for the optimization of agro-industrial processes. In: Response Surface Methodology in Engineering Science. IntechOpen; 2021.
- [Google Scholar]
- Advances and emerging research trends for modulation of centelloside biosynthesis in Centella asiatica (L.) urban-a review. Ind. Crops Prod.. 2019;141:111768
- [Google Scholar]
- Biological activities and stability of a standardized pentacyclic triterpene enriched Centella asiatica extract. Nat. Prod. Sci.. 2016;22(1):20-24.
- [Google Scholar]
- Recent advances of greener pretreatment technologies of lignocellulose. Current Res. Green Sustainable Chem.. 2020;3:100035.
- [Google Scholar]
- Two ammonium ionic liquids as efficient catalysts for the one-pot Green synthesis of 3, 4, 5-substituted furan-2 (5h)-ones. Bul. Chem. Commun.. 2016;48(3):364-368.
- [Google Scholar]
- Deep eutectic solvent-based microwave-assisted extraction of phytochemical compounds from black jamun pulp. J. Food Process Eng. 2021;44(8):e13750.
- [Google Scholar]
- Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett.. 2021;19:3409-3443.
- [Google Scholar]
- Green extraction optimization of triterpenoid glycoside-enriched extract from Centella asiatica (L.) Urban using response surface methodology (Rsm) Sci. Rep.. 2021;11(1):1-11.
- [Google Scholar]
- Encapsulation of Centella asiatica leaf extract in liposome: study on structural stability, degradation kinetics and fate of bioactive compounds during storage. Food Chem. Adv.. 2023;2:100202
- [Google Scholar]
- Recent trends in extraction, identification and quantification methods of Centella asiatica phytochemicals with potential applications in food industry and therapeutic relevance: A review. Food Biosc. 2022:101864.
- [Google Scholar]
- Preparation of simple ammonium ionic liquids and their application in the cracking of dialkoxypropanes. Green Chem.. 2006;8(7):603-607.
- [Google Scholar]
- Cutting-edge research for a greener sustainable future. Green Chem.. 2013;15:550-583.
- [Google Scholar]
- Antimicrobial activity of different types of Centella asiatica extracts against foodborne pathogens and food spoilage microorganisms. LWT. 2021;142:111026
- [Google Scholar]
- Potential therapeutic strategies of phytochemicals in neurodegenerative disorders. Curr. Top. Med. Chem.. 2021;21(31):2814-2838.
- [Google Scholar]
- Microwave-assisted extraction of lactones from ligusticum chuanxiong hort. Using Protic Ionic Liquids. Green Chem.. 2011;13(3):666-670.
- [Google Scholar]
- Modeling and optimization of microwave-assisted extraction of pentacyclic triterpenes from Centella asiatica leaves using response surface methodology. Ind. Crop. Prod.. 2020;147:112231
- [Google Scholar]
- A Review of ultrasound-assisted extraction for plant bioactive compounds: phenolics, flavonoids, thymols, Saponins and Proteins. Food Res. Int. 2022:111268.
- [Google Scholar]
- triethylammonium hydrogen sulfate ionic liquid as a low-cost solvent: A short review of synthesis, analysis and applications. MATEC Web Conf.. 2018;00006
- [Google Scholar]
- Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit. Rev. Food Sci. Nutr.. 2014;54(10):1283-1297.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102863.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







