Translate this page into:
Use of agroindustrial wastes for the production of cellulases by Penicillium sp. FSDE15
⁎Corresponding author at: Department of Sugar and Alcohol Technology, Federal University of Paraíba, 58058-600 Paraiba, Brazil. laisctcg@ctdr.ufpb.br (Laís Campos Teixeira de Carvalho-Gonçalves)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cellulase refers to a group of enzymes like endoglucanases (CMCase), exoglucanases and β-glucosidase that have great industrial importance for the production of second-generation ethanol, e.g. The present study aimed to evaluate the CMCase and β-glucosidase activities of the fungal strain Penicillium sp. FSDE15 using different agroindustrial wastes (wheat bran, sugarcane bagasse and sugarcane straw) as carbon sources to the basal medium during Submerged Fermentation (SFm). When the medium presented wheat bran, highest CMCase and β-glucosidase yields were 1.03 U/mL (96 h) and 1.68 U/mL (168 h), respectively. For sugarcane bagasse medium, maximum enzymatic activity values were observed in 120 h culture (1.38 U/mL for CMCase and 0.55 U/mL for β-glucosidase). Sugarcane straw medium presented highest enzymatic yields after 120 h culture (1.02 U/mL of CMCase and 0.39 U/mL of β-glucosidase). The response surface methodology showed highest CMCase (1.3 U/mL) and β-glucosidase (6.5 U/mL) activities in the presence of lower levels of wheat bran (1%) (w/w), inoculum concentration of 105 spores/mL, temperatures of 41 °C and 33 °C, respectively. Penicillium sp. FSDE15 presented two isoforms of CMCase (150 kDa and around 30 kDa) and one isoform of β-glucosidase (70 kDa). Our study demonstrated that the production of cellulases with high activity by Penicillium sp. FSE15 in a culture medium with agroindustrial wastes, such as wheat bran, might be a promising alternative to reduce the cost of these molecules and facilitate their use in various industrial processes.
Keywords
Fungi
CMCase
β-glucosidase
Biomass
Submerged fermentation
- CMCase
-
Endoglucanase
- MW
-
Mandels and Weber medium
- RSM
-
Response Surface Methodology
- WB
-
Wheat Bran
- SFm
-
Submerged Fermentation
- SB
-
Sugarcane Bagasse
- SS
-
Sugarcane Straw
Abbreviations
1 Introduction
Lignocellulosic materials are mainly composed of lignin, cellulose and hemicellulose. They have low cost and potential use in the production of biomolecules and biofuels that offer benefits, both environmental and strategic. Among these materials, sugar cane bagasse, sugar cane straw, corn cob, wheat bran, among others (Zoghlami and Paës, 2019).
Cellulose is a fibrous, stringent, crystalline and water insoluble organic carbon source that can be completely hydrolyzed into cellobiose and glucose monomers by cellulases. These enzymes systems consisting of three main components: endoglucanases (CMCases), exoglucanases and β-glucosidases (Thapa et al., 2020). Cellulases are essential enzymes for the conversion of lignocellulosic biomass into fermentable sugars for the production of second-generation ethanol (Vaishnav et al., 2018).
Fungi of the genus Penicillium are also profitable options for the production of cellulases on an industrial scale, in addition to producing β-glucosidases with high activity (Vaishnav et al., 2018). β-Glucosidases are often present at lower levels than the other two enzymatic components – endo and exoglucanases - in microbial preparations. Maintaining optimal amounts of this enzyme is essential for achieving high yields of glucose during biomass degradation (Gao et al., 2012). CMCase and β-glucosidase are key enzymes in the process of cellulose degradation to glucose. Recently, our research group reported the Penicillium FSDE15 isolate as an outstanding producer cellulase (Santos et al., 2021). However, these results prompted the question, is it possible to improve cellulase production in Penicillium sp. FSDE15? It is evident that there are several variables that can be manipulated for this purpose, such as carbon source with an experimental design by statistical models. Therefore, the aim of this study was to evaluate the CMCase and β-glucosidase production by the fungal strain Penicillium sp. FSDE15 using different agroindustrial wastes such as wheat bran, sugarcane bagasse and sugarcane straw as well as to optimize the enzymatic activity.
2 Materials and methods
2.1 Microorganism
This study was carried out with a new fungal strain Penicillium sp. FSDE15 obtained from the collection of cultures of the Laboratory of Applied Microbiology (UFPB, Brazil). The strain was isolated by screening soil samples from sugarcane plantation located at Santa Rita, Paraiba, Brazil (6°99′20.67′’S 35°02′55.61′’) and stored at 4 °C on potato dextrose agar plates (Sigma Aldrich, St. Louis, USA). The microorganism was maintained in Petri dishes containing PDA medium under sterile conditions at 37 °C for seven days. Spore suspension was counted at 106 spores/mL according to Santos et al. (2021).
2.2 Cellulases production by submerged state fermentation (SFm)
The basal medium was adapted from Mandels and Weber medium (MW) (1969). Three different lignocellulosic residues, wheat bran (WB), sugarcane bagasse (SB) and sugarcane straw (SS), were used as carbon source. Before their use, each residue underwent milling processes in a knife mill (Wiley Mill, Solab, SL-31) and sieving (mesh 32). Subsequently, residues were submitted to thermal pretreatment in autoclave (121 °C, 1 atm for 15 min). A volume of 50 mL of MW medium were added to 125 mL Erlenmeyer flasks, as well as 5% (w/v) of pretreated WB, SB and SS, separately, final pH was 5.7. The medium was autoclaved for 15 min at 121 °C and, after cooling, received an inoculum of 106 spore/mL. Enzymatic production under SmF occurred in duplicates in rotary shaker (Solab, SL-223) at 150 rpm, 37 °C for 8 days. Every 24 h, two flasks were removed and the sample were filtrated using Whatman n° 1 filter paper and centrifuged at 5000 rpm, 10 °C for 5 min (Quimis, Q222RT). Afterwards, the pH of all centrifuged samples was measured. The crude enzymatic extract was used for determination of enzymatic activities.
2.3 Enzymes activities measurement
CMCase activity was assayed in triplicate according to Santos et al. (2021). Calibration curve for CMCase was performed using different glucose concentrations, each level in three replicates (Miller, 1959). One unit (U) of CMCase activity corresponded to 1 µmol of glucose released per minute.
β-glucosidase activity was determined using 2 mM p-nitrophenyl-β-D-glucopyranoside (PNPG, Sigma-Aldrich) in 50 mM sodium acetate buffer (pH 5.0) and 0.125 mL of the crude extract. The trials were in triplicate according to Gao et al. (2008). Calibration curve for β-glucosidase was made similarly as CMCase, although using p-nitrophenol as substrate (Shi et al., 2018). One unit of β-glucosidase was defined as the releasing of 1 µmol of p-nitrophenol per minute.
2.4 Experimental design and optimization of cellulase production by response surface methodology (RSM)
In order to optimize the cultivation conditions and enhance CMCase and β-glucosidase production by Penicillium sp. FSDE15, experimental design was performed using RSM by the software Statistica (Statsoft 7.0, EUA). Then a 23 experimental design was used with 3 repetitions in the central point and 8 unique combinations, totaling 11 assays. The factors were inoculum concentration (X1) (105 –107 spores/mL), temperature (X2) (30–40 °C) and wheat bran concentration (X3) (1–5%). Each factor in the design matrix was studied at three different levels (-1, 0, +1) and their level values are shown in Table 1. The variables or responses were activity of CMCase (U/mL) and β-glucosidase (U/mL) are depicted in Table 2.
Independent variables
Symbol
Codified levels
−1
0
1
Inoculum concentration (spore/mL)
Incubation temperature (°C)
Wheat bran concentration (%)X1
X2
X3
105
33
1106
37
3107
41
5
Test
X1
X2
X3
CMCase
β-glucosidase
Observed
Predicted
Observed
Predicted
1
2
3
4
5
6
7
8
9
10
11105
107
105
107
105
107
105
107
106
106
106
33
33
41
41
33
33
41
41
37
37
371
1
1
1
5
5
5
5
3
3
31.05
0.74
1.30
0.90
0.12
0
0.62
0.40
0.80
0.78
0.811.09
0.78
1.31
0.94
0.16
0.04
0.66
0.44
0.68
0.68
0.686.50
5.99
0.52
1.29
0.69
3.82
2.49
0.80
3.53
4.18
3.136.74
6.22
0.75
1.52
0.92
4.05
2.69
1.13
3
3
3
The fungal strain was cultivated in 125 mL Erlenmeyer flasks containing 50 mL of modified MW medium with 5% (w/v) wheat bran for 4 days, under constant agitation in orbital shaker at 150 rpm. The enzymatic extract was centrifuged at 12.000 rpm at 10 °C for 5 min and enzymatic activity was assayed.
2.5 Enzymatic profile analysis with SDS-PAGE electrophoresis
For CMCase profile analysis, the cell-free supernatant was assayed using polyacrylamide denaturing gel (12% w/v; 0.75 mm) (Padilha et al., 2015). The β-glucosidase gel was washed with distilled water and incubated at 55 °C for 15 min in 0.5 mM 4-methyl-lumbelliferyl β-D-glucopyranoside (MUG) diluted in citrate buffer 50 mM (pH 4.8). The molecular mass markers used were those for low molecular weight ranges (GE, USA) including phosphorylaseb (97 kDa), serum albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa), and α-Lactalbumin (14.4 kDa). Finally, for enzymatic activity visualization and photography, the CMCase gel was submerged in Congo Red (0.1 %) for 15 min and decolored in NaCl 1 M, while β-glucosidase gel was visualized under UV 365 nm (UltraBright transilluminator) (Ng et al., 2010).
2.6 Statistical analysis
The statistical analysis was performed by variance analysis (ANOVA) aiming to obtain the regressions’ significance at a 95 % level of confidence. Softwares such as Graph Prism 5.0 (GraphPad software, CA, EUA) and Statistica (Statsoft 7.0, EUA) were used to analyze the data obtained.
3 Results
3.1 CMCase and β-glucosidase production by SmF
All three carbon sources (WB, SB and SS) used in our study induced growth and production of CMCase and β-glucosidase by the Penicillium sp. FSDE15. The initial activity of CMCase and β-glucosidase before 24 h, in all culture media, was zero. When the medium was composed of WB, maximum CMCase and β-glucosidase yields were 1.03 (96 h) and 1.68 (168 h), respectively (Fig. 1A). With the SB medium, maximum values of enzymatic activity observed were after 120 and 168 h culture to CMCase (1.38 U/mL) and β-glucosidase (0.55 U/mL), respectively (Fig. 1B). Although, when the SS medium was used, the highest enzymatic activity was 1.02 U/mL of CMCase and 0.39 U/mL of β-glucosidase after 120 h growth (Fig. 1C).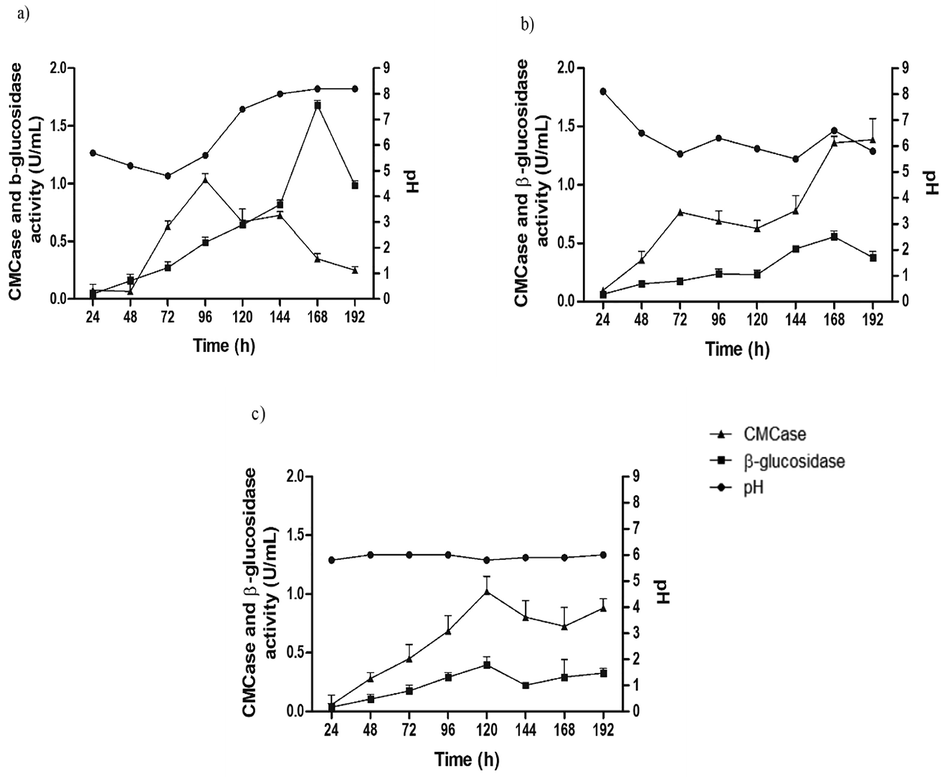
Enzymatic activity (EA) of Penicillium sp. FSDE15 in basal media containing wheat bran (a), sugarcane bagasse (b) and sugarcane straw (c).
Regarding the pH variation, alkalinization of the WB medium was directly related to the reduction in CMCase activity, where the pH increased from 5.7 (96 h) to 7.4 (120 h) and reduced about 35% of the enzyme activity (Fig. 1). On the order hand, highest β-glucosidase activity were observed at pH 8.2, 5.7 and 6.6, when the medium WB, SB and SS were used, respectively.
3.2 Cellulase production by RSM
The results presented in Table 2 showed a wide variation of CMCase activity (0 to 1.3 U/mL) and β-glucosidase activity (0.52 to 6.5 U/mL), which reflects the importance to optimize the process parameters. Both CMCase and β-glucosidase showed highest activity in the presence of lower levels of WB (1%) (w/w), inoculum concentration of 105 spores/mL and temperatures of 41 and 33 °C, respectively (Table 2).
In this study, R2 for CMCase and β-glucosidase were 0.96 and 0.95, respectively. Analysis of variance (ANOVA) was used to assess the fit of the polynomial model to CMCase and β-glucosidase activity. The results tabulated in Table 3 showed that calculated F value was 1.28 for CMCase and 0.92 for β-glucosidase (P < 0.05 at 95% confidence level), indicating that the model was significant only to CMCase. F0.05; 7; 3 = 8.89.
Source of Variation
Sum of Squares
Degrees of Freedom
Mean Square
F-value
CMCase
β-glucosidase
CMCase
β-glucosidase
CMCase
β-glucosidase
CMCase
β-glucosidase
Regression
1.4193
40.922
7
7
0.2028
5.846
11.39
8.19
Residual
0.0532
2.141
3
3
0.0178
0.714
Lack of Fit
0.0527
1.580
1
1
Pure Error
0.0005
0.562
2
2
Total
1.4725
43.063
10
10
R2
0.96
0.95
Statistical analysis showed that the two independent variables (WB concentration and temperature) were significant for CMCase. For β-glucosidase, independent variable temperature and interaction between temperature-WB concentration was significant at 5% significance level, as observed in X3 and X2 bars (Fig. 2A) and X2 and X2*X3 bars (Fig. 2B).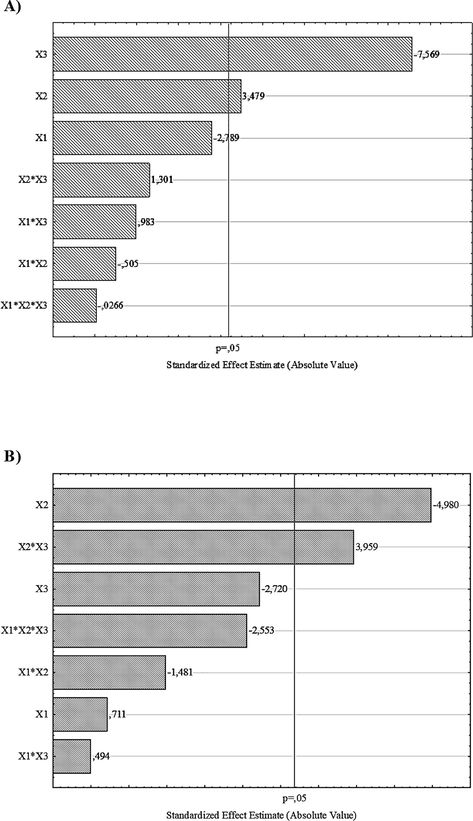
Pareto chart showing the significance of variables X1 (inoculum concentration), X2 (temperatura) and X3 (wheat bran concentration) on the CMCase (a) and β-glucosidase (b) activity.
The final response function to predict CMCase activity was:
For β-glucosidase, the final response function for activity prediction was:
Fig. 3 shows the optimized response surface, built based on the model for the CMCase activity. It is possible to verify that with the increase in temperature and decrease in the concentration of WB, CMCase activity increases, for any spore concentration used, obtaining a maximum value of 1.3 U/mL under the conditions studied in this work.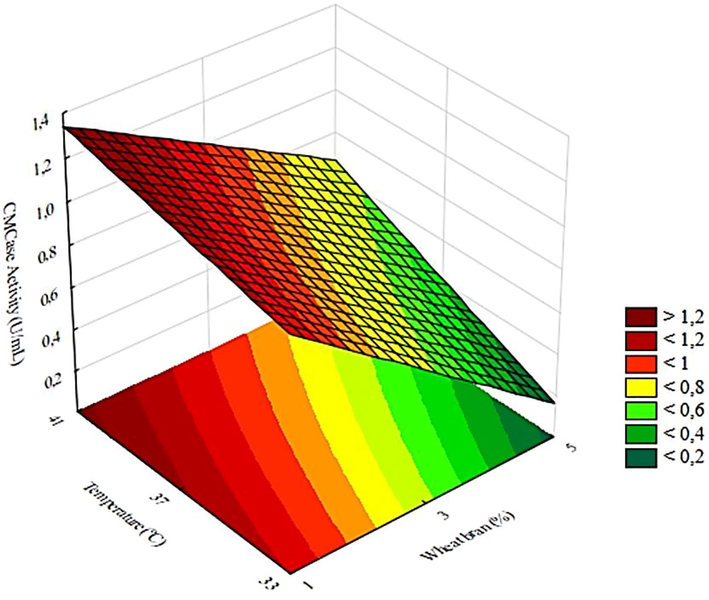
Effect of temperature and wheat bran concentration on CMCase activity for the fixed spore concentration of 105.
3.3 Enzymatic profiling by SDS-PAGE electrophoresis
The enzymatic profile of Penicilium sp. FSDE15 in the crude enzymatic extract by zymogram presented two isoforms of CMCase and one isoform of β-glucosidase. The CMCase isoforms were highly different in molecular weight with 150 kDa and between 20 and 30 kDa. β-glucosidase isoform weighed closely to 70 kDa (Fig. 4).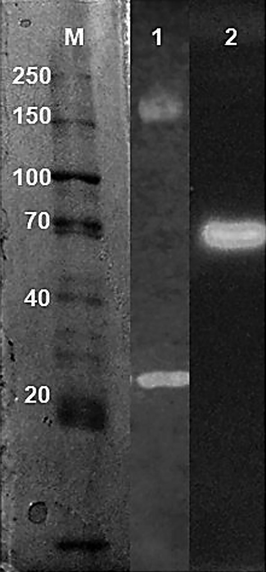
Enzymatic profile of cellulases from Penicilium sp. FDSE15. CMCase and β-glucosidase isoforms are shown in lanes 1 and 2, respectively. M: molecular marker (bp).
4 Discussion
4.1 Cellulase production using different agroindustrial wastes
In this study, the agroindustrial wastes (WB, SB and SS) induced the production of CMCase and β-glucosidase by Penicillium sp. FSDE15. While the SB medium was considered exceptional for CMCase activity, β-glucosidase yield was low for this carbon source. Thus, the WB medium was selected for the experimental design.
Before the experimental design by RSM, the highest CMCase and β-glucosidase activity were 1.03 U/mL and 1.68 U/mL in the WB medium, respectively. Zimbardi et al. (2013) investigated the action of agroindustrial wastes such as corn straw, wheat bran, corn cob, cassava meal and sugarcane bagasse in the production of CMCase and β-glucosidase. They observed that wheat bran was the best induction source for the production of these enzymes. Studies presented by Kumar et al. (2011) demonstrated that the highest production of CMCase occurred in 126 h, pH 4.6, and in a carbon source (a combination of wheat bran, corn bran and kinnow peel at the proportion of 2:1:2, respectively) concentration of 65 g/L in SmF. Prasanna et al. (2016) reported that the highest β-glucosidase activity presented by Penicillium sp. was 1.04 U/mL, when the culture medium presented a mixture of cellulose at 0.5%, lactose at 0.5% and sawdust at 0.5% and that at the morphological changes, enzyme secretion and product stability in the medium can be affected by pH change during the growth of microorganisms.
The present study showed that the highest CMCase production by the microorganism evaluated in WB, SB and SS medium was observed at pH 5.7, showing that these enzymes are more active at acidic pH, independent of the carbon source. Sohail et al. (2009) showed that the maximum CMCase yield by A. niger MS82 was obtained at pH 4.0, while at pH 5 enzyme production decreased drastically and remained constant up to 7. Unlike CMCase, the highest levels of β-glucosidase were obtained at pH 4.0, followed by a reduction in its activity as pH increases to 5, and a continuous activity decrease while pH reaches 7.
Sulyman et al. (2020) demonstrated that the optimum pH for maximum CMCase and β-glucosidase production by Aspergillus niger in the submerged fermentation was 4.0. Prasanna et al. (2016) observed that the highest CMCase and β-glucosidase production by Penicillium sp. was at pH 5.0. The highest β-glucosidase activity by Penicillium roqueforti ATCC 10,110 occurs in pH 5.5 (Neves et al., 2020).
The results obtained after experimental design by RSM demonstrate a significant effect on the optimization of CMCase and β-glucosidase activity. After carrying out the experimental design by RSM, using WB as carbon source, the largest CMCase activity was observed at 41 °C (1.3 U/mL), with an increase of 26% in relation to the initial values of 1.03 U/mL for this same carbon source.
Matkar et al. (2013) optimized SmF conditions for Aspergillus sydowii using solely lactose as carbon source and reported an optimal CMCase activity (0.39 U/mL) at 40 °C. Gomathi et al. (2012), on the other hand, studied CMCase production by Aspergillus flavus using wheat bran as carbon source and registered the highest production of cellulases (0.8 U/mL) at 30 °C.
For β-glucosidase, optimal activity was observed at 33 °C (6.5 U/mL), with an increase of 287% in relation to the initial values of 1.68 U/mL for WB medium. Saini et al. (2015) obtained similar results in optimization studies with Penicillium oxalicum, where temperature of cultivation was held at 28 °C. Our results are also comparable to those obtained by Singhvi et al. (2011) in SmF experiments with mutant strains (EMS-UV-8, 5.1 U/mL and EU-2D-21, 3.1 U/mL) and the parent strain (6.8 U/mL) of Penicillium janthinellum.
Few studies have been made to compare the β-glucosidase production between the widely used T. reesei and other fungal strains, like Penicillium and Aspergillus. Singhania et al. (2013) performed comparative studies of SmF between a reference strain of T. reesei and four strains of Penicillium. The maximum yield of 0.03 U/mL of β-glucosidase was obtained from the T. reesei strain, while the Penicillium strains produced between 0.97 and 2.45 U/mL. Results like these demonstrate the importance of screening studies for finding novel biological sources of industrially applicable enzymes.
The β-glucosidase is as a “rate-limiting” enzyme due to its key role in determining efficiency of biomass hydrolysis. If its amount during hydrolysis is low, cellobiose concentration rises and provokes feedback inhibition of the other components of the cellulolytic system, reducing the rate of glucose release (Monteiro et al., 2020).
4.2 Enzymatic profiling
Various fungi are known for producing multiple cellulases. The presence of different isoforms of CMCase can be explained by different genes that encode enzymes of the same type and this can happen when the fungus grow in different culture media and culture conditions (Bonfá et al., 2018). The existence of multiple isoforms may be important for the microorganism’s ability to respond well and rapidly to environmental changes (Oberoi et al., 2014).
5 Conclusion
The present study aimed to evaluate the CMCase and β-glucosidase activities of the fungal strain Penicillium sp. FSDE15. We have demonstrated how different variables, such as spore and carbon source concentration and temperature, can drastically affect CMCase and β-glucosidase production by fungal microorganisms, and that the agroindustrial wastes such as wheat bran are potential inducers in the production of these enzymes. Our findings revealed the Penicillium sp. FSDE15, obtained from the soil of sugarcane plantation, as a promising fungal strain to be submitted to scale-up studies for industrial cellulase production, especially for enhancement of CMCase and β-glucosidase yields.
Acknowledgments
The authors acknowledge the Brazilian funding agencies Coordination for the Improvement of Higher Education Personnel (CAPES) and National Council for Scientific and Technological Development (CNPq) for the financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biochemical characterization of an isolated 50 kDa beta-glucosidase from the thermophilic fungus Myceliophthora thermophila M.7.7. Biocatal. Agric. Biotechnol.. 2018;13:311-318.
- [Google Scholar]
- Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresour. Technol.. 2008;99(16):7623-7629.
- [CrossRef] [Google Scholar]
- The production of β-glucosidases by Fusarium proliferatum NBRC109045 isolated from Vietnamese forest. AMB Express. 2012;2(1):49.
- [CrossRef] [Google Scholar]
- Submerged fermentation of wheat bran by Aspergillus flavus for production and characterization of carboxy methyl cellulase. Asian Pac. J. Trop. Biomed.. 2012;2(1):S67-S73.
- [CrossRef] [Google Scholar]
- Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in Submerged (SmF) and Solid State Fermentation (SSF) Food Sci. Biotechnol.. 2011;20(5):1289-1298.
- [CrossRef] [Google Scholar]
- Production of cellulase by a newly isolated strain of Aspergillus sydowii and its optimization under submerged fermentation. Int. Biodeterior. Biodegrad.. 2013;78:24-33.
- [CrossRef] [Google Scholar]
- Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem.. 1959;31(3):426-428.
- [CrossRef] [Google Scholar]
- A highly glucose tolerant β-Glucosidase from Malbranchea pulchella (MpBg3) enables cellulose saccharifcation. Sci. Rep.. 2020;10:6998.
- [CrossRef] [Google Scholar]
- Production and biochemical characterization of halotolerant β-glucosidase by Penicillium roqueforti ATCC 10110 grown in forage palm under solid-state fermentation. Biomass Convers. Biorefin.. 2020;1:1-12.
- [CrossRef] [Google Scholar]
- High-level production of a thermoacidophilic β-glucosidase from Penicillium citrinum YS40-5 by solid-state fermentation with rice bran. Bioresour. Technol.. 2010;101(4):1310-1317.
- [CrossRef] [Google Scholar]
- Response surface optimization for enhanced production of cellulases with improved functional characteristics by newly isolated Aspergillus niger HN-2. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol.. 2014;105(1):119-134.
- [CrossRef] [Google Scholar]
- Production and characterization of thermophilic carboxymethyl cellulase synthesized by Bacillus sp. growing on sugarcane bagasse in submerged fermentation. Brazilian J. Chem. Eng.. 2015;32(1):35-42.
- [CrossRef] [Google Scholar]
- Optimization of cellulase production by Penicillium sp. 3. Biotech. 2016;6:1-11.
- [CrossRef] [Google Scholar]
- Enhanced cellulase production by Penicillium oxalicum for bio-ethanol application. Bioresour. Technol.. 2015;188:240-246.
- [CrossRef] [Google Scholar]
- Evaluation of the production of cellulases by Penicillium sp. FSDE15 using corncob and wheat bran as substrates. Bioresour. Technol. Rep.. 2021;14:100648.
- [CrossRef] [Google Scholar]
- Data on thermostableβ-glucosidase immobilizedby Zn2+. Data Brief.. 2018;18:873-876.
- [CrossRef] [Google Scholar]
- Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour. Technol.. 2013;127:500-507.
- [CrossRef] [Google Scholar]
- Comparative production of cellulases by mutants of Penicillium janthinellum NCIM 1171 and its application in hydrolysis of Avicel and cellulose. Bioresour. Technol.. 2011;102(11):6569-6572.
- [CrossRef] [Google Scholar]
- Cellulase production from Aspergillus niger MS82: effect of temperature and pH. N. Biotechnol.. 2009;25(6):437-441.
- [CrossRef] [Google Scholar]
- Isolation, purification and characterization of cellulase produced by Aspergillus niger cultured on Arachis hypogaea shells. Helion. 2020;6:1-10.
- [CrossRef] [Google Scholar]
- Microbial cellulolytic enzymes: diversity and biotechnology with reference to lignocellulosic biomass degradation. Rev. Environ. Sci. Biotechnol.. 2020;19(3):621-648.
- [CrossRef] [Google Scholar]
- Penicillium: The next emerging champion for cellulase production. Bioresour. Technol. R.. 2018;2:131-140.
- [CrossRef] [Google Scholar]
- Optimization of β-Glucosidase, β-Xylosidase and Xylanase Production by Colletotrichum graminicola under solid-state fermentation and application in raw sugarcane trash saccharification. Int. J. Mol. Sci.. 2013;14:2875-2902.
- [CrossRef] [Google Scholar]
- Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front Chem.. 2019;7:1-11.
- [CrossRef] [Google Scholar]







