Translate this page into:
Unveiling the salinity tolerance potential of Armenian Dandur (Portulaca oleracea L.) genotypes: Enhancing sustainable agriculture and food security
⁎Corresponding authors at: Faculty of Biology, Yerevan State University, Yerevan 0025, Armenia (Abhishek Singh); Department of Botany and Microbiology, College of Science, King Saud University, 11451, Riyadh, Saudi Arabia (Mohamed S. Elshikh). sinxabishik@ysu.am (Abhishek Singh), athanasios.th.alexiou@gmail.com (Athanasios Alexiou), melshikh@ksu.edu.sa (Mohamed S. Elshikh),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study examines the adaptation of an undocumented halophyte, Dandur, to salt stress in different soil mediums. The Dandur plant physiological, morphological, and biochemical analyses show that it can withstand salinity stress. Dandur plants grown in clay and clay loam soil experienced significant decreases in height and stem diameter under extreme salinity conditions compared to non-saline soil. The fresh weight of root and shoot decreased by 70.4% and 84.8%, respectively, and dry weight by 48.5% and 79.7%. These findings underscore the importance of maintaining saline soil conditions for plant growth. Dandur unique ability to avoid dehydration and maintain growth traits, particularly in clay soil, holds promise for further exploration in agricultural research and food security-related issues caused by climate change in saline areas of Armenia and other European countries.
Keywords
Dandur
Salinity stress
Armenia
Europe
Food security
Soil
1 Introduction
The world population is projected to reach 10 billion by 2050, leading to an urgent need for optimal food production (Kumar and Sharma, 2020a, 2020b). Modern agriculture has relied on intensive practices to produce high-yielding crops, but improvements in productivity may not be enough to meet this demand. Climate change has reduced farmable land availability, leading to the salinization of one-third of the world's agricultural fields. About 6 % of arable land becomes infertile due to climate change-related salinization (Kumar and Sharma, 2020a). Salinity affects 50 % of irrigated regions, with over 20 % exhibiting elevated salinity. Salinity in the soil is detrimental to plant growth and development, causing osmotic tension and ion toxicity. Researchers are exploring novel plants that can survive in extreme environments and use them as an alternative to conventional food with higher nutrient value (Dhankher and Foyer, 2018; Kumar and Sharma, 2020). Halophytes, which can thrive in soil conditions with more than 200 mM NaCl, are an estimated 1 % of all plants on Earth. Their physiological functions, including ion compartmentalization, salt inclusion, salt excretion, ion transportation, antioxidants, and osmotic management, help them survive under extreme saline conditions. With practical commercial standards, such as food, fodder, energy, medicines, and revegetation, halophytes can meet the growing population's fundamental requirements in developing and developed nations in the climate change era.
Purslane, or P. oleracea L., is a member of the family Portulaceceae Juss (Ocampo and Columbus, 2012). Purslane is an annual, succulent, dicot, and powerful medicinal plant (Ocampo and Columbus, 2012). Although it is predominantly grown in the tropics and subtropics, humans exploit it as an edible agricultural product (Carrascosa et al., 2023). Tropical and subtropical regions are the native habitats of succulent purslane (P. oleracea). Since prehistoric times, it has been widely used in traditional medicine and as a nutritious herb for potting and salads (Mitich, 1997). The nutritional value of the purslane plant and its therapeutic properties make purslane a significant resource (Zhou et al., 2015). Purslane high concentrations of total phenolic compounds (TPC), ascorbic acid (ASC), vitamins and dietary minerals have stimulated farmers' interest in growing it as a vegetable crop (Alam et al., 2014; He et al., 2021; Murray, 2014; Simopoulos et al., 1992). It is a C4 halophyte, like purslane. In response to abiotic stresses such as drought or salt stress, this plant can transition from the C4 to the CAM pathway, allowing for more efficient water usage (He et al., 2021). The crop has gained significance as a model crop for investigating the evolution of facultative C4/CAM photosynthesis and its potential impact on food security in the future (D’Andrea et al., 2014; He et al., 2021; Petropoulos et al., 2016). There are an estimated 3,800 vascular plant species that compose the Armenian flora, divided into 160 families and 913 genera, and 146 of these are endemic to Armenia (Nanagulyan et al., 2020). The ability of many horticultural and agricultural crops to withstand abiotic stresses like salt, drought, and heavy metals has not been adequately investigated. Given the facts mentioned above and the necessity to uncover more concerning the morphological, physiological, and biochemical responses of the local Armenian genotype of P. oleracea (Dandur) to external salinity stressors factors, the current study endeavors to examine the responses of an undocumented P. oleracea (Dandur) to multiple soil mediums (clay and clay loam soil) and salt concentrations (0-2 dS m-1 (non-saline) to 2-4 dS m-1 (slightly saline) to 4-8 dS m-1 (moderately saline) to 8-16 dS m-1 (strongly saline) to >16 dS m-1 (extremely saline) during the seedling, reproductive, and harvesting stages in a laboratory setting. This was undertaken in preparation for potential applications in biosaline agriculture.
2 Materials and methods
2.1 Experimental sites
In 2022, the Department of Ecology and Nature Conservation in the Faculty of BiologyYerevan State University (40° 18′ 29.74′' N, 44° 52′ 66.55′' E) conducted a controlled trial of our pot experiment.
2.2 Soil and plant material collection
In 2022, soil from the middle Ararat plain in Armenia was collected for experiments, focusing on clay (40° 06.076′, E 44° 22.78′) and clay loam (40° 06.422′, E 44° 19.975) soils due to their abundance and non-saline nature, with ECe values below 2.0 dS/m (Bernstein et al., 1954).
2.3 Growth conditions
The study involved experiments on P. Oleracea (Dandur) plant seeds in a climate-controlled area near the Department of Ecology and Nature Conservation in the Faculty of Biology. The seeds were surface-sterilized in 10 % sodium hypochlorite and rinsed in distilled water. A 20 kg soil sample was divided among sterile PVC pots, and one seed was sown in each. The seedlings were given salt treatments when rapid growth was observed. Soil salinity was modified by adding sodium chloride to varying concentrations [(from 0 to 2 dS m−1 (non-saline) to 2–4 dS m−1 (slightly saline) to 4–8 dS m−1 (moderately saline) to 8–16 dS m−1 (strongly saline) to > 16 dS m−1 (extremely saline)]) in distilled water (Bernstein et al., 1954). Salt concentrations were gradually increased over 15 days to prevent an osmotic shock. Nonperforated plastic pots were used to prevent salt leaching and maintain the soil's consistent electrical conductivity. The trial lasted around ten weeks in a vegetative state.
2.4 Morphological, physiological, and biochemical parameters
2.4.1 Morphological indices
The undocumented genotype of P. oleracea (Dandur) was evaluated based on two key morphological traits: shoot length and stem diameter under control and salt groups.
2.4.2 Physiological indices
-
Shoot length
During the study, shoot length was measured after 60 days in non-saline and saline soils to find the effect of salt stress on plant height.
-
Biomass
Salt-stressed plants and control plants both had their biomass measured. At harvest time, the roots and the shoots were collected from each plant and replicated individually. Fresh weight measurements were taken of the shoots and roots. After drying the samples at 70 °C in an oven until they acquired a consistent weight, each plant's RDW and SDW were calculated.
-
Water content of roots and shoots
Whole plants were split into their roots and shoots, cleaned, and then heat-dried at 70 °C until the dry weights of the roots and shoots stabilized. The following formula (4) was applied to compute the root and shoot WC:
-
Chlorophyll content index (CCl)
The chlorophyll content index (CCI) was used to assess the adversity plants face in salinized soil, assessing the CCI of 10 leaves from the top using a CCM-200 plus chlorophyll content meter.
-
Gas exchange parameters
A portable photosynthesis system (CI-340) was used to measure gas exchange parameters like transpiration rate (E), photosynthetic rate (Pn), and water usage efficiency (WUE) (Rabhi et al., 2012):
-
Leaf succulence index (LS)
The LS was determined by using the sum of the areas of 10 leaves from each plant and their fresh weight and provided the following method for calculating leaf succulence (Agarie et al., 2007; Jennings, 1976):
2.4.3 Biochemical attributes
-
Relative electrolytic leakage (REL)
Relative electrolytic leakage of plant cells was observed (Bajji et al., 2002). Fresh leaf tissue (0.5 g) was incubated at room temperature in 50 ml of deionized water. Samples were autoclaved at 121 °C for 15 min after recording initial electrical conductivity (EC1). Final conductivity (EC2) was assessed. We calculated REL as:
-
Uptake nutrient content, K+/Na+ ratio, and TDS
The study involved grinding dried plant tissues into a powder, digesting them in an HNO3 solution for 30 min and then filtrating the sample. The concentration of Cl- was measured using an ionometer (I-160 M ionometer), while Na+, K+, and Ca2+ were quantified using a flame photometer (FP-I6431) and TDS with an ionometer.
2.4.4 Salt tolerance index (STI)
The salt tolerance index (STI) was calculated by comparing plant dry weight under different salt concentrations to the control. The salt shoot toxicity index, shoot length stress index, and shoot weight stress index were also calculated (Tao et al., 2021):
2.4.5 Statistical analysis
Both Microsoft Excel 2021 and SPSS-19 served as tools to analyze the data. The statistically significant differences were determined using Fisher's least significant difference (LSD) test. Error bars represent 95 % confidence intervals within the figures.
3 Results
3.1 Morphological traits
The study reveals that plants grown in extreme salinity levels experience a decrease in shoot length, with clay soil typically having 14.6 % less height than clay loam soil (Fig. 1A–B). The shoot length of plants in clay soil and clay loam soil decreases by 36.0 % and 26.2 %, respectively, compared to non-saline soil. The NaCl treatment also impacts the stem diameter of P. oleracea, with plants grown in clay and clay loam soils experiencing decreased stem diameters at higher salinity levels (66.7 % and 70.8 %, respectively) (Fig. 1 C-D).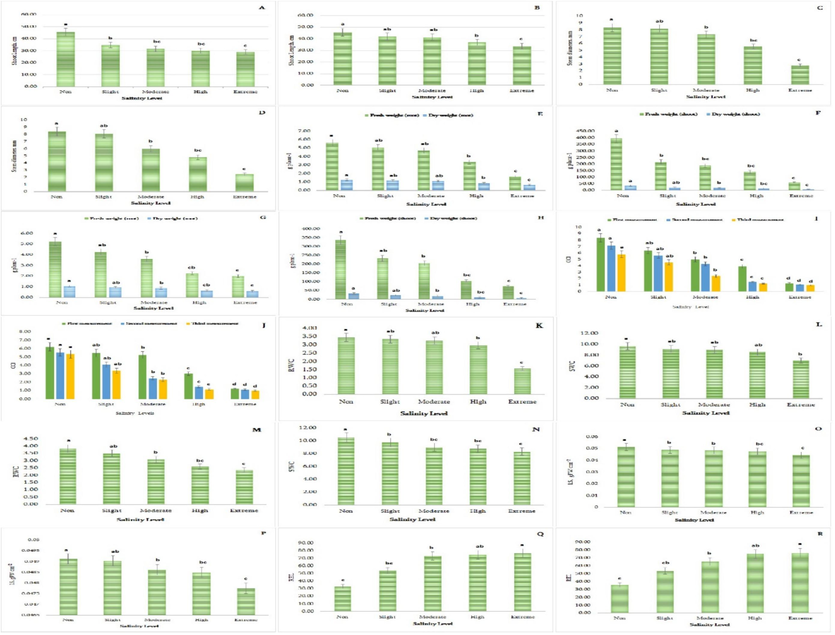
Effect of salinity on some morphological and physiological parameters of P. oleracea during salt treatment (A-shoot length of plants in clay soil, n = 5, P < 0.05, B-shoot length of plants in clay loam soil, n = 5, P < 0.05, C-stem diameter of plants in clay soil, n = 5, P < 0.05, D-stem diameter of plants in clay loam soil, n = 5, P < 0.05, E-fresh and dry weight of root of plants in clay soil, n = 5, P < 0.05, F-fresh and dry weight of shoot of plants in clay soil, n = 5, P < 0.05, G-fresh and dry weight of root of plants in clay loam soil, n = 5, P < 0.05, H-fresh and dry weight of shoot of plants in clay loam soil, n = 5, P < 0.05, I-CCI of plants in clay soil, n = 50, P < 0.05, J-CCI of plants in clay loam soil, n = 50, P < 0.05, K-RWC of plants in clay soil, n = 50, P < 0.05, L-SWC of plants in clay soil, n = 50, P < 0.05, M-RWC of plants in clay loam soil, n = 50, P < 0.05, N-SWC of plants in clay loam soil, n = 50, P < 0.05, O-LS of plants in clay soil, n = 10, P < 0.05, P-LS of plants in clay loam soil, n = 10, P < 0.05, Q-REL of plants in clay soil, n = 10, P < 0.05, R-REL of plants in clay loam soil, n = 10, P < 0.05.
3.2 Physiological traits
3.2.1 Effect of salinity stress on P. oleracea biomass
The study found that different NaCl concentrations had varying effects on plant biomass, depending on the soil texture (Tables S1 and S2). Plants grown in clay and clay loam soil experienced a reduction in root and shoot fresh and dry weight at higher salinity levels. In extremely saline clay soil, the fresh weight of roots and shoots declined by 70.4 % and 84.8 % respectively (Fig. 1E–F). The dry weight was reduced by 48.5 % and 79.7 % in clay soil (Fig. 1E–F). In clay loam soil, the fresh weight declined by 61.7 % and 77.5 %, and the dry weight by 45.2 % and 80.9 % (Fig. 1G–H). In soil without salt stress, the highest values of fresh and dry weight were observed, and statistical evaluation confirmed a significant drop in plant biomass compared to the control.
3.2.2 Evaluation of chlorophyll content and gas exchange index
As soil salinity increased, P. oleracea photosynthetic pigment decreased (Fig. 1I–J). Three vegetative phase observations showed a significant reduction in CCI. With increasing salinity, the decline was greater. A decline of 46.3 % and 37.2 % was observed in clay and clay loam soil as salinity increased from slight to moderate. Plants grown in clay soils showed higher CCI values than plants in clay loam soil.
Results showed that P. oleracea Pn was reduced in both soil types (Tables S3 and S4). It may be because plants developed the ability to adjust to stimuli throughout time. E values followed a similar pattern. Generally, clay soil plants showed greater decreases in Pn and E values (71.5 % and 61.3 %) than non-saline soil plants. In clay loam soils, these indices decreased by 78.2 % and 79.6 %. However, clay and clay loam soil plants showed different WUE dynamics within the same period. Plants grown in clay loam soils use water more efficiently, leading to a 6.7 % rise in WUE value due to higher salinity tolerance and enhanced defense mechanisms. However, plants in clay soils showed a 26.5 % lower WUE value.
3.2.3 Evaluation of osmotic tolerance and water status of P. oleracea under salinity stress
To evaluate P. oleracea osmotic tolerance and water status, root (RWC) and shoot water content (SWC) (Tables S1 and S2) (Fig. 1K–L, M–N), and leaf succulence index (LS) were evaluated (Fig. 1O–P). Dehydration of roots and shoots in clay and clay loam soil sometimes results in decreased WC values. The breakpoint was shown when RWC drastically decreased following a high salinity level. Clay and clay loam soil plants' SWC values dropped significantly after a moderate salinity level. Under extreme salinity level, roots and shoots water content of plants grown in clay soil collapsed by 54.8 % and 27.6 %, respectively, and in clay loam soil, 38.1 % and 21.0 %. At non-salinity level, plants in two soil types showed the highest leaf succulence index values. Plants in clay soil differed 13.3 % from non-saline soil plants and 2.7 % from plants in clay loam soil. Fig. 1O–P shows that in clay soil leaf succulence index decreased proportionally with salinity level increased. In clay loam soils after reaching a slight salinity level, the leaf succulence index decreased greatly.
3.3 Biochemical traits
3.3.1 REL, ions content and TDS analysis
The magnitude of REL increased notably with an increased salinity level in both types of soil. On the other hand, the increase in clay loam soils was more proportional; however, in clay soil, a significant shift in the REL value was noted once the soil reached a moderate salinity level (Fig. 1 Q-R). Compared to plants grown in non-saline soils, the value of REL increased by 2.1 times in plants grown in higher salinity levels. Tables 1 and 2 demonstrate that, as opposed to control plants, plants cultivated in extreme salinity levels of both soil types had an overall reduction in the total K+ concentration both in roots and shoots.
Salinity degree
K+
Na+
Ca2+
Cl−
TDS
K/Na
root
shoot
root
shoot
root
shoot
root
shoot
root
shoot
root
shoot
Non
30.83 ± 3.62
77.24 ± 3.87
7.38 ± 1.23
2.35 ± 1.21
1.05 ± 0.10
6.17 ± 1.23
0.38 ± 0.01
5.12 ± 2.12
65.42 ± 3.11
133.7 ± 3.65
4.17 ± 2.14
32.91 ± 3.17
Slight
21.62 ± 3.11
76.75 ± 3.64
15.63 ± 3.11
8.23 ± 2.36
0.65 ± 0.03
6.53 ± 1.31
1.45 ± 0.12
16 ± 2.41
73.04 ± 3.38
166.03 ± 3.74
1.38 ± 0.13
9.32 ± 2.10
Moderate
19.92 ± 2.85
75.72 ± 3.54
20.9 ± 3.55
12.75 ± 2.45
0.65 ± 0.04
8.21 ± 1.52
2.49 ± 1.26
22.89 ± 2.65
69.22 ± 3.05
168.53 ± 3.71
0.95 ± 0.05
5.94 ± 1.98
High
17.22 ± 2.89
74.64 ± 3.36
23.08 ± 3.58
16.34 ± 2.56
0.65 ± 0.03
8.77 ± 1.61
2.86 ± 1.41
28.37 ± 2.78
63.29 ± 3.10
176.6 ± 3.92
0.74 ± 0.03
4.56 ± 1.84
Extreme
9.9 ± 1.14
66.85 ± 3.21
39.55 ± 3.87
31.52 ± 2.93
1.71 ± 0.15
8.86 ± 1.65
5.79 ± 1.36
59.56 ± 3.13
23.61 ± 2.76
221.83 ± 3.98
0.25 ± 0.01
2.12 ± 1.45
Salinity degree
K+
Na+
Ca2+
Cl−
TDS
K+/Na+
root
shoot
root
shoot
root
shoot
root
shoot
root
shoot
root
shoot
Non
28.08 ± 2.46
70.25 ± 3.68
8.53 ± 1.36
2.32 ± 0.18
0.92 ± 0.04
6.98 ± 2.13
0.24 ± 0.01
4.43 ± 1.47
58.41 ± 3.32
124.23 ± 3.78
3.29 ± 1.14
30.28 ± 2.17
Slight
18.7 ± 2.23
68.62 ± 3.52
23.23 ± 2.12
9.56 ± 1.26
1.18 ± 0.08
7.12 ± 2.36
0.65 ± 0.03
15.83 ± 2.86
65.79 ± 3.41
158.13 ± 3.80
0.80 ± 0.04
7.18 ± 1.94
Moderate
15.32 ± 2.15
65.54 ± 3.48
34.1 ± 2.23
25.65 ± 1.85
1.6 ± 0.09
7.66 ± 2.41
1.8 ± 0.08
28.59 ± 2.96
57.39 ± 3.12
167.35 ± 3.94
0.45 ± 0.02
2.55 ± 0.42
High
10.32 ± 1.55
64.16 ± 3.40
36.27 ± 2.29
25.86 ± 1.79
1.87 ± 1.11
7.88 ± 2.59
2.19 ± 1.14
45.33 ± 3.21
37.93 ± 2.25
193.67 ± 4.11
0.28 ± 0.01
2.48 ± 0.41
Extreme
7.31 ± 1.37
61.95 ± 3.27
46.06 ± 2.47
42.36 ± 2.37
3.21 ± 1.32
8.47 ± 2.22
3.88 ± 1.21
82 ± 3.76
45.79 ± 2.87
276.53 ± 4.35
0.16 ± 0.009
1.46 ± 0.07
In clay soil, salinity raised Na+ concentration by 13.4 times in shoots and 5.35 times in roots, whereas decreased K+ and K+/Na+ ratio in shoots (1.15 and 15.5 times, respectively) and roots (3.11 and 16.6 times, respectively) in response to salt stress. Clay loam soil followed a similar pattern. Salt raised Na+ content by 18.2 times in shoots and 5.39 times in roots, while decreasing K+ and K+/Na+ ratio in shoots (1.13 and 20.70 times, respectively) and roots (3.83 and 20.72 times, respectively). This data shows that P. oleracea shoots tolerated high salinity levels and kept reduced Na+ levels. Along with enhanced salinity levels, root and shoot Cl− concentrations increased, while Ca2+ content was less dramatic. Plants grown in extremely salinized soil demonstrate a greater change in Na+ and Cl− concentrations in roots and shoots compared to those grown in non-saline soils. The study found that P. oleracea shoots in both soil types showed a significant increase in TDS levels as soil salt levels increased. Compared to plants cultivated in non-saline soils, plants growing in clay soils with extreme salinity levels raised shoot TDS by 1.65 times and in clay loam soils by 2.22 times (Tables 1 and 2). The roots of P. oleracea in both types of soil showed the opposite pattern. When compared to non-saline soils, plants in clay soil with higher salinity level lowered root TDS by 2.77 times and clay loam soils by 1.27 times.
3.3.2 Phytodesalination potential of P. oleracea
After 30 days of salt exposure, plants roots and shoots accumulated more Na+ and Cl− than control plants (Tables 1 and 2). A plant grown in clay soil could eliminate 241.3 mg Na+ and 433.2 mg Cl− from moderate salinity levels, 242.0 mg Na+ and 420.2 mg Cl− from high salinity levels, and 236.1 mg Na+ and 446.2 mg Cl− from extreme salinity levels. A plant grown in clay loam soil could remove 490.9 mg Na+ and 547.1 mg Cl− from moderate salinity levels, 291.4 mg Na+ and 510.7 mg Cl− from high salinity levels, and 277.7 mg Na+ and 537.6 mg Cl− from extreme salinity levels.
4 Evaluation of the salt tolerance index (STI)
4.1 Salt tolerance index
Plants cultivated in two distinct kinds of soil displayed the greatest values of the salt tolerance index at the non-saline level (Fig. 2A–B). The difference was 63.9 % in clay soil and 70.4 % in clay loam soil when comparing plants grown in slightly and extremely salinized soil. As Fig. 2A–B illustrates, the salt tolerance index value considerably plummeted as salinity levels increased.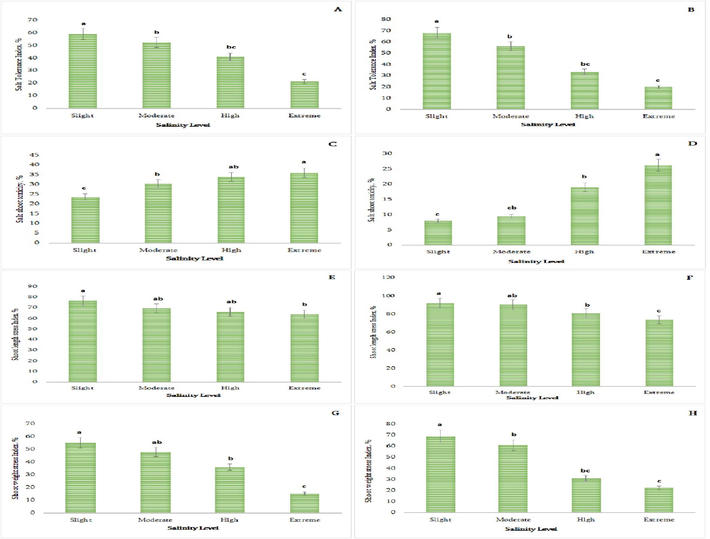
Effect of salinity on some stress indices of P. oleracea (A-salt tolerance index of P. oleracea in clay soil, n = 10, P < 0.05, B-Salt tolerance index of P. oleracea in clay loam soil, n = 10, P < 0.05, C-Salt shoot toxicity of P. oleracea in clay soil, n = 10, P < 0.05, D-Salt shoot toxicity of P. oleracea in clay loam soil, n = 10, P < 0.05, E-Shoot Length Stress Index of P. oleracea in clay soil, n = 10, P < 0.05, F-Shoot Length Stress Index of P. oleracea in clay loam soil, n = 10, P < 0.05, G-Shoot Weight Stress Index of P. oleracea in clay soil, n = 10, P < 0.05, H-Shoot Weight Stress Index of P. oleracea in clay loam soil, n = 10, P < 0.05.
4.2 Salt shoot toxicity %
Salt toxicity on P. oleracea shoots increases with salinity levels, with extreme clay soils enhancing it by 6.5 times and clay loam soils by 3.2 times (Fig. 2C–D).
4.3 Shoot length stress index %
The shoot length stress index, a crucial metric for assessing P. oleracea tolerance, decreases with increased saline levels in both soil types. The decline is more proportionate in clay soil, with a sharp decline in clay loam soil after moderate salinity (Fig. 2 E-F). This difference is 19.8 % in clay loam soil and 16.3 % in clay soil.
4.4 Shoot weight stress index %
The shoot weight stress index declined in both slightly and extremely saline soils as salinity levels increased, with a 72.3 % difference in clay soil and a 67.5 % difference in clay loam soil (Fig. 2 G-H).
5 Correlation matrix
P. oleracea demonstrates increased tolerance against salinity stress, with inverse correlations between salinity in clay loam soils and toxic ions accumulation (Table S 5–6). This is reflected in correlations between various parameters such as biomass, photosynthetic frequency, transpiration rates, stem diameter, shoot length, chlorophyll content, and K+ content.
6 Discussion
6.1 Status of growth, water content and productivity of shoot and root
For water from drainage reuse systems, purslane (P. olereacea) has been widely proposed as a desirable halophytic crop with an excellent nutritional profile (Grieve and Suarez, 1997). It was reported that P. olereacea could develop with greater biomass in low-salinity environments and possibly complete its life cycle in high-salinity environments. Furthermore, the plant's shoots revealed no manifestations of harm or nutrient deficiencies arising from the excess salt (Grieve and Suarez, 1997). This study examined the morphological, physiological, and biochemical changes of the undocumented Dandur (P. oleracea L.) plant genotype under two soil conditions (clay and clay loam) along with different concentrations of salt (0-2 dS m-1 (non-saline) to 2-4 dS m-1 (slightly saline) to 4-8 dS m-1 (moderately saline) to 8-16 dS m-1 (strongly saline) to >16 dS m-1 (extremely saline) (Fig. 3). The results demonstrated that shoot length and steam diameter were not much affected at 100 mM salt stress compared to control (Fig. 1 A-B, C-D). Still, these morphological features declined with increasing salinity levels from moderate to extreme in Dandur purslane plants (Fig. 1A–B). Some previous studies also suggested that NaCl above 350 mM affected the growth and development of purslane plants (Grieve and Suarez, 1997). Some reports also support our finding that higher NaCl concentrations at 200 mM negatively impacted some species of purslane plants, decreasing their stem length and diameter (Borsai et al., 2020a; Zaman et al., 2020). Plant biomass, both fresh and dry, was also negatively affected by salinity stress (Singh et al., 2022). Succulent purslane has a water content of 90 % or more with its leaves, steams, and roots. During our experiment, we also analyzed the water content in the root and shoot of Dandur purslane plants (Tables S1 and S2). Therefore, in comparison to its dry weight, the fresh weight of purslane was higher. A sharp decline in purslane's fresh weight was seen in response to the high salinity stress, and this decline was predictable as the salinity enhancement level increased. At 350 mM salinity stress, sugar beetroot cultivars dry matter content decreased significantly (Dadkhah and Grrifiths, 2006). In contrast, hybrid maize varieties' shoot and root dry matter contents decreased significantly at 250 mM salinity (Eker et al., 2006), while in Pennisetum alopecuroide plant found that dry matter content increased at 100 mM salinity level (Mane et al., 2011). The spike in dry matter production in salinity is presumably attributed to the accumulation of inorganic ions and organic solutes for osmotic acclimatization, while the decrease in dry matter content at the most concentrated soil levels could be driven by the inhibition of reserved foods' hydrolysis and vascular translocation to developing shoots (Xu et al., 2008). On the other hand, after applying salt, several of the accessions further increased the fresh weight of purslane. Dandur grown in slightly to moderate NaCl treatments had the highest shoot and root biomass compared to strong and extreme NaCl treatments levels (Fig. 1E–F, G-H and Tables S1–S2). Flowers et al. (Flowers et al., 1986) observed that most halophytes survive in saline environments. We found that undocumented Dandur purslane plant genotypes cultivated with slight NaCl treaments yielded more root and shoot biomass. In addition, we found that Dandur purslane plants can finish their lifespan between slight to moderate NaCl treatments; hence, Dandur may be classified as a halophyte crop (Yuan et al., 2019).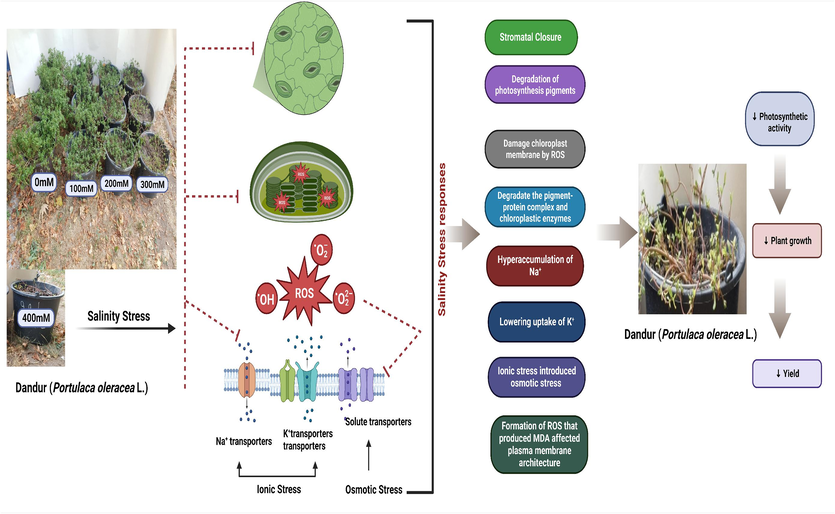
The study found that Dandur plants adapt well under 100–200 mM salinity stress concentrations, but experience reduced growth and development when salinity concentrations increase from 300-400 mM.
6.2 Photosynthesis pigments and efficiency
Purslane, a C4 plant with palisade layers on both leaves, is affected by water usage efficiency and CO2 adaptation strategies during photosynthesis in various abiotic stress conditions (Bromham and Biology, 2014; Jin et al., 2016). The purslane plant's cell membrane serves as an essential element for preserving cell turgor pressure and other physiological features against various salt stress scenarios. Plants respond to over-abundance of water or prolonged flooding by eliciting a wide range of biochemical and physiological responses. Salinity stress may impact photosynthetic efficiency, with studies showing that leaves accumulate salt, while total chlorophyll levels decline. Researcher was found that chlorophyll content of Arabidopsis and Thellungiella decreased in response to salt, while chlorophyll content of Thellungiella showed no discernible shift under the same salinity conditions (Stepien and Johnson, 2009). Undocumented Dandur plants exhibited less affected chlorophyll content under salinity stress at concentrations of slight to moderate NaCl saline level, while a substantial decrease was seen at concentrations of strong to extreme NaCl treatments. Research by Rabhi et al. (2012) found that two obligatory halophytes, Tecticornia indica and Sesuvium portulacastrum, have much higher total chlorophyll levels when cultivated in environments with slight to extreme NaCl saline level, respectively (Rabhi et al., 2012). In increasing salinity circumstances, salt-tolerant species display an increase or maintenance of chlorophyll content, while salt-sensitive species exhibit a reduction in chlorophyll levels.
Salinity stress reduces gas exchange properties, impacting plants ability to photosynthesize (Hnilickova et al., 2021). In this study found that Dandur Pn, E, and WUE levels were significantly decreased under conditions of water deficit or osmotic imbalance caused by salinity stress (Fig. 1 I-J, Tables S3 and S4). Stomatal closure, which is the openings for CO2 and water exchange, is induced by root signals in dry soil, reducing transpirational water loss (Linglan et al., 2008). As transpiration decreases with decreasing the water content in the stem and roots. This decrease in water leads to restriction of stomatal function that directly hindering photosynthesis. A decrease in water results in a decrease in Pn, suppressing overall photosynthesis (Tables S3–S4). This is illustrated in Tables S5 and S6. Stomata shut in response to a fall in water potential and water content in osmotic-stressed leaves, reducing CO2 efficacy, Pn, and E (Tardieu and Simonneau, 1998; Zhao et al., 2020). The water use efficiency (WUE) of a crop is a measure of its water-saving potential and water productivity, representing the final performance of water consumption and crop production. Water stress enhances WUE across a range of plant species. Stomatal closure reduces transpiration, photosynthesis, and leaf conductivity when plants are under water stress. Water-deficient plants are more likely to use water conservatively, leading to higher WUE (Liu et al., 2016). WUE is a crucial physiological adaptation mechanism that can increase agricultural yields when water is limited (Mashilo et al., 2017). This study found that WUE increased when subjects were subjected to mild stress (slight to moderate NaCl solution) (Tables S3–S4).
6.3 Plant ion toxicity and ion imbalance
Salinity stress impacts plant nutrient concentrations, potentially leading to cytoplasmic toxicity and plant death. Plants regulate homeostasis through ion transport, absorption, and distribution. Halophytes store harmful ions in vacuoles, while glycophytes decrease root-level Na+ and Cl−uptake. Osmotic adjustment is the primary mechanism for salinity changes in halophytes. Both Na+ and Cl− levels increase in response to NaCl concentration suggesting identical Na+ absorption and transport from root to shoot in Dandur cultivars (Tables 1 and 2).
The Dandur Na+ ion concentration was consistently greater in the stem than the root across all salinity levels, indicating that this cultivar cannot prevent Na+ ions from moving from the roots to steam. Cl- and Na + ions had similar distributions despite the greater salinity. Plants quickly absorb sodium and chloride and transfer them to their leaves as osmotica. We found similar results in cotton (Gossypium hirsutum L.) plants (Zhang et al., 2014). Dandur plants can only tolerate slight to moderate salinity levels and Na+ and Cl− ions beforehand that photosynthesis and plant growth are impaired, as shown in Tables S6 and S7. In salty environments, Cl/NO3 antagonism may reduce shoot nitrogen intake due to increased absorption and Cl-accumulation (Muchate et al., 2016). Phosphorus intake, essential for energy transmission, storage, and photosynthesis, is affected by salt stress. Soil with high Na+, Cl− and SO42 concentrations may have decreased phosphorus absorption due to high ionic strength and limited solubility of Ca and P compounds (Borsai et al., 2020). Protein production and water balance need potassium, an inorganic solute. Absorbing too much Na+ and Cl− can cause cation imbalance and K+ and Ca2+ deficiency (Tables 1 and 2). At the same time, Dandur K+ and Ca2+ levels decreased significantly. However, shoot loss was greater than root loss as NaCl concentration increased (Tables 1 and 2). Due to molecular similarities, large exogenous Na+ concentrations restrict K+ absorption (Kaya et al., 2007). Plant development in saline soil requires a precise Na+/K + ratio in plant cells. In current study analysis show that increasing salinity stress also increased Na+ ions which lowered the K+/Na+ ratio (Tables 1 and 2). Borsai et al. (2020a, 2020b) found that a greater K+/Na+ ratio influenced K+ and other ionic concentrations in Portulaca and Purslane cultivars under salinity stress. Na+ cannot replace K+ in cellular functions due to their chemical similarities. As Na+ increases, K+ and Ca2+ decrease because they compete for root absorption sites. Na + inhibits plant K + absorption by a competitive mechanism regardless of solution concentration of Na+, Cl−, or SO42 salts (Borsani et al., 2003). Salinity decreases wheat, Manihot esculenta, Zea mays, G. hirsutum, and other plant yields and creates nutritional imbalances (Cruz et al., 2018; Hasana and Miyake, 2017). Ramos et al. (2004) observed that membrane integrity worsened with increased salinity, and our work demonstrates that this causes Na+ buildup and biomass loss (Ramos et al., 2004). To activate enzymes and synthesize proteins, tissue K+ and Ca2+ concentrations must be constant because Na+ cannot fill the gap of K+ and Ca2+.
6.4 Salt stress tolerance activity
When exposed to salt, Dandur shows a wide range of morpho-physiological and biochemical responses, and as halophytes, salt-tolerant genotypes often perform well at an initial concentration of salinity. All the morpho-physiological and biochemical variables were shown to be STI-related in this study. The correlation matrix displays the morpho-physiological and biochemical features that correlate significantly with the STI (Fig. 2A–H). For plants to produce new tissues, which involves adjusting osmosis, it is necessary for them to keep the shoot turgor positive. We found that tolerance for salt in saline conditions was negatively connected with increasing concentrations of salinity stress (strong to extreme NaCl saline levels), suggesting that osmotic adjustment is a key component of salt tolerance. There is less STI in the early stages of salinity in the salt-sensitive genotypes when compared to earlier results. However, genotypes that are resilient to salt stress exhibit an increase in shoot length stress index and STI as well as a decrease in salinity shoot toxicity during the early stages of salinity stress (Fig. 2 A-H).
7 Conclusions and future perspectives
The adaptability and competitive nature of halophytes present a significant challenge to agricultural productivity. However, some halophytes can be used as edible agricultural products due to their valuable nutrients and easy-to-grow process. Halophytes, naturally salt-tolerant plants, can grow in coastal and arid-saline environments. Climate change exacerbates abiotic stressors experienced by halophyte plants, making understanding their adaptation to stressful environments essential. This study investigated the response of an undocumented halophyte, Dandur, in different soil mediums to salt stress. Biochemical, physiological, and morphological analyses confirmed the plant's capacity to withstand stress. Plants in clay soils showed superior adaptive responses compared to those in clay loam soils. However, at high salinity levels, signs of stress became evident across all growth parameters. Dandur unique ability to avoid dehydration and maintain growth traits, particularly in optimal conditions, holds promise for further exploration in agricultural research and food security-related issues caused by climate change in saline areas of Armenia and other European countries.
CRediT authorship contribution statement
Gohar Margaryan: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Abhishek Singh: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Hrant Khachatryan: Writing – review & editing, Writing – original draft. Vishnu D Rajput: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Tatiana Minkina: Writing – review & editing, Writing – original draft. Dimitrios Petropoulos: Writing – review & editing, Writing – original draft. Athanasios Kriemadis: Writing – review & editing, Writing – original draft. Athanasios Alexiou: Writing – review & editing, Writing – original draft. Mohamed S. Elshikh: Writing – review & editing, Writing – original draft, Methodology, Funding acquisition, Conceptualization. Abd El-Zaher M.A. Mustafa: . Karen Ghazaryan: Writing – review & editing, Writing – original draft, Methodology, Supervision, Conceptualization.
Acknowledgements
KG is supported under grant numbers 21AG-4C075. AS is supported by the 23PostDoc-4D007 grant provided by the Science Committee of the Republic of Armenia. VDR and TM are supported by the Strategic Academic Leadership Program of Southern Federal University known as “Priority 2030” and the Ministry of Science and Higher Education of the Russian Federation (grant number: FENW-2023-0008). The authors extend their appreciation to the Researchers supporting project number (RSPD2024R941), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Salt tolerance, salt accumulation, and ionic homeostasis in an epidermal bladder-cell-less mutant of the common ice plant Mesembryanthemum crystallinum. J. Exp. Bot.. 2007;58:1957-1967.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant compounds, antioxidant activities, and mineral composition of 13 collected purslane (Portulaca oleracea L.) accessions. Biomed. Res. Int.. 2014;2014
- [CrossRef] [Google Scholar]
- Osmotic and ionic effects of NaCl on germination, early seedling growth, and ion content of Atriplex halimus (Chenopodiaceae) Botany. 2002;80:297-304.
- [CrossRef] [Google Scholar]
- Allison L Bernstein, C.A. Bower, J.W. Brown, M. Fireman, J.T. Hatcher, H.E. Hayward, G.A. Pearson, R.C. Reeve, L.E., Richards Wilcox, L.A. Richards, A.L., 1954. Diagnosis and Improvement of United States Salinity Laboratory Staff.
- Borsai, O., Al Hassan, M., Negrușier, C., Raigón, M.D., Boscaiu, M., Sestraș, R.E., Vicente, O., 2020a. Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms. Plants 2020, Vol. 9, Page 1660 9, 1660. doi: 10.3390/PLANTS9121660.
- Borsai, O., Al Hassan, M., Negrușier, C., Raigón, M.D., Boscaiu, M., Sestraș, R.E., Vicente, O., 2020b. Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms. Plants 2020, Vol. 9, Page 1660 9, 1660. doi: 10.3390/PLANTS9121660.
- Developing salt tolerant plants in a new century: A molecular biology approach. Plant Cell Tiss. Org. Cult.. 2003;73:101-115.
- [CrossRef] [Google Scholar]
- Bromham, L., Biology, T.B.-J. of E., 2014, undefined, 2014. Salt tolerance evolves more frequently in C4 grass lineages. Wiley Online LibraryL Bromham, TH BennettJournal of Evolutionary Biology, 2014•Wiley Online Library 27, 653–659. doi: 10.1111/jeb.12320.
- Salinity reduces nutrients absorption and efficiency of their utilization in cassava plants. Ciência Rural. 2018;48:e20180351.
- [Google Scholar]
- Deciphering the mechanisms involved in Portulaca oleracea (C4) response to drought: metabolic changes including crassulacean acid-like metabolism induction and reversal upon re-watering. Physiol. Plant.. 2014;152:414-430.
- [CrossRef] [Google Scholar]
- The effect of salinity on growth, inorganic ions and dry matter partitioning in sugar beet cultivars. J. Agric. Sci. Technol.. 2006;8:199-210.
- [Google Scholar]
- Climate resilient crops for improving global food security and safety. Plant Cell Environ.. 2018;41:877-884.
- [CrossRef] [Google Scholar]
- Effect of Salinity Stress on Dry Matter Production and Ion Accumulation in Hybrid Maize Varieties. Turk. J. Agric. For.. 2006;30:365-373.
- [Google Scholar]
- Halophytes.. 1986;61:313-337.
- [CrossRef]
- Purslane (Portulaca oleracea L.): A halophytic crop for drainage water reuse systems. Plant Soil. 1997;192:277-283.
- [CrossRef] [Google Scholar]
- Salinity Stress Alters Nutrient Uptake and Causes the Damage of Root and Leaf Anatomy in Maize. KnE Life Sci.. 2017;3:219.
- [CrossRef] [Google Scholar]
- High Salinity Reduces Plant Growth and Photosynthetic Performance but Enhances Certain Nutritional Quality of C4 Halophyte Portulaca oleracea L. Grown Hydroponically under LED Lighting. Front. Plant Sci.. 2021;12:651341
- [CrossRef] [Google Scholar]
- Hnilickova, H., Kraus, K., Vachova, P., Hnilicka, F., 2021. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, Vol. 10, Page 845 10, 845. doi: 10.3390/PLANTS10050845.
- The effects of sodium chloride on higher plants. Biol. Rev.. 1976;51:453-486.
- [CrossRef] [Google Scholar]
- Physiological and metabolic changes of purslane (Portulaca oleracea L.) in response to drought, heat, and combined stresses. Front. Plant Sci.. 2016;6
- [CrossRef] [Google Scholar]
- Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environ. Exp. Bot.. 2007;60:397-403.
- [CrossRef] [Google Scholar]
- Soil Salinity and Food Security in India. Front. Sustain. Food Syst.. 2020;4:533781
- [CrossRef] [Google Scholar]
- Soil Salinity and Food Security in India. Front Sustain Food Syst.. 2020;4:174.
- [CrossRef] [Google Scholar]
- Rubisco activase mRNA expression in spinach: Modulation by nanoanatase treatment. Biol. Trace Elem. Res.. 2008;122:168-178.
- [CrossRef] [Google Scholar]
- Effects of water stress on photosynthetic characteristics, dry matter translocation and WUE in two winter wheat genotypes. Agric. Water Manage.. 2016;167:75-85.
- [CrossRef] [Google Scholar]
- Studies on the effects of salinity on growth, polyphenol content and photosynthetic response in Vetiveria zizanioides (L.) Nash. 2011;23:59-70.
- [Google Scholar]
- Drought tolerance of selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol. Biochem.. 2017;120:75-87.
- [CrossRef] [Google Scholar]
- Muchate, N.S., Nikalje, G.C., Rajurkar, N.S., Suprasanna, P., Nikam, T.D., 2016. Plant Salt Stress: Adaptive Responses, Tolerance Mechanism and Bioengineering for Salt Tolerance. The Botanical Review 2016 82:4 82, 371–406. doi: 10.1007/S12229-016-9173-Y.
- ω-3 polyunsaturated fatty acids and their metabolites as inhibitors of mammalian tumorigenesis. Phytochem. Rev.. 2014;13:139-156.
- [CrossRef] [Google Scholar]
- Wild plants and fungi sold in the markets of Yerevan (Armenia) J. Ethnobiol. Ethnomed.. 2020;16:1-27.
- [CrossRef] [Google Scholar]
- Molecular phylogenetics, historical biogeography, and chromosome number evolution of Portulaca (Portulacaceae) Mol. Phylogenet. Evol.. 2012;63:97-112.
- [CrossRef] [Google Scholar]
- Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci. Technol.. 2016;55:1-10.
- [CrossRef] [Google Scholar]
- Photosynthetic responses to salinity in two obligate halophytes: Sesuvium portulacastrum and Tecticornia indica. S. Afr. J. Bot.. 2012;79:39-47.
- [CrossRef] [Google Scholar]
- Effect of NaCl and KCl salts on the growth and solute accumulation of the halophyte Atriplex nummularia. Plant and Soil. 2004;259:163-168.
- [CrossRef] [Google Scholar]
- Common purslane: a source of omega-3 fatty acids and antioxidants. J. Am. Coll. Nutr.. 1992;11:374-382.
- [CrossRef] [Google Scholar]
- Prominent Effects of Zinc Oxide Nanoparticles on Roots of Rice (Oryza sativa L.) Grown under Salinity Stress. Stresses. 2022;3:33-46.
- [CrossRef] [Google Scholar]
- Contrasting Responses of Photosynthesis to Salt Stress in the Glycophyte Arabidopsis and the Halophyte Thellungiella: Role of the Plastid Terminal Oxidase as an Alternative Electron Sink. Plant Physiol.. 2009;149:1154-1165.
- [CrossRef] [Google Scholar]
- Evaluating and Screening of Agro-Physiological Indices for Salinity Stress Tolerance in Wheat at the Seedling Stage. Front. Plant Sci.. 2021;12:646175
- [CrossRef] [Google Scholar]
- Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J. Exp. Bot.. 1998;49:419-432.
- [CrossRef] [Google Scholar]
- The effect of salt stress on the chlorophyll level of the main sand-binding plants in the shelterbelt along the Tarim Desert Highway. Chin. Sci. Bull.. 2008;53:109-111.
- [CrossRef] [Google Scholar]
- Reproductive physiology of halophytes: Current standing. Front. Plant Sci.. 2019;9:432034
- [CrossRef] [Google Scholar]
- Zaman, S., Bilal, M., Du, H., Che, S., 2020. Morphophysiological and Comparative Metabolic Profiling of Purslane Genotypes (Portulaca oleracea L.) under Salt Stress. Biomed. Res. Int. 2020. doi: 10.1155/2020/4827045.
- Morphological and Physiological Responses of Cotton (Gossypium hirsutum L.) Plants to Salinity. PLoS One. 2014;9:e112807.
- [Google Scholar]
- Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation. 2020;1:100017
- [CrossRef] [Google Scholar]
- Zhou, Y.X., Xin, H.L., Rahman, K., Wang, S.J., Peng, C., Zhang, H., 2015. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. Biomed. Res. Int. 2015. doi: 10.1155/2015/925631.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103332.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







