Two-step chromium photo-precipitation in the sequential UV/Sulfite/Manganese dioxide processes: Efficiency, kinetic, energy-economic evaluation, and sludge survey
⁎Corresponding author at: Social Determinants of Health Research Center, Mashhad University of Medical Sciences, Mashhad, Iran. Marya.sarkhosh@yahoo.com (Maryam Sarkhosh),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, removal of Chromium (Cr) in a novel process includes reduction, adsorption, oxidation and complexion in a UV/Sulfite/Manganese dioxide (USM) investigated. In our study, in the optimal condition was MnO2 = 1 mM, Na2SO3 = 0.4 mM, 6 min reaction time (synthetic sample) and at pH 7, and 10 mg L−1 Cr removed completely. In the first stage, the sulfite-sulfate radicals react with Cr, and then Cr-S are removed from the solution by forming a complex with MnOOH•. Advantage of this method against other process include less time, higher efficiency, less use of reactive materials, and no need for large pH changes without release of sulfite or sulfate. At pH 7 about 60% and 40% of reaction species were reduction and oxidative species respectively. Considering the better efficiency at pH 7, it shows that reducing species have a more important and primary role in Cr removal. Also, the amount of energy consumed decreases from 16.14 to 3.25 kWh per cubic meter, Kobs (min−1) 0.533to 0.1837 and robs (mg/L.min) increase from 26.68 to 45.2 with change of Cr concentration from 50 to 250 mg L−1 respectively. The total cost of the USM process is much lower than other methods. In the UV, U Manganese dioxide, UV/Sulfite, and USM methods, the total cost were estimated 9.80–18.75, 5.74–8.75, 5.72–1.51, and 2.74–0.55 $ when the Cr concentration increase 100 to 250 mg L−1, respectively.
Keywords
Chromium
Concern
Reduction
Photo-sedimentation
Real sample
Radicals
1 Introduction
The presence of heavy metals in water due to its high toxicity and non-degradability by microorganisms and their high depletion in water resources, has created many risks to the environment and human health (Ding et al., 2021). Chromium (Cr) is one of the main mineral water pollutants in aqueous media, that hexavalent Cr has high solubility and toxicity compared to trivalent Cr and can be very dangerous to humans due to mutagenicity, teratogenicity and carcinogenicity (Liu and Yu 2021). Increasing the use of chromium in the chemical industry, metal plating, tanning and leather dyeing processes, and discharging their wastewater into the aqueous medium has increased the concentration of this pollutant in water sources (Ozcelik et al., 2021). To this end, the introduction of new methods to remove Cr from wastewater is crucial to protecting the environment and human health. Various methods including chemical sedimentation (Harper and Kingham 1992), adsorption (Liu et al., 2020; Sadani et al., 2020), ion exchange (Lee et al., 2017), electrochemical treatment (Syam Babu and Nidheesh 2021), and membrane (Hao et al., 2018), advanced treatment processes (ATPs) (Babu et al., 2019) technologies for separation of chromium from contaminated water have been proposed. Advanced Treatment Processes (ATP) are able to achieve removal of specific organic and inorganic ingredients in solution, which are not normally achieved by other treatment options (Rasolevandia et al., 2020) Inorganic anions such as Iodide(Rasolevandia et al., 2020), Sulfite (Sarkhosh et al., 2019), and Carboxylates (Massoudinejad et al., 2020) are stimulated to produce electrons and reducing species when exposed to a UV activator. In the last decade, a new advanced reduction process has been developed and patented using soluble sulfur (IV) (sulfite) as the photo absorber to produce large amounts of electrons (eaq−) (Zaw and Emett 2002; Azarpira et al., 2019). In this study Sulfite chosen due to two very important properties: UV absorption and electron production per photon are absorbed, which in sulfite are 253.7 nm (εi, 254 = 220 M−1 cm−1, respectively), and eaq•− (eg, 0.286 mol E−1). Manganese oxide has been widely studied as one of the most important oxidizing agents for oxidation due to its strong oxidation ability and strong stability. In this study UV254 rays was used to stimulate sulfite and Manganese dioxide ions to create both regenerative species or react directly with pollutants. Therefore, in this study, UV rays were used to stimulate sulfite and remove direct and indirect chromium in photo reactor.
2 Martial and methods
2.1 Reactor configuration
A UV lamp (nominal power: 11 W; light intensity: 78 µW/cm2) with wavelength emission in the range λmax = 254 nm was used as UV irradiation source with quartz sleeve fixed in center of the tubular glass (distance of 1 cm from the quartz). Arsenic solution was entered in the empty space between the quartz and tubular glass as reaction space. The experiments were performed in a 500 mL container. To prevent the effect of the lamp temperature on the reaction and disturbance in the results obtained, water was rotated around tubular glass.
2.2 Analytical methods
In As photo-sedimentation, the effects of initial Cr concentration (50 to 250 mg L−1), initial pH (3 to 11) and photo-reaction time (0 to 50 min) were investigated. Graphite atomic absorption spectroscopy was used to measure the residual chromium concentration. The following equation was used to determine the photo-sedimentation of Cr.
Cr0 and Crt are defined as the Cr concentration (mg L−1) at the beginning and time of the reaction, respectively. Also the effect of anions on the photo-sedimentation of Cr was investigated.
2.3 Kinetic model, energy and effective cost analyses
Because it is almost impossible to determine the amount of reducing species in solutions, according to Eqs. (7)–(8), the Pseudo-first-order (PFO) was used to study the Cr reduction model.
In this equation, k is the constant coefficient of reaction rate.
Important factors such as economy, wastewater quality, cost, etc. also play a key role in choosing a treatment technology. The major part of the cost of photo processes in the removal of water pollutants is related to electrical energy, electrical energy represents a major part of operating costs. Although there are several methods in determining the AORP electrical energy consumption in the literature and it depends on the type of pollutant, effluent quality, the reactor configuration and the type of light source used, etc., it is necessary to study the AORP electrical energy consumption under experimental conditions. The International Union of Pure and Applied Chemistry (IUPAC) has introduced the parameter of energy consume for each order (Figure-of-merit) that briefly called EEO to determine the amount of electrical energy (kW⋅h) in UV-based reactions. Electrical energy per order EEo allows to quickly determine the electrical energy consumption and they show the total energy required. For the purpose of comparison, the treatment efficiency for different processes is evaluated through EEo values. This means that energy used to almost 90% of photo-remove contaminants in one cubic meter of contaminated water(Rasolevandi et al., 2019).
In this equation, P is the amount of power (Kw), V is the volume (L), t is the photo-reaction time (minutes) of water in the photo-reactor. By combining and rearranging Eqs. (21) and (23), new EEO obtains based on kinetic equation (Lee et al., 2018).
2.4 Reduction and oxidation species activity tests
The photocatalytic tests were carried out in UV-only, UV/Sulfite, UV/Manganese dioxide and there of them, in percent of Oxidative and reductive scavengers at the optimal condition. The initial concentration of Cr (III) was 50, 100 and 250 µg l−1. To understand the role of HO•, h+ and O2•− and reductive species in the mechanism of the Cr (III) removal, experiments with the addition of oxidative and reductive species scavengers including isopropyl alcohol, sodium oxalate and benzoquinone and nitrate (10 mL scavengers in 100 mL of Cr (III) aqueous solution) were performed(Gekko et al., 2012; Alam et al., 2019). For all tests, the reactor was in the dark for 1 h before the UV lamp was turned on. During the test, several samples were collected at constant time and the concentrations of Cr (III) and Cr (V) were analyzed.
3 Result and discussion
3.1 Effect of factors in Cr photo-sedimentation
3.1.1 Effect of pH
The effect of pH on Cr removal in UV/Manganese dioxide (UV/M), UV/Sulfite (US) and UV/Sulfite/Manganese dioxide USM were investigated over a wide pH range (3–11). Cr shown in Fig. 1, without irradiation, the removal of Cr (III) at pH 3 was significant (about 20). It can be concluded that the adsorption rate of Cr by Manganese dioxide particles is 20%. The effect of the presence of sulfite is also different at various pH values shows that decreased with increasing pH. In the presence of sulfite, kobs is significantly higher than that in the absence of sulfite over the whole pH range investigated (Xu et al., 2016a,b). In many researches, the rate of Cr removal increased in low pH level, which is consistent with the findings of our study (He et al., 2020; Kong et al., 2020; Kong et al., 2021). At pH > 3.1, the surface of MnO2 negative charge while positive charge is corresponded to pH < 3.1. That’s means in pH < 3.1, surface of MnO2 prepare to complex with Cr-S (ALAH ABADI et al., 2021). Generation of hydrated electrons (eaq−) and hydrogen radicals (H•) in the stimulation of sulfite ions by UV which causes reduction of pH and release of Cr (V) in solution. Hexavalent chromium can react with SO3−• and SO4−• radicals in the aqueous medium (Zhang et al., 2018), and then S-Cr can be react by the MnOOH • and form a stable complex.
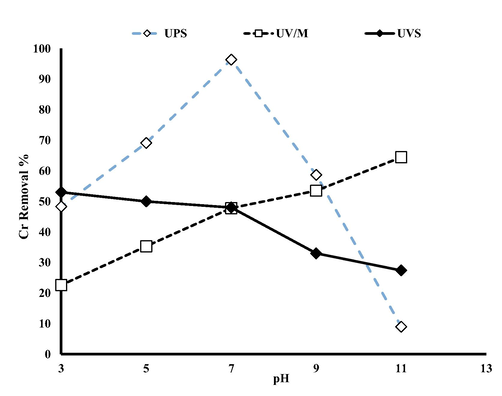
- Effect of pH on the removal of Cr (The experimental conditions: MnO2:1 Mm, Sulfite: 0.4 Mm, Cr = 10 mg L−1, 6 min reaction time).
Step1:
Step2: Where MnOOH• is a reactive hydroxyl group on the manganese oxide surface and the 2MnO2.CrOOH is a surface complex. Also, radical chain reactions can occur in medium reaction (Kuo et al., 2006, Xu et al., 2016a,b).
At lower pH, HSO3− as the dominant ion can produce •H as a weak reducing agent by either direct photolysis (Eq. (25)) or in the reaction of eaq− and H+ (Eq. (11)) (Yu et al., 2019).
Also, at higher pH, almost all sulfur species are present as SO3•2−, meaning that eaq- and SO3−• are the predominant reducing radicals (Eq. (8)) (Cao et al., 2021). It is important to note that the performance of strong species including eaq− and •H with the potential −2.9 V and −2.3 V. Therefore, low pH is not suitable for this process. On the other hand, at high pH, production of oxidizing radicals can disrupt the process. According to the Fig. 2 robs of different process shows that the UV/S/M with 32 mg/L.min, UVS about 8.2 mg/L.min, and UV/Mn 5.97 mg/L.min are proved. In a study conducted by Yu et al. on the efficiency and mechanism of diclofenac degradation by the advanced UV/Sulfite reduction process, the effect of pH on diclofenac degradation at pH 3 to 11 was investigated, and pH 7 was optimal pH. By increasing the pH from 6.0 to 10 the constant reaction rate for diclofenac degradation decreased from 0.2294 to 0.1303 min−1. Also, they proved that direct photolysis, kobs is pH independent, and the values of kobs 0.0743 min−1, min−1 0.0795 and min−1 0.0720 are proved at pH 6, 7 and 9, respectively. This study proves that the effect of pH on DCF degradation is mainly due to radical reactions, not direct photolysis (Yu et al., 2019). In addition, Cr (VI) depletion is increased in the presence of several complexing agents including sulfite, citrate, EDTA, and humic acid. The positive effect of this substance is due to their ability to complexes with Cr (III) (Cao et al., 2021).
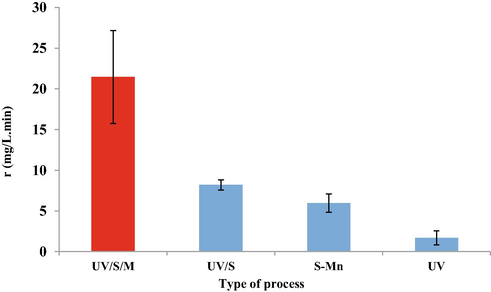
- Reaction rate of different processes of Cr photo- sedimentation at various initial pH values.
3.1.2 Reactive species identification in Cr photo- sedimentation
To gain further insight into the oxidation mechanism in the UV/sulfite/Manganese dioxide process, we investigated the presence of different reactive species at different pH by Scavengers to explore of reactive species nature at pH 3, 7 and 11. Inspection of Fig. 3 shows that the contribution of reactive species at pH 7 with best performance about 45% and 55% of reaction species were reduction and oxidative species respectively. On the other hand at pH 11, about 95% of reaction species were oxidative species. This shows that reductive agents are important effective species to prepare Cr-S to formation complex with MnO2 particle. At pH 7, a significant difference is observed, probably due to the low percentage of soluble sulfite as well as the presence of sulfate radical in reaction medium and formation •OH radicals at this pH. •OH radicals can be a scavenger to •H radicals and other reductive agents (Rinklebe et al., 2016).
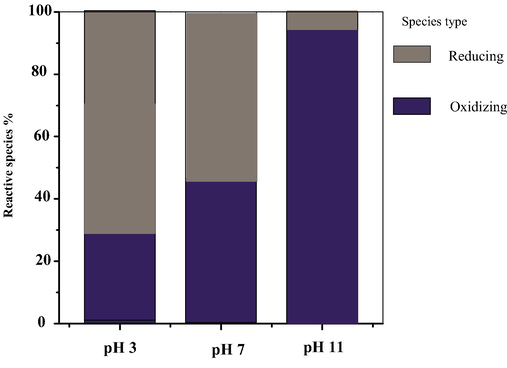
- Contribution of different reactive species in Cr photo- sedimentation at various initial pH values.
3.1.3 Effect of different sulfite dose
Different amounts of sulfite were used to investigate the effect of sulfite dosage on Cr (III) removal at pH 3, 7 and 11) in optimal condition and fixed MnO2 dose at 1 mM. C/Co values decrease initially with the increase in sulfite dosage, reached a maximum value at 0.4 mM, and then decreased. According to Eqs. (13)–(23), sulfite can cause chain reactions that produce sulfur oxy and HO radicals, and increasing the sulfite concentration can improve the initial oxidation rate. However, they can react with sulfite by over-increasing the produced radicals SO4−• and HO• when used at much higher concentrations.
3.2 Sequential addition of Manganese dioxide
After 4 min of reaction time MnO2 is added to the reaction solution, respectively (Fig. 4). In general, the rate of Cr-S complex formation and excess MnO2 showed higher efficiency in Cr oxidation. In fact, sulfite is not recyclable because it is irreversibly converted to sulfate. Therefore, additional MnO2 is required to promote sequential treatment. In the sequential addition of MnO2, excess Cr (III) oxidation may be induced by the UV/Sulfite/MnO2 process.
![Effect of time at high Cr levels. Initial conditions: pH 7, [Cr (III) = 10 mg L−1, [MnO2] = 1 mM, [Na2SO3] 0.4 mM.](/content/185/2022/34/3/img/10.1016_j.jksus.2022.101894-fig4.png)
- Effect of time at high Cr levels. Initial conditions: pH 7, [Cr (III) = 10 mg L−1, [MnO2] = 1 mM, [Na2SO3] 0.4 mM.
3.3 Kinetics and energy- economic evaluation in the photo-sedimentation process
In cost-effective factors, energy consumption is a limiting factor in the use of AORPs process. In this study kinetic and IUPAC methods were used to calculate photo-process energy consumption. The calculated of R2 (0.95) in Table 1 indicate the fact that the Cr photo-sedimentation process follows the Pseudo first-order (PFO) kinetics. The values of R2 are given in Table 1 and are calculated according to Fig. 5. The reaction rate increased with increasing concentration, which is due to the oxidizing and reducing species colliding with Cr and sediment formation. Because of need to more time or insufficient generate of reactive species to sedimentation of Cr in high concentrations so, the reaction rate decreased at a concentration of 250 mg L−1. In Kinetic and IUPAC methods, energy consumption has increased with increasing Cr concentration, which according to the formula is clear that more Cr sedimentation at higher concentrations requires more energy. Also, the proximity of the energy obtained by the kinetic method in the range of energy obtained from the IUPAC method, which indicates the accuracy of the calculations. Constant reduction and reaction rate was probably due to the lower chance of Cr colliding with reactive species or insufficient generation of reactive species to sediment of high Cr concentrations. It leads to a decrease in the efficiency of the sedimentation process and as a result, the constant and reaction rate are reduced (Rastogi et al., 2009). Also, the amount of energy consumed decreases from 16.14 to 3.25 kWh per cubic meter, Kobs (min−1) 0.5337 to 0.1837 and robs (mg/L.min) increase from 26.68 to 45.92. Because the amount of energy consumed varies depending on the concentration of the pollutant. In many studies, energy consumption increases with increasing pollutants, but in some studies it is quite the opposite and the amount of energy consumption decreases with increasing pollutant concentration (Moussavi et al., 2015; Xiao et al., 2017). Total cost of the system (TCS) was investigated at different concentrations. As shown in Table 1, the total cost of the MN process is much lower than other methods. In the UV, UVM, UVS, and USM methods, the total cost were estimated 9.80–18.75, 5.74–8.75, 5.72–1.51, and 2.74–0.55 $ when the Cr concentration increase 100 to 250 mg L−1, respectively.
| Process | Photo-degradation-PFO | ||||||
|---|---|---|---|---|---|---|---|
| Cr (mg L−1) | R2 | Kobs (min−1) | robs (mg/L.min) | Kinetic EEO (kwh//m3) |
figure-of-merit EEO (kwh /m3) |
TCS ($ g−1) | |
| 50 | 0.9793 | 0.0748 | 3.74 | 125.23 | 119.6–107.41 | 18.75 | |
| UV | 100 250 |
0.9647 0.9832 |
0.0518 0.0218 |
5.18 5.45 |
78.61 65.37 |
70.26–76.15 63.24–68.12 |
11.79 9.80 |
| 50 | 0.9761 | 0.1258 | 6.29 | 51.48 | 50.66–57.25 | 8.75 | |
| UV/M | 100 250 |
0.9853 0.9722 |
0.0825 0.0672 |
8.25 16.80 |
41.27 33.82 |
0.26–43.1537 31.24–36.12 |
7.01 5.74 |
| 50 | 0.9763 | 0. 1671 | 8.355 | 35.76 | 92.23–97.54 | 5.72 | |
| UV/S | 100 250 |
0.9963 0.9756 |
0.0905 0.0673 |
9.05 16.82 |
28.54 9.47 |
20.14–32.16 8.24–11.02 |
4.56 1.51 |
| 50 | 0.9946 | 0.5337 | 26.68 | 16.14 | 21.67–29.69 | 2.74 | |
| UV/S/M | 100 250 |
0.9652 0.9765 |
0.3269 0.1837 |
32.69 45.92 |
10.22 3.25 |
16.26–21.74 4.28–6.18 |
1.73 0.55 |
![Ct/Co versus t to calculate reaction rate constant (conditions: [MnO2] = 0.1 mM, [Na2SO3] = 0.4 mM).](/content/185/2022/34/3/img/10.1016_j.jksus.2022.101894-fig5.png)
- Ct/Co versus t to calculate reaction rate constant (conditions: [MnO2] = 0.1 mM, [Na2SO3] = 0.4 mM).
3.4 Sludge investigation
To investigate the sludge produced in the process, a concentration of 1000 mg L−1 chromium was used for 1–5 min reaction time. As shown in Fig. 6, over time, the amount of chromium removed increases and more sludge is produced. FTIR analyses was performed on the obtained sludge. As shown in Fig. 7 the broad band at 3450 cm−1 was observed in the spectra of all samples ascribed to surface hydroxyl groups and adsorbed water molecules. Pure MnO2 nanoparticles have characteristic IR peaks at 1626 cm−1 corresponding to the bending vibrations of O–H groups. Furthermore, two peaks were observed in FTIR spectra of Cr at 1064 and 917 cm−1 for Cr–O and Cr=O, respectively. This is supported by new peaks in FTIR spectra of Cr (VI) 523 cm−1, suggesting the formation of Cr (OH)3 and, also the peak at 576 cm−1was associated with the stable Cr2O3.

- The generated sludge in different reaction time of USM.
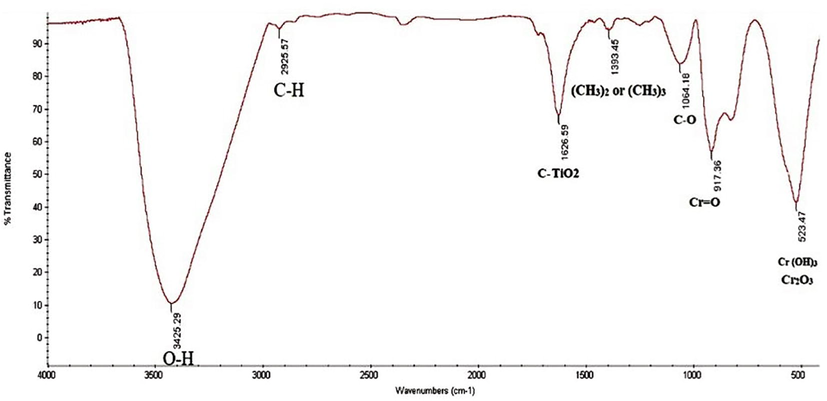
- The FTIR of generated sludge in USM process.
3.5 The impact of anion in real sample on Cr photo-sedimentation
Common anions in water and wastewater interfere with AORPs and reduce their effectiveness. The amount of water ions may also change after AORPs processes, especially photo-sedimentation. To explore the USM process application for treatment of Cr contaminated water, experiments in tow water matrix, including distillated and underground water (real sample) were carried out and the results were presented in Table. 2. Obviously, the USM process effectively removed Cr from different water matrix and all Cr concentrations were reduced to a value less than 273 µg L (the maximum permissible Cr level) after 1 min of USM process, Suggesting the great potential of the USM process on practical applications for Cr removal. The ions increase the time to reach the standard from 1 to 2 min as when the actual sample is applied. The increase in time can be due to three reasons:
| Parameters | Row water | After MnO2 addition | UV/SO3− |
|---|---|---|---|
| pH (unit) | 7.77 | 7.26 | 7.13 |
| Cr (µg L−1) | 273 | 89.75 | 3.26 |
| HCO3− (mg L−1) | 97.36 | 94.17 | 90.65 |
| CO32− (mg L−1) | 65.17 | 44.87 | 43.59 |
| NO2− (mg L−1) | 14.18 | 10.65 | 7.38 |
| Cl− (mg L−1) | 84.23 | 64.78 | 63.25 |
| SO42− (mg L−1) | 51.94 | 126.34 | 87.16 |
| HPO42− (mg L−1) | 10.27 | 8.63 | 2.58 |
Reaction of ions in water and production of weak radicals
-
Absorption of UV irradiation on the surface of the liquid and not reaching it to a greater depth
-
Ions such as phosphate can replace Cr in the process and disrupt the production of radical’s cycles and may inhibit Cr oxidation by affecting the oxidative agents’ process (Azarpira et al., 2019; Azizi et al., 2021).
As can be seen in Table 3 for comparing different catalytic methods with the present method, the present method has many advantages. These include less time, higher efficiency, less use of reactive materials, and no need for large pH changes. Also, sulfite and sulfate are completed with Manganese dioxide particles and therefore are not released into the environment.
| processes | Concentration of Cr | Reactive Concentration | pH | Time (min) | Performance | References |
|---|---|---|---|---|---|---|
| UV/H2O2/Fe0/S2O8 | 10 mg L 1 | H2O2/Fe0 molar ratio, 2.0; S2O8, 0.75 mM L−1 | 3 | 4 | 100% | (Yazdanbakhsh et al., 2020) |
| UV/TiO2/Formate | 30 mg L1 | TiO2: 10 mg L 1, Formate: 6 mg L 1 | 8 | 150 | 60% | (Massoudinejad et al., 2020) |
| Visible/pristine TiO2 | 10 mg L1 | TiO2 dosage (anatase) = 0.5 g L1 | 5.36 | 50 | 100% | (Sun et al., 2021) |
| Sunlight/N, S-codoped Carbon Dot | 100 mg L1 | 0.5 mg.mL−1 | 7.4 | 350 | 98% | (Saini et al., 2020) |
| Present study | 100 mg L1 | [MnO2] = 1 mM, [Na2SO3] 0.4 mM | 6 | 6 | %90 |
4 Conclusions
In this study, removal of Cr in a novel process includes reduction and complexion, adsorption and oxidation in a UV/Sulfite/Manganese dioxide investigated. Information shows that the UV/S/M is very rapid process against other process (About twice as much UV/Mn and about six times much more than UV/S. Comparison of different catalytic methods prove that the USM process less time, higher efficiency, less use of reactive materials, and no need for large pH changes. This method investigated in a real well sample with anions, and more effective anions were Phosphate and Sulfate. Phosphate reacts with eaq− and react with sulfite radical and does not allow the formation of complexes, on the other hand Sulfate help to convert Cr+6 to Cr+3 and direct removal.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Removal of Acetaminophen from Aqueous Solutions by H2O2 + UV in the Presence of Zinc Oxide Nanoparticles Using Response Surface Methodology wastewater treatment. J Res. Environ Health. 2021;7:105-119.
- [CrossRef] [Google Scholar]
- One-pot ultrasonic assisted sol-gel synthesis of spindle-like Nd and V codoped ZnO for efficient photocatalytic degradation of organic pollutants. Sep. Purif. Technol.. 2019;212:427-437.
- [Google Scholar]
- Photo-catalytic degradation of Trichlorophenol with UV/sulfite/ZnO process, simultaneous usage of homogeneous reductive and heterogeneous oxidative agents generator as a new approach of Advanced Oxidation/Reduction Processes (AO/RPs) J. Photochem. Photobiol., A. 2019;374:43-51.
- [Google Scholar]
- Degradation of Codeine Phosphate by simultaneous usage of eaq− and •OH radicals in photo-redox processes: Influencing factors, energy consumption, kinetics, intermediate products and degradation pathways. Optik 2021167415
- [Google Scholar]
- Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ.. 2019;696:133961
- [Google Scholar]
- Review on UV/sulfite process for water and wastewater treatments in the presence or absence of O2. Sci. Total Environ.. 2021;765:142762
- [Google Scholar]
- Degradation of ethyl paraben in aqueous medium using advanced oxidation processes: efficiency evaluation of UV-C supported oxidants. J. Cleaner Prod.. 2018;180:505-513.
- [Google Scholar]
- Removal performance and mechanisms of toxic hexavalent chromium (Cr (VI)) with ZnCl2 enhanced acidic vinegar residue biochar. J. Hazard. Mater.. 2021;420:126551
- [Google Scholar]
- Photocatalytic reduction of nitrite to dinitrogen in aqueous suspensions of metal-loaded titanium (IV) oxide in the presence of a hole scavenger: an ensemble effect of silver and palladium co-catalysts. Phys. Chem. Chem. Phys.. 2012;14(22):7965-7970.
- [Google Scholar]
- Arsenic removal from water and river water by the combined adsorption-UF membrane process. Chemosphere. 2018;202:768-776.
- [Google Scholar]
- Removal of arsenic from wastewater using chemical precipitation methods. Water Environ. Res.. 1992;64(3):200-203.
- [Google Scholar]
- Arsenic (III) removal from a high-concentration arsenic (III) solution by forming ferric arsenite on red mud surface. Minerals. 2020;10(7):583.
- [Google Scholar]
- Specific H2S release from thiosulfate promoted by UV irradiation for removal of arsenic and heavy metals from strongly acidic wastewater. Environ. Sci. Technol.. 2020;54(21):14076-14084.
- [Google Scholar]
- Improving removal rate and efficiency of As (V) by sulfide from strongly acidic wastewater in a modified photochemical reactor. Environ. Technol. 2021:1-25.
- [Google Scholar]
- The chemistry of aqueous S (IV)-Fe-O2 system: state of the art. J. Sulfur Chem.. 2006;27(5):461-530.
- [Google Scholar]
- Arsenic (V) removal using an amine-doped acrylic ion exchange fiber: kinetic, equilibrium, and regeneration studies. J. Hazard. Mater.. 2017;325:223-229.
- [Google Scholar]
- Lee, C.-G., H. Javed, D. Zhang, et al., 2018. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol.
- A review of functional sorbents for adsorptive removal of arsenic ions in aqueous systems. J. Hazard. Mater.. 2020;388:121815
- [Google Scholar]
- Removal of recalcitrant trivalent chromium complexes from industrial wastewater under strict discharge standards. Environ. Technol. Innovation 2021101644
- [Google Scholar]
- Enhancing photo-precipitation of chromate with carboxyl radicals: Kinetic, energy analysis and sludge survey. Process Saf. Environ. Prot.. 2020;134:440-447.
- [Google Scholar]
- Advanced reduction of Cr (VI) in real chrome-plating wastewater using a VUV photoreactor: Batch and continuous-flow experiments. Sep. Purif. Technol.. 2015;151:218-224.
- [Google Scholar]
- Calixarene-tethered textile fabric for the efficient removal of hexavalent chromium from polluted water. Colloids Surf., A: Physicochem. Eng. Aspects 2021127045
- [Google Scholar]
- Photo-degradation of dexamethasone phosphate using UV/iodide process: Kinetics, intermediates, and transformation pathways. J. Mol. Liq.. 2019;295:111703
- [Google Scholar]
- Modeling and optimizing chromate photo-precipitation with iodide exciting under UV irradiation. Desalin. Water Treat.. 2020;195:369-376.
- [Google Scholar]
- Sulfate radical-based ferrous–peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems. Appl. Catal. B. 2009;85(3–4):171-179.
- [Google Scholar]
- Trace Elements in Waterlogged Soils and Sediments. CRC Press; 2016.
- Arsenic selective adsorption using a nanomagnetic ion imprinted polymer: Optimization, equilibrium, and regeneration studies. J. Mol. Liq.. 2020;317:114246
- [Google Scholar]
- N, S-codoped carbon dots for nontoxic cell imaging and as a sunlight-active photocatalytic material for the removal of chromium. ACS Appl. Bio Mater.. 2020;3(6):3656-3663.
- [Google Scholar]
- Photo-biological degradation of Bisphenol A, UV/ZnO/Iodide process at the center of biological reactor. J. Photochem. Photobiol., A. 2019;374:115-124.
- [Google Scholar]
- Simultaneous removal of colorless micropollutants and hexavalent chromium by pristine TiO2 under visible light: An electron transfer mechanism. Chem. Eng. J.. 2021;405:126968
- [Google Scholar]
- A review on electrochemical treatment of arsenic from aqueous medium. Chem. Eng. Commun.. 2021;208(3):389-410.
- [Google Scholar]
- An overview of advanced reduction processes for bromate removal from drinking water: Reducing agents, activation methods, applications and mechanisms. J. Hazard. Mater.. 2017;324:230-240.
- [Google Scholar]
- Rapid catalytic oxidation of arsenite to arsenate in an iron (III)/sulfite system under visible light. Appl. Catal. B. 2016;186:56-61.
- [Google Scholar]
- Mineralization of sucralose by UV-based advanced oxidation processes: UV/PDS versus UV/H2O2. Chem. Eng. J.. 2016;285:392-401.
- [Google Scholar]
- Application of the enhanced sono-photo-Fenton-like process in the presence of persulfate for the simultaneous removal of chromium and phenol from the aqueous solution. J. Water Process Eng.. 2020;34:101080
- [Google Scholar]
- Efficiency and mechanism of diclofenac degradation by sulfite/UV advanced reduction processes (ARPs) Sci. Total Environ.. 2019;688:65-74.
- [Google Scholar]
- Arsenic removal from water using advanced oxidation processes. Toxicol. Lett.. 2002;133(1):113-118.
- [Google Scholar]
- Enhanced removal of arsenite and arsenate by a multifunctional Fe-Ti-Mn composite oxide: photooxidation, oxidation and adsorption. Water Res.. 2018;147:264-275.
- [Google Scholar]







