Translate this page into:
Treatment of oily bilge waste water using marine fungi
⁎Corresponding author. fuadameen@ksu.edu.sa (Fuad Ameen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Oily bilge waste waters released from ships cause an emerging environmental problem in the seas because they are toxic to many sea organisms. Bilge waters need new treatments that are efficient, economic, and local. The crude oil degradation efficacy of five marine fungal isolates (Acremonium sp., Ceratocystis sp., Cladosporium sp., Emericellopsis sp., Fusarium magnifereae) was studied in controlled conditions. The plate culture technique was used to measure the degradation of crude oil in five concentrations (100, 200, 300, 400, 500 mg/L). In addition, the degradation of oily bilge water (320 mg/L) collected from ships was measured using broth culture technique. The fungi F. magnifereae had the highest crude oil degradation efficacy; the crude oil concentration of bilge water was reduced to one-tenth: from the original 320 mg/L to 32 mg/L. Turbidity (350 NTU) and biological oxygen demand (380 mg/L) were reduced to one-tenth of the original. Acremonium sp. and Ceratocystis sp. degraded crude oil slightly less (to 1/7) and two of the isolates poorly (to 1/5) (Emericellopsis sp. and Cladosporium sp.). The phytotoxicity of bilge water was studied with a seed germination test. The seeds of mung bean did not germinate in bilge water (0% germinated) while 90% of the seeds germinated in F. magnifereae-treated bilge water. The germination in tap water was 81% and in distilled water 74%. Seed germination and crude oil concentration correlated strongly (r = 0.90) giving a significant regression equation. F. magnifereae could degrade crude oil in bilge water and remove phytotoxic compounds. Treated bilge water can be used for irrigation.
Keywords
Bilge water
Marine fungi
Crude oil
BOD
COD
Seed germination
1 Introduction
Motorized transportation of raw materials and products is increasing continuously. In the twenty-first century, 80% of the world’s trade has been delivered by ships meaning over ten billion tons of cargo transported across the world’s seas (Walker et al., 2019). It has been forecasted that global shipping traffic will increase more than tenfold by 2050 (Sardain et al., 2019). Along with the increasing traffic, oil pollution in the seas is a growing environmental concern. Motorized vessels release oily bilge water to the sea. Although these waters are dilute, they are toxic to sea organisms (Afshar et al., 2019).
Ships generate bilge water that is a byproduct of various ship operations like condensation, oil leakage, and wastewater from various onboard activities. Bilge water contains fats, oils, greases, inorganic compounds, and highly toxic polycyclic aromatic hydrocarbons (PAHs) (Wei et al., 2021). Bilge water pollutants are toxic to aquatic flora, fauna, and microbes (Afshar et al., 2019; Tiselius and Magnusson, 2017). The organization of the International Convention for the Prevention of Pollution from Ships (MARPOL) has set regulations for the discharge of oil residues into the marine environment. The oil concentration of the effluent without dilution must not exceed 15 mg/L. The limit set by MARPOL has not succeeded to limit pollution enough. In many cases, the treatment of oily waste is not efficient enough compared to the MARPOL requirements and more efficient onboard treatment techniques must be developed. Due to the enormously increasing maritime traffic also smaller discharges such as oily bilge waters from vessels must be cleaned throughout the world.
Numerous techniques have been developed to treat industrial oily wastewater as reviewed recently (Adetunji and Olaniran, 2021). Chemical and physical techniques such as chemical- and electrocoagulation, carbon adsorption, membrane filtration, and gravity separation are commonly used. Biological treatments use microorganisms that can degrade crude oil using it for their growth (Tahri et al., 2013). Bacteria are thought to be the most efficient degraders of crude oil and they are known to convert toxic substances into non-toxic forms (Xu et al., 2018). Crude oil is also known to be degraded by certain fungal species (Al-Hawash et al., 2018). While most oily waste treatments use bacteria, also fungi may have certain benefits that are increasingly studied. It is important to select natural species that are adapted to local conditions. Indigenous fungi, for instance in coastal mangrove habitats, was shown to degrade oil on site (Ameen et al., 2015; 2016; 2022). Thus, indigenous microbes have the potential to be utilized to treat oily waste water and oily port soils. Microbial degradation is an economic and simple method to treat bilge water, and importantly, the treated water could be further utilized in irrigation if shown to be non-phytotoxic. This aspect is extremely important in an arid area such as the Arabian Peninsula. In the Arabian Peninsula where farming is based on artificial irrigation, the utilization of treated waste water would be of great value. Our study aims to find efficient fungal species to treat bilge water that can be used for irrigation in farming after the treatment.
2 Materials and methods
2.1 Fungi
The hydrocarbon degradation study was conducted using previously isolated and identified marine fungi (Ameen et al., 2022). The marine fungi Fusarium magnifereae (OM487085 NCBI accession number), Acremonium sp. (OM510382), Emericellopsis sp. (OM510377), Cladosporium sp. (OM510301), Ceratocystis sp. (OM510413) had been collected from Red sea coast, Saudi Arabia and maintained in the laboratory of the Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia. The fungi were first aseptically transferred on potato dextrose agar (PDA) (HiMedia) and incubated at 37 °C for 3–4 days. Single colonies were then subcultured on PDA plates and preserved.
2.2 Oil degradation experiment
Bilge water (10 L) was collected from ships located in Aljubail port, east of Saudi Arabia, and transferred aseptically to the laboratory. Crude oil was collected from Saudi Aramco, Ltd., Riyadh refinery.
All experiments were carried out as three replicates. In the experiments, the crude oil concentration was measured using a crude oil analyzer (Bruker ASTM D7575), turbidity using an electronic turbidity meter (Hach turbidimeter) in NTU (nephelometric turbidity units), BOD (biological oxygen demand) using a BOD meter (BD600) and COD (chemical oxygen method) using a COD meter (Lovibond®).
Minimal salt (MS) (HiMedia) medium was used for both agar plate and broth techniques. The degradation of five different concentrations of crude oil (100, 200, 300, 400, and 500 mg/L) was tested using agar plate technique. MS agar media were prepared with five different concentrations of crude oil. The five fungi were inoculated on their plates and incubated at 37 °C for 4 days. After the incubation, the zone of clearance was measured with a ruler.
For the bilge water treatment experiment, the fungi were first inoculated into 500 mL of MS broth and incubated in a rotary shaker at 37 °C for 4 days. The fungal mycelia were separated using a sterile cheesecloth and the spores (300 mL) were collected. Then the spore suspension was inoculated into 5 L of experimental bilge water, mixed thoroughly, incubated at 37 °C in an orbital shaker for 4 days, and measured for the crude oil concentration and other water properties mentioned above.
2.3 Seed germination analysis
The seeds of mung beans (Vigna radiata, green gram) (100 g) were submerged into 100 mL of untreated and treated bilge water, tap water, and distilled water for ten hours. After the soaking, the seeds were place on Petri dishes to germinate. The percentage of seeds germinated was calculated.
2.4 Statistical analysis
Regression analysis was carried out to see the relation between seed germination and crude oil concentration after the fungal treatments using R software Version 2023.03.0 + 386 (Core Team., 2021).
3 Results
3.1 Oil degradation
The test with different concentrations of crude oil showed that the higher the concentration the lower the degradation of oil measured as the zone of clearance (ZOI) (Table 1). The reduction in the degradation efficiency was observed for all fungal species. The highest crude oil degradation efficiency was obtained by Fusarium magnifereae: the agar plate assay gave ZOI of 55 mm in 100 mg/L crude oil and 25 mm in 500 mg/L crude oil (Table 1). The poorest degrader, Emericellopsis sp, reached half of the efficiency, the respective clearance zone values being 23 mm and 6 mm.
Crude oil concentration mg/L
Fusarium magnifereaemm
Acremonium sp.mm
Emericellopsis sp.mm
Cladosporium sp.mm
Ceratocystis sp.mm
0
0.6 ± 0.1
0.8 ± 0.2
0.5 ± 0.3
0.8 ± 0.2
0.7 ± 0.2
100
55 ± 3
35 ± 3
23 ± 2
27 ± 3
32 ± 4
200
40 ± 3
32 ± 4
19 ± 6
23 ± 1
28 ± 2
300
34 ± 3
27 ± 1
15 ± 2
19 ± 1.8
24 ± 3.6
400
29 ± 5
21 ± 4
10 ± 1
15 ± 1
19 ± 1
500
25 ± 2
17 ± 3
6 ± 2
11 ± 4
15 ± 3
Bilge water contained 320 mg/L crude oil. Turbidity was 350 NTU, BOD 210 mg/L, COD 1088 mg/L. Fusarium magnifereae reduced the values to 34 mg/L, 33 NTU, 40 mg/L, and 188 mg/L, respectively (Table 2). Acremonium sp. and Ceratocystis sp. reduced the crude oil concentration to 43 mg/L and 47 mg/L, respectively. The rest of the fungi reduced the values less.
Variable
Pre-
treatmentAfter the fungal treatments
Fusarium magnifereae
Acremonium sp.
Emericellopsis sp.
Cladosporium sp.
Ceratocystis sp.
Crude oil (mg/L)
320 ± 7
34 ± 2
43 ± 3
61 ± 2
54 ± 2
46 ± 3
Turbidity (NTU)
350 ± 2
33 ± 3
59 ± 2
57 ± 1
53 ± 3
63 ± 5
BOD (mg/L)
380 ± 3
40 ± 4
58 ± 3
46 ± 5
69 ± 6
60 ± 4
COD (mg/L)
1088 ± 5
187 ± 7
449 ± 5
548 ± 4
280 ± 2
350 ± 3
3.2 Seed germination
The seeds did not germinate (0%) in raw bilge wastewater of which crude oil concentration was 320 mg/L (Fig. 1). Germination was 81% and 74% in tap water and distilled water, respectively. The highest germination was observed after the F. magnifereae treatment, when 90% of the seeds germinated (Fig. 2). The germination after the Acremonium sp. treatment was 82%, and after Ceratocystis sp. 78%. Seed germination and crude oil concentration correlated strongly (r = 0.90, n = 15). The significant regression model explained 81% of the variation, and thus, showed high dependence between the variables (Table 3). The equation was as follows. Seed germination = −2.5 × Crude oil concentration + 183. The equation indicated a significant, strong, and relatively sharp decrease in germination when the crude oil concentration increased.
Seed germination in A) bilge water; B) tap water, C) distilled water, and D) bilge wastewater after the treatment with the fungi F. magnifereae.
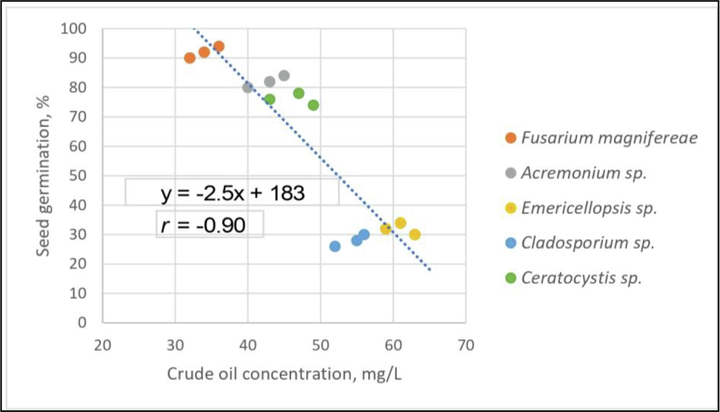
Relation of seed germination and crude oil concentration after the treatments with five different fungi. The line refers to the linear regression equation.
ANOVA
df
SS
F
P
Regression
1
8720
57.3
4.04E-06
Residual
13
1975
Total
14
10,696
4 Discussion
The treatment of oily bilge wastewater must be developed to be more efficient due to the increasing oil pollution caused by the increasing motorized traffic in all seas. Around the Arabian Peninsula, many busy ports with high traffic pollute coastal waters. The seas around the Arabian Peninsula suffer from oil accidents and also from oil pollution originating from ordinary operating ships (Suneel et al., 2019). Local solutions to treat bilge waters onboard the vessels and near the ports with indigenous microbes are needed.
Economic and local solutions to treat bilge wastewater are offered by microorganisms such as fungi. Previously, sixty different fungal isolates from 13 genera were isolated from crude oil-contaminated fields in Iran (Dawoodi et al., 2016). This shows that the potential to find local fungi to be utilized in bilge wastewater treatment is high. Efficient oil degraders were found also in India; the marine fungi Fusarium sp. and Acremonium sp. (Barnes et al., 2018). In our experiment, the same genera were the most efficient degraders. F. magnifereae reduced the crude oil concentration of bilge water to ca. one-tenth of the original concentration (320 mg/L) in bilge water. Other bilge wastewater properties (turbidity, biological and chemical oxygen demand) were also reduced to ca. one-tenth of the original values. These values confirm that the oil has been degraded to one-tenth of the original concentration. In our experiment, the crude oil concentration was originally 320 mg/L. It appeared that the concentration was suitable to be degraded efficiently. Namely, the results showed that the higher the oil concentration the lower is the degradation of oil; higher than 320 mg/L would have been degraded inefficiently. When the concentration was as high as 500 mg/L, the degradation efficiency was half of the lowest tested 100 mg/L concentration. This was shown by the ZOI for our best species F. magnifereae. ZOI was 55 mm in the lowest concentration and 25 mm in the highest concentration. The two other efficient species, Acremonium sp. and Ceratocystis sp., showed the same trend, the degradation decreased to half when the oil concentration increased from 100 to 500 mg/L.
Several species and isolates need to be studied locally to find the most efficient indigenous strains. For instance, Cladosporium sp., which was relatively inefficient in our experiment, degraded crude oil efficiently when isolated from the Gulf of Mexico (Al-Hawashet al., 2018). From the Red Sea Coast of Saudi Arabia, the isolates of Cladosporium sphaerospermum degraded diesel fuel efficiently (Ameen et al., 2016). This shows that the molecular identification of the isolates, which we had carried out, is needed in the reports.
Many other efficient fungal isolates have also been presented, Aspergillus oryzae isolated from the Saudi Arabian coast degraded crude oil almost totally (99%) (Amr et al., 2017). Penicillium sp. isolated from an oil field in Iraq was shown to degrade crude oil (Al-Hawash et al., 2018). Several fungi (Alternaria alternata, Aspergillus terreus, Cladosporium sphaerospermum, Eupenicillium hirayamae, and Paecilomyces variotii) isolated from mangrove habitat on the Red Sea coast of Saudi Arabia degraded diesel hydrocarbons (Ameen et al., 2016). In addition to above mentioned, at least the genera Cephalosporium, Rhizopus, Paecilomyces, Torulopsis, Pleurotus, Mucor, Talaromyces, Gliocladium, Rhodotorula, and Geotrichum include oil degraders (Dawoodi et al., 2015; Al-Nasrawi, 2012; AI-Jawhari, 2014; Hanafy et al., 2016; Marchand et al., 2017). Degradation of oil and hydrocarbons derivatives by isolated yeast strains from Ras Tanura in Saudi Arabia were studied by Al-Dhabaan (2022).
The final crude oil concentration of the treated bilge wastewater in our experiment was 34 mg/L, which is still too high compared to the MARPOL regulations for bilge wastewater to be released back into the sea (15 mg/L). Increasing the incubation time from four days would most probably reduce the concentration above the limit and needs further studies.
Petroleum hydrocarbons have been shown to be phytotoxic and inhibit the germination of seeds (Fasih et al., 2021). However, the effect of bilge wastewater on seed germination has been studied scarcely. Seed germination in bilge wastewater has been shown to vary greatly between plant species. Of different crop species studied, Sorghum bicolor seeds were most affected, with less than 30% of the seeds germinating in raw bilge wastewater (Olorunfemi and Idada, 2013). The germination of two Solanum species was inhibited greatly, less than 10% were germinated in bilge water (Olorunfemi et al., 2012). In our experiment, the germination of V. radiata seeds was totally inhibited by bilge wastewater. The difference may be due to the relatively high oil concentrations in our experiment. However, V. radiata as an indicative species can be recommended to be used to test the phytotoxicity of crude oil solutions.
Surprisingly, the germination of seeds was slightly lower in distilled and tap water than in cleaned bilge water. This may be due to the lack of nutrients and other elements in distilled or tap water. Bilge water was shown to contain nitrogen and other elements (Olorunfemi et al., 2012) that possibly improved the germination. The germination below 90% was observed also previously in distilled water (Olorunfemi et al., 2012, Olorunfemi and Idada, 2013).
Regarding cleaned bilge wastewater, we found no studies on phytotoxicity. Phytotoxicity seemed to be reduced only slightly when experimental petroleum refinery wastewater was treated using an electrochemical process; the germination of wheat seeds increased from 17% to 23% (Gousmi et al., 2016). Our microbial treatment increased the germination from 0% to 90%, and thus, was shown to reduce phytotoxicity remarkably. The relation between the crude oil concentration and seed germination was negative and strong. The statistically significant regression analysis indicated the remarkable difference between ca 50 – 65 mg/L concentration and 30–40 mg/L concentration. While the former concentration inhibited the germination remarkably, the latter concentration allowed almost all seeds to germinate. It shows that the oil concentration needs to be reduced to 30 – 40 mg/L to be satisfactory. Our results show that the treatment with F. magnifereae was successful and efficient in reducing phytotoxic compounds. Our F. magnifereae-treatment seemed to remove the phytotoxic compounds present in bilge wastewater. This result is outstanding in the arid Arabian Peninsula where the possibility to utilize treated wastewater will be of great value.
5 Conclusion
Many methods are used to treat and detoxify bilge wastewater. We applied five marine fungi and found that F. magnifereae had a high crude oil reduction ability. F. magnifereae was the most efficient fungus not only to decrease the crude oil concentration but to detoxify bilge water. Bilge water was detoxified as assessed with the seed germination test. The phytotoxicity of untreated bilge wastewater was high and shown to be reduced remarkably by the treatment with the marine fungi F. magnifereae. The result gives promising implications that the cleaned water could be used for irrigation in farming.
Author contributions
All authors participated equally in data analysis, authoring, and revising the article. The final version of the manuscript has been reviewed and approved by all authors.
Acknowledgment
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (2-17-01-001-0063).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Treatment of industrial oily wastewater by advanced technologies: a review. Applied Water Sci.. 2021;11:98.
- [Google Scholar]
- Ability of some soil fungi in biodegradation of petroleum hydrocarbon. J of Applied Enviro Micro.. 2014;2(2):46-52.
- [Google Scholar]
- Degradation of oil and hydrocarbons derivatives by isolated yeast strains from Ras Tanura in Saudi Arabia. J of King Saud University - Science.. 2022;34(7):102263
- [CrossRef] [Google Scholar]
- Isolation and characterization of two crude oil-degrading fungi strains from Rumaila oil field, Iraq. Biotech Reports.. 2018;17:104-109.
- [CrossRef] [Google Scholar]
- Biodegradation of crude oil by fungi isolated from Gulf of Mexico. J of Bioremed and Bio degra.. 2012;3(04)
- [Google Scholar]
- Biodegradation of engine oil by fungi from mangrove habitat. J of Gene and Appl Microb.. 2015;61(5):185-192. PMID: 26582288
- [CrossRef] [Google Scholar]
- Biodegradation of diesel fuel hydrocarbons by mangrove fungi from Red Sea Coast of Saudi Arabia. Saudi J of Biolo Sci.. 2016;23(2):211-218.
- [CrossRef] [Google Scholar]
- Marine fungi showing multifunctional activity against human pathogenic microbes and cancer. PLOSONE.. 2022;17(11):e0276926.
- [Google Scholar]
- Amr, Abd-EL-Mooti, EL-Hanafy., Yasir, Anwar., Jamal, S.M. Sabir., Saleh, A. Mohamed., Saleh, M.S. Al-Garni., Osama, A.H. Abu, Zinadah., Mohamed, Morsi, Ahmed., 2017. Characterization of native fungi responsible for degrading crude oil from the coastal area of Yanbu, Saudi Arabia. Biotech & Biotechnological Equip. 31:1, 105-111, DOI: 10.1080/13102818.2016.1249407.
- Bioremediation potential of hydrocarbon-utilizing fungi from select marine niches of India. 3Biotech.. 2018;8(1)
- [CrossRef] [Google Scholar]
- Core ,Team, R. A.. 2021. Language and environment for statistical computing. R. Foundation for statistical computing, Vienna, Austria. http://www.R-project.org/.
- The study of heterotrophic and crude oil-utilizing soil fungi in crude oil contaminated Regions. J of Biorem and Biodeg.. 2015;6:270.
- [CrossRef] [Google Scholar]
- Fasih, Ullah, Haider., Mukkaram, Ejaz.., Sardar, Alam, Cheema., Muhammad, Imran, Khan., Baowei, Zhao., Cai, Liqun., Muhammad, Arslan, Salim., Muhammad, Naveed., Naeem, Khan., Avelino, Nunez-Delgado., 2021. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Envir Rese. 197.
- Purification and detoxification of petroleum refinery wastewater by electrocoagulation process. Envir Tech.. 2016;37(18):2348-2357.
- [Google Scholar]
- EL Hanafy, Amr., Anwar, Yasir.; Sabir, Jamal, Saleh., Al-Garni, Saleh., Zinadah, Osama., Ahmed, Mohamed., 2016. Characterization of native fungi responsible for degrading crude oil from the coastal area of Yanbu, Saudi Arabia. Biotech & Biotechnological Equip. 31. 10.1080/13102818.2016.1249407.
- Petroleum biodegradation capacity of bacteria and fungi isolated from petroleum-contaminated soil. Inter Biodeterioration & Biodegradation.. 2017;116:48-57.
- [CrossRef] [Google Scholar]
- Impact of bilge water on seed germination and seedling growth of some vegetable plants. NISEB Journal.. 2012;12:81-165.
- [Google Scholar]
- Germination and Seedling Growth of Zea mays L., Sorghum bicolor L. and Pennisetum americanum L.under varying concentrations of bilge water. J of Sci Res.. 2013;12:125-134.
- [Google Scholar]
- Global forecasts of shipping traffic and biological invasions to 2050. Nat Sust.. 2019;2:274-282.
- [CrossRef] [Google Scholar]
- Oil pollution in the Eastern Arabian Sea from invisible sources: A multi-technique approach. Marine Poll Bull.. 2019;146:683-695.
- [CrossRef] [Google Scholar]
- Tahri, N., Bahafid, W., Sayel, H., El, Ghachtouli, N., 2013. Biodegradation: Involved Microorganisms and Genetically Engineered Microorganisms. Biodeg - Life of Sci, InTech. 289-309. doi:10.5772/56194.
- Toxicity of treated bilge water: The need for revised regulatory control. Marine Poll Bull.. 2017;114(2):860-866.
- [Google Scholar]
- Environmental effects of marine transportation. World Seas: An Environmental Evaluation (Second Edition).. 2019;505–530
- [CrossRef] [Google Scholar]
- Degradation performance of petroleum-hydrocarbon-degrading bacteria and its application in remediation of oil contaminated soil. IOP Conference Series: Earth and Environmental Science.. 2021;766:012096
- [CrossRef] [Google Scholar]
- Petroleum Hydrocarbon-Degrading Bacteria for the Remediation of Oil Pollution Under Aerobic Conditions: A Perspective Analysis. Frontiers in Microb.. 2018;9
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102929.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







