Translate this page into:
Toxins profiles, toxicological properties, and histological alteration potentiality of Trimeresurus erythrurus venom: In vitro and in vivo experiments
⁎Corresponding authors at: Department of Zoology, University of Chittagong, Chattogram 4331, Bangladesh (IKA Haidar, MAW Chowdhury) and Molecular Biology and Protein Science Laboratory, Department of Genetic Engineering & Biotechnology, University of Rajshahi, Rajshahi 6205, Bangladesh (MA Reza). ibrahimalhaidar88@gmail.com (Ibrahim Khalil Al Haidar), wahed.chowdhury@cu.ac.bd (Mohammad Abdul Wahed Chowdhury), reza.gen@ru.ac.bd (Md Abu Reza)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Analysis of T. erthrurus venom.
Abstract
Objectives
Trimeresurus erythrurus (Red-tailed bamboo pit viper) contributes significantly to snakebite envenoming cases in Bangladesh, India, Myanmar, and Nepal. However, the absence of specific antivenom and challenging socio-economic conditions in these regions necessitate the development of symptom-based medication to mitigate the severity of T. erythrurus envenoming. Therefore, documenting the effects of T. erythrurus venom on mammalian organs can establish a foundation for further research on symptom-based medication.
Methods
We profiled T. erythrurus venom toxins using SDS-PAGE. Additionally, we assessed the venom's toxicological properties through lethality, hemorrhagic, edematogenic, hemolytic, and phospholytic assays. Furthermore, we examined the histological alterations induced by crude venom on various organs of mice.
Results
SDS-PAGE profiling confirmed the presence of Snake Venom Metalloproteases (SVMPs), Snake Venom Serine Proteases (SVSPs), Phospholipase A2 (PLA2), L-amino Acid Oxidases (LAAOs), Myotoxins, and Disintegrins as toxic components in T. erythrurus venom. The venom exhibited a Median Lethal Dose (LD50) of 1.131 mg/kg, Minimum Hemorrhagic Dose (MHD) of 0.21 mg/kg, Minimum Edematogenic Dose (MED) of 0.17 mg/kg, and Median Hemolytic Dose (HD50) of 2.527 mg/L. Moreover, the venom induced significant pathological changes in mammalian skin, skeletal muscle, lung, heart, kidney, and intestine.
Conclusions
This study elucidates the major toxins, critical toxicological indices, and histopathological effects of T. erythrurus venom on mammalian organs. These findings provide valuable insights on the development of effective treatment strategies for pathophysiological complications that could manifest post T. erythrurus envenoming.
Keywords
Red-tailed bamboo pit viper
Snake venom
Venom profile
Venom toxicity
Snakebite management
1 Introduction
Snakebite envenoming presents a significant threat to rural communities in tropical regions, contributing to high mortality and morbidity rates worldwide. Globally, an estimated 5.4 million snakebite incidents occur annually, with 2.7 million resulting in envenoming bites and 81,000–138,000 deaths each year (Bawaskar and Bawaskar, 2019). Tropical South Asia bears a huge snakebite burden due to the diverse venomous snake population, agrarian economy, dense human population, inadequate public health infrastructure, and low awareness (Alirol et al., 2010). Addressing this challenge requires multidisciplinary strategies to reduce mortality and morbidity rates in the region (Gutiérrez et al., 2017).
In Bangladesh alone, there are 710,159 incidences of snakebites and 6,041 deaths yearly estimated through a country-wide community survey (Ghose and Faiz, 2014). Approximately 25 % of reported cases exhibit varying degrees of severity (Ghose and Faiz, 2014). A retrospective survey conducted at Chittagong Medical College reveal that Green pit vipers (Trimeresurus spp.) account for 57–62 % of total snakebite envenoming cases in the southeastern region of Bangladesh, with similar patterns expected in the northeast and northwest regions where these vipers are prevalent (Sarmin et al., 2013). The scenario perhaps is the same in the northeast and northwest parts of this country, where Green pit vipers are distributed. There are two green pit vipers, Trimeresurus erythrurus and T. popeiorum are found in the mixed evergreen forest of the southeast (Chattogram, Cox’s Bazar, Bandarban, Rangamati, Khagrachari, and Feni), northeast (Sylhet, Habiganj, and Moulavibazar), and in mangrove forest of southwest (the Sundarbans) parts of Bangladesh (Hassan et al., 2014). Of the pit vipers, T. erythrurus is commonly available (IUCN status: LC) not only in Bangladesh but also in neighboring countries (India, Myanmar, and Nepal) (Hassan et al., 2014; Tun-Pe et al., 2000; Wallach et al., 2014). Despite high bite incidences of this viper (Tun-Pe et al., 2000), minimum attention has been paid to the clinical management and the development of specific antivenom (Tun-Pe et al., 2000; Yee et al., 2020).
Snake venom toxicity, biochemistry, and pharmacological effects vary both inter- and intra-specifically (Shashidharamurthy et al., 2002). Viper envenoming typically causes local pathophysiological changes including edema, blistering, hemorrhage, coagulopathy, lymphatic vessel damage, and extracellular matrix degradation due to myotoxic and hemotoxic components (Gutiérrez et al., 2009). Without prompt intervention, the synergistic impact of those components may lead to the death of the victim. Unlike other viperid snakes, pit viper venom contains more enzymatic proteins aimed to immobilize mammalian prey over non-enzymatic peptides to kill the prey (Peterson and Talcott, 2006). In humans, T. erythrurus envenomation commonly manifests as hemorrhage associated with the formation of edema, local tissue damage, and severe organ dysfunction (Aung et al., 2010).
Detail characterization of the venoms of 10 Trimeresurus species. (i.e., T. albolabris, T. borneensis, T. gramineus, T. hageni, T. insularis, T. macrops, T. nebularis, T. puniceus, T. purprureomaculatus and T. Stegnegeri) indicated that the venom of pit vipers are species-specific with unique venom proteomes and toxin compositions (Kumkate et al., 2020; Tan et al., 2019). T. erythrurus envenoming causes severe pain, local swelling, profuse sweating, vomiting, necrosis with ecchymosis leading to abscess and gangrene formations, low blood pressure, low platelet count, hemoglobin fluctuation, and high urea concentration (Tun-Pe et al., 2000). However, the histopathological impact of T. erythrurus venom on mammalian organs remain largely unexplored. Therefore, detailed scientific documentation on the effects of the venom of this viper on mammalian organs is a primary demand.
In this study, we conducted a thorough analysis of T. erythrurus venom, including toxin profiling and assessment of its toxicological properties, such as median lethal dose, hemorrhagic, edematogenic, hemolytic, and phospholytic activity. Additionally, we investigated the histopathological alterations induced by T. erythrurus venom on the skin, muscle, lung, kidney, heart, intestine, and liver of Swiss albino mice. These findings contribute to a better understanding of the pathophysiological mechanisms underlying T. erythrurus envenomation and may inform the development of effective treatment strategies.
2 Materials and methods
2.1 Snake collection
The snakes used in this study were obtained in response to rescue calls from the Chittagong University Campus, while venom extraction was carried out at the Venom Research Centre Bangladesh (VRC,B). VRC,B is officially authorized by the Bangladesh Forest Department to handle venomous snake species for venom collection purposes and to undertake activities aimed at initiating the production of high-quality anti-snake venom for Bangladesh. This authorization is formalized under permission letter no.: 22.01.0000.101.23.2019.3173.
2.2 Venom Collection, Preservation, and transportation
The venom extraction process involved gently pressing the venom gland by securely holding the snake's head and allowing the fangs to penetrate a glass jar wrapped with parafilm, following the method described by (Tare et al., 1986). In brief, after removing the parafilm from the glass jar, the liquid venom was placed in an airtight container with a cloth-covered bedding of calcium chloride and stored at a temperature of 4 °C until complete drying. Once dried, the venom was transferred into cryovials and promptly preserved at −20 °C for long-term storage. During transportation from VRC,B, Department of Medicine, Chittagong Medical College to the Molecular Biology and Protein Science Laboratory, Department of Genetic Engineering and Biotechnology, University of Rajshahi, Bangladesh, the lyophilized venom was kept in dry ice at −80 °C to maintain its integrity until analysis of the venom components was conducted. Subsequently, the extracted venom was lyophilized, preserved, and transported in accordance with the 'Venom extraction, preservation, and transportation protocol v.1.1′ established by the Venom Research Centre Bangladesh (VRC,B).
2.3 Animals and ethics clearance
A total of 140 young and healthy Swiss albino mice (20–25 g) were used in this study following the International Council of Medical Organizations (CIOMS) guidelines for animal experiments (CIOMS, 1985). In vivo studies were carried out in the Molecular Biology and Protein Science laboratory of the University of Rajshahi under the ethical approval from the Institute of Biological Sciences, University of Rajshahi, Bangladesh (Ethics license number: 225/ 320 IAMEBBC/ IBSc). The mice were kept in commercially manufactured mice-rearing cages and acclimatized for two weeks at room temperature (25 ± 2 °C) and natural humidity (60–80 %) maintaining 12/12 h day/night cycle. The mice were fed with commercially available standard formulated food and fresh vegetables (potato, cucumber, carrots etc.) were also given as supplementary food. Pure drinking water was offered continuously in standard water bottles designed for drinking of mice and remaining water in a bottle was changed at least once in a day.
2.4 Materials and chemicals
Mass Spectrometry grade trypsin protease and HPLC grade solvents used for RP-HPLC, and Spectra™ Multicolor Broad Range Protein Ladder (10 to 260 kDa) used for SDS-PAGE were purchased from Thermo Scientific™ Pierce™ (USA). Other chemicals and reagents of analytical grade were purchased from Sigma-Aldrich (USA).
2.5 Venom profiling by SDS-PAGE
The protein components present in the crude venom of T. erythrurus from Bangladesh were investigated based on their molecular weight using SDS-PAGE (Bio-Rad, CA, USA) (Gutiérrez et al., 1988). The venom (25 µg in each well) was loaded onto a 12 % gel and electrophoresis was performed under the non-reducing condition at 100 V for 2.5 h. Coomassie Brilliant Blue R-250 was used as a staining agent. Quantitative analysis of the venom proteins was carried out using the visual image of the polyacrylamide gel in ImageJ (version: 2.1.0/1.53c; Java 1.8.0_172 [64-bit]).
2.6 Determination of venom lethality
A total of 36 healthy male Swiss albino mice (randomly assigned into 5 treatment groups and one control group, each group having 6 mice) were subjected to the lethality assay. The crude venom was diluted in the Phosphate Buffered Saline (PBS, pH 7.2) and doses were formulated at 0.2, 0.4, 0.8, 1.6, and 3.2 mg/kg concentrations in 200 µL PBS. The treatment groups were injected with venoms at different concentrations intraperitoneally (IP), while the control group received only PBS. The number of mice that survived in each experimental group after 24 h of envenoming was recorded and Median Lethal Dose (LD50) values were calculated using probit analysis (Finney, 1971; Hayes and Kruger, 2014).
2.7 Haemorrhagic activity of T. erythrurus venom
The Minimum Hemorrhagic Dose (MHD) was determined following a standard method (Kondo et al., 1960), that was later modified (Takahashi and Omori-Satoh, 2002). Three different doses of venom (0.25 mg/kg, 0.5 mg/kg, and 1.0 mg/kg in 30 μL of PBS) were injected into three healthy Swiss albino mice subcutaneously in the dorsum. Another mouse was injected with an equal volume of PBS solution used as a control. The treated mice were euthanized by injecting Sodium Barbitone (30 mg/L) intraperitoneally after three hours of treatment following the standard ethical approach (Close et al., 1997). The diameter of the skin area with hemorrhage was measured by a vernier scale and the MHD was calculated. The method was replicated thrice to find out more precise result.
2.8 Edematogenic activity T. erythrurus venom
The Minimum Edematogenic Dose (MED) was evaluated as previously described method (Vishwanath et al., 1988). Three different doses of venom (0.25 mg/kg, 0.5 mg/kg, and 1.0 mg/kg in 30 μL of PBS) were injected into the left foot pads of three healthy Swiss albino mice. Another mouse was injected with an equal volume of PBS solution used as a control. The treated mice were euthanized using Sodium Barbitone (30 mg/L) after one hour of treatment following the ethical guidelines (Close et al., 1997). The feet of the sacrificed mice were removed from the ankle and both paws were weighted. The MED was estimated as the amount of venom causing an edema ratio of 30 % (Vishwanath et al., 1988). This method was replicated thrice to find out more precise result.
2.9 Hemolytic activity of T. erythrurus venom
The hemolytic activity of venom was assessed by following scientific principles as described by Harrigan and Buxton (Buxton, 2005; Harrigan and McCance, 1966). Human blood was collected aseptically from a healthy volunteer after signing a consent form and stored in sodium citrate (3.2 %) tubes and 3 ml EDTA. Fresh blood (pH: 7.4 ± 0.2) was centrifuged at 3000 rpm for 5 min, the supernatant was discarded. The pellet containing RBCs was washed three times with PBS at 3000 rpm for 5 min each. The agar suspension was prepared using 28 g of nutrient agar powder in 1000 ml of distilled water. The mixture was heated while stirring to dissolve the components. Then the mixture was autoclaved at 121 °C for 15 min. The autoclaved mixture was allowed to cool at 45–50 °C. Then 8 % sterile RBC was added to the mixture and mixed gently avoiding air bubble generation (92 ml agar + 8 ml RBC). The mixture was dispensed into sterile plates as liquid and after solidification small wells (8 mm) were created using a cork borer. The wells were loaded with 20 μL of test solutions with different concentrations of venom (1.25, 2.5, 5, 10, 20, 40, 80, and 160 mg/L). 20 µL PBS was used as negative control and an equal volume of Triton X-100 was used as a positive control. The agar medium was observed for hemolysis after 24 h of incubation at room temperature. The percentage of hemolysis was calculated by the following formula- Then Hemolytic Dose (HD50) was calculated using probit analysis (Finney, 1971; Hayes and Kruger, 2014) and the mean result was obtained after replication of the produre for three times.
2.10 Phospholipase A2 activity assay
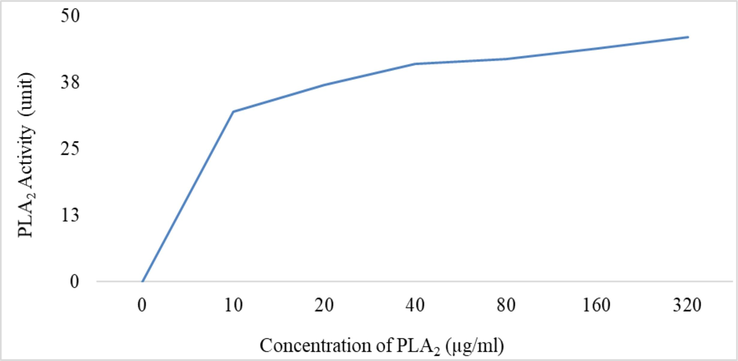
Phospholipase A2 activity of T. erythrurus venom was determined using two different assays, namely, turbidometric assay (Marinetti, 1965) and modified egg yolk-agar plate assay (Joubert and Taljaard, 1980). The turbidometric assay was carried out using a spectrophotometer (GENESYSTM 10S UV–Vis, Thermo ScientificTM, Waltham, USA). In brief, one egg yolk was separated from a fresh chicken egg and dissolved in 250 ml solution of 0.9 % NaCl with 0.02 % (w/v) sodium azide. The egg yolk solution which would be used as a substrate for PLA2, was stored at 4 °C temperature for further use. For the experiment, 1 ml of egg suspension was mixed with 10 ml of 20 mM Tris-HCl buffer (pH 7.4), and absorbance at 740 nm was adjusted to 1 optical density (OD). For the PLA2 activity assay, various concentrations of crude venom (10 μg, 20 μg, 40 μg, 80 μg, 160 μg, and 320 μg) were mixed with a reaction mixture, and absorbance was monitored at 740 nm over a 10 min against blank. The unit activity of PLA2 is described as a decrease in the turbidity of the solution by 0.01 absorbance unit at 740 nm (Joubert and Taljaard, 1980). The PLA2 was also evaluated on egg yolk-agar plates. Three doses of T. erythrurus venom (5 μg, 10 μg, 20 μg) in PBS were assessed along with negative control of PBS and positive control of Naja naja venom (10 μg). After four hours of incubation of the samples on plates at 37 °C, phospholipase activity was measured in agar plates by their sizes (in mm) of the translucent area around each well.
2.11 Histopathological study
To check the effects of T. erythrurus venom on different mammalian organs histopathological studies were carried out. Three healthy Swiss albino mice were treated with ½LD50 dose of venom (0.5655 mg/kg) dissolved in 50 μL PBS. The control mouse was treated with the same volume of PBS solution without venom. After six hours of venom injection, mice were euthanized by injecting Sodium Barbitone (30 mg/L) intraperitoneally following the standard ethical approach guideline (Close et al., 1997), and the autopsies of mice were done immediately after sacrifice. The tissues excised from skin, muscle, lung, kidney, liver, heart, and intestine were fixed in 10 % neutral buffered formalin. Subsequently, samples were dehydrated in ascending series of ethanol, cleaned with methyl benzoate, and embedded in paraffin wax. The paraffin was sectioned with 5 μm thickness by a rotary microtome and stained with hematoxylin-eosin for microscopic observation (Lendrum, 1968). An inverted microscope (Optika IM-3, Italy) was used to observe and capture photomicrographs of tissue sections at 400X magnification.
3 Results
3.1 Venom composition
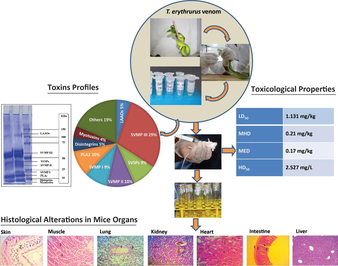
The venom of T. erythrurus from Bangladesh consists of several key components, including Snake Venom Metalloproteases (SVMPs), Phospholipase A2 (PLA2), myotoxins, disintegrins, serine proteases, and L-amino acid oxidases, with approximately 19 % of the venom components remaining unidentified (Fig. 1). A semi-quantitative analysis of the venom revealed that SVMP III comprises the most abundant component at approximately 28.85 %, followed by SVMP II and PLA2, each constituting around 10 % of the venom composition. Additionally, SVSPs and SVMP I each contribute approximately 9 % to the overall venom composition. Other constituents present in the venom include L-amino acid oxidases (∼5%), disintegrins (∼5%), and myotoxins (∼4%) (refer to Fig. 1).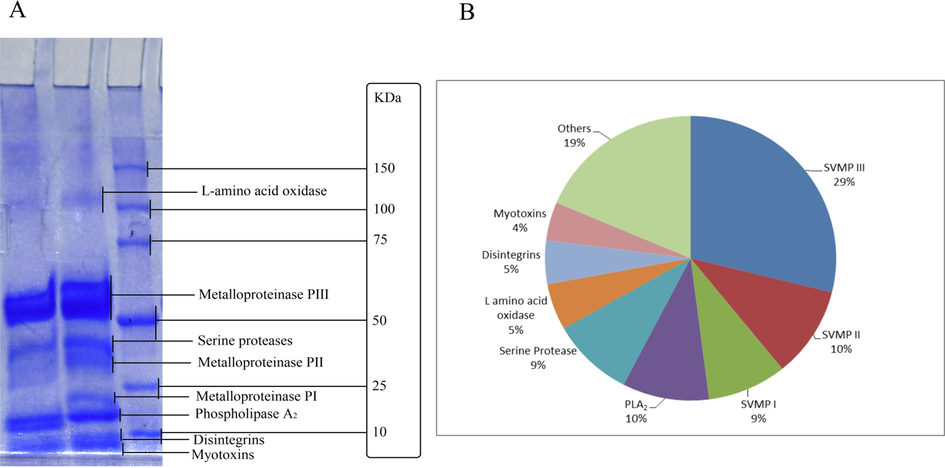
Composition of T. erythrurus venom. (A) The T. erythrurus venom composition was investigated using 12% SDS-PAGE analysis. (B) The venom is composed of (1) snake venom metalloproteases-III (SVMPs-III), (2) snake venom metalloproteases-II (SVMPs-II), (3) snake venom metalloproteases-I (SVMPs-I), (4) Phospholipase A2 (PLA2), (5) snake venom serine protease (SVSPs), (6) L-amino acid oxidase (LAAO), (7) disintegrins, (8) myotoxins, and (9) other unidentified components.
3.2 Toxicity of venom
In the Swiss albino mice model, the Median Lethal Dose (LD50) of the venom was determined to be 1.131 ± 0.079 mg/kg (mean ± SE) upon intraperitoneal (IP) injection of different concentrations of venom (Table 1). The Minimum Hemorrhagic Dose (MHD) was found to be 0.21 ± 0.071 mg/kg when the venom was introduced subcutaneously (SC) (detail in methods). Similarly, the Minimum Edematogenic Dose (MED) of the venom was determined to be 0.17 ± 0.069 mg/kg in a similar mice model. Additionally, the Hemolytic Dose (HD50) was recorded as 2.527 ± 0.072 µg/L (Table 1). Analysis conducted through dose-dependent turbidimetric assays on egg yolk suspension and egg yolk in agar plate revealed that the Phospholipase A2 (PLA2) enzyme activity increased proportionally with the escalating venom concentration (Figs. 2 & 3). Importantly, this activity was notably higher compared to the PLA2 activity observed in N. naja venom from Bangladesh, which was used as a positive control.
Toxicity assessments
T. erythrurus venom from Bangladesh
T. erythrurus venom from Myanmar (Tun-Pe et al., 2000)
Median lethal dose (LD50)
1.131 ± 0.09 mg/kg
4.69 mg/kg
Minimum hemorrhagic dose (MHD)
0.21 ± 0.04 mg/kg
0.025 mg/kg
Minimum edematogenic dose (MED)
0.17 ± 0.02 mg/kg
–
Median hemolytic dose (HD50)
2.527 ± 0.12 mg/L
–
Phospholipase A2 activity of T. erythrurus venom. The PLA2 activity was assayed against egg yolk suspension by the Turbidometric method and the PLA2 activity is found dose-dependent.
Dose-dependent PLA2 activity of T. erythrurus venom. (A) The comparisons of PLA2 by three different doses of venom with negative control (PBS) and positive control (10 µg N. naja venom), and (B) the area of lysis of egg yolk phospholipids by Control (1), 5 µg (2), 10 µg N. naja venom (3), 10 µg (4), and 20 µg (5) of T. erythrurus venom.
3.3 Histopathological effects of venom
The histopathological effects of T. erythrurus venom were observed across various tissues of Swiss albino mice, including the skin, skeletal muscle, lung, heart, kidney, and liver (Fig. 4). Upon subcutaneous administration of the venom, severe dermonecrosis was evident, characterized by blood vessel congestion, hemorrhage, gland and hair follicle disruption, endothelial line swelling, and epidermal strata destruction (Fig. 4B). Notably, bruising on the skin became prominent six hours post subcutaneous introduction of T. erythrurus venom. In skeletal muscle tissue, inflammatory cell infiltration and collagen fiber degeneration resulted in severe myonecrosis (Fig. 4D). Similar infiltrations were observed in pulmonary tissue, accompanied by distorted alveolar septae and hemorrhage following blood vessel congestion (Fig. 4F).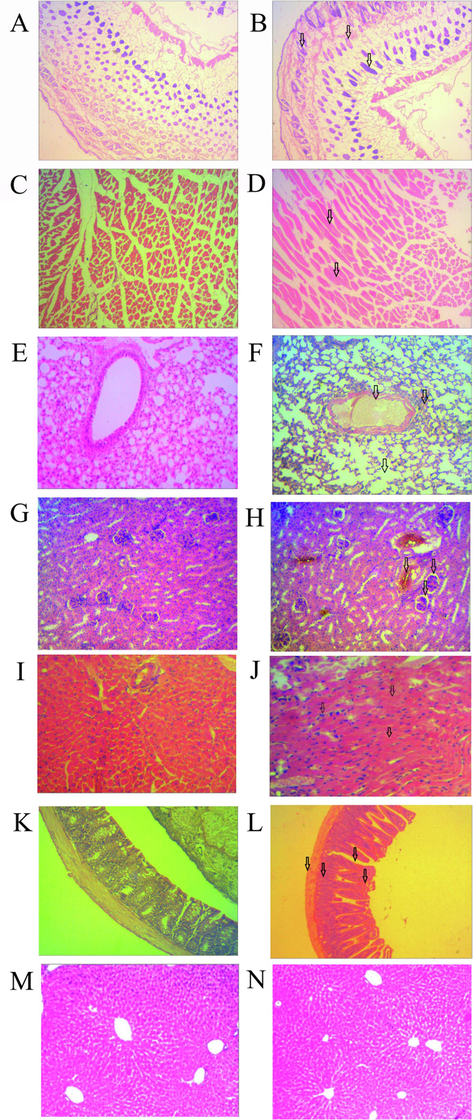
Venom-induced histopathological changes in skin, muscle, lung, kidney, heart, intestine, and liver. Mice envenomed with sublethal dose and tissues after 24 h of envenomation are shown in this figure. The skin tissues of control (A) and venom-treated (B) mice are shown. Regular structures of dermis and epidermis became significantly disordered following envenomation. Similarly, muscle, lung, kidney, heart, intestine, and liver tissues of venom-treated (panels D, F, H, J, L, and N, respectively) and control mice (panels C, E, G, I, K, and M, respectively) are different. Envenomation induced severe myonecrosis with inflammatory infiltration in muscle, hemorrhage in alveoli with congestion in blood vessels in the lung, destruction of glomerular capillaries in the kidney, massive clumping of erythrocytes with myonecrosis in the heart, and inflammatory cellular infiltration in the intestine with the irregular formation of villi. However, T. erythrurus venom did not affect the hepatic tissues. Histopathological changes in different tissues are indicated by arrows in photomicrograph (Magnification 400×).
Nephrotoxic effects were apparent, featuring shrunken glomeruli, vascular congestion, renal tubule vacuolation, renal tissue hemorrhage, and moderate inflammatory infiltration (Fig. 4H). Cardiotoxic effects were also noted in cardiac tissue, marked by blood vessel congestion, erythrocyte clumping, necrotic fibers, and severe multifocal myonecrosis (Fig. 4J). In intestinal tissue, hemorrhage, mucosal and submucosal necrosis, swelling, and villi disassociation were observed following venom exposure (Fig. 4L). However, the hepatic tissue exhibited minimal impact from T. erythrurus venom (Fig. 4N).
4 Discussion
The analysis of T. erythrurus venom composition through SDS-PAGE revealed significant quantities of Snake Venom Metalloproteases (SVMPs), Snake Venom Serine Proteases (SVSPs), and Phospholipase A2 (PLA2). Furthermore, all three sub-classes of SVMPs (P-I, P-II, and P-III) known for their hemorrhagic and coagulopathic activities (Kini and Koh, 2016) were abundant in T. erythrurus venom. In addition, SVSPs are associated with edema, hemorrhage, and coagulopathy were also present along with PLA2 (Slagboom et al., 2017) contributing to myonecrosis, inflammation, edema, pain, allodynia, mechanical hyperalgesia, hemorrhage, neurotoxicity, and coagulopathy following the envenoming viper bite (Costa et al., 2017). Additionally, small amount of L-amino acid oxidases (LAAO) are found that can contribute to the pathophysiology of edema, platelet aggregation, hemorrhage, and coagulopathy (Lazo et al., 2017). Disintegrins and myotoxins known for cytotoxicity and destruction of skeletal muscle were identified in T. erythrurus venom, which align with previous reports on its toxicological effects from Bangladesh and Myanmar (Tun-Pe et al., 2000).
The lethality of T. erythrurus venom, as determined by its Median Lethal Dose (LD50), was notably higher compared to the venom of T. erythrurus from Myanmar (LD50 1.131 versus 4.688 mg/kg), T. puniceus (1.21 mg/kg) from Indonesia, T. flavoviridis (5.066 mg/kg), T. gramineus (4.00 mg/kg), and T. venustus (3.0 mg/kg) from Thailand, T. nebularis (2.0 mg/kg) from Malaysia, T. albolabris (3.3 mg/kg) from China, and T. malabricus (20 mg/kg) from India. Furthermore, its Minimum Hemorrhagic Dose (MHD) indicated a higher hemorrhagic potential (MHD 0.21 mg/kg) than other pit vipers from southeastern Asia, namely, T. wiroti (MHD 0.59 mg/kg), T. puniceus (MHD 0.47 mg/kg), and T. nebularis (MHD 1.67 mg/kg) (Tan et al., 2019). These differences highlight the variability in venom lethality and hemorrhagic activity among inter-specific and geographically distant populations of the same species.
Histopathological examination revealed profound effects of T. erythrurus venom on vital mammalian organs including the lung, kidney, heart, and intestine along with local hemorrhage in the skin which is fully corroborated with the clinical findings of the envenoming bite of other crotalids (Efrati and Reif, 1953). Clinically, internal hemorrhage has long been considered a prime lethal symptom following Trimeresurus sp. envenoming (Reid, 1967). Furthermore, Such pathophysiological changes like disintegration of blood vessels by the hemorrhagins and metalloproteinases are also present in pit viper venom (Mehta and Sashindran, 2002). Interestingly, the edematogenic effect of T. erythrurus venom is almost fully corroborated with the similar impact of crotalid and viperid snake envenoming in human victims (Gutiérrez and Cerdas, 1984; Sarmin et al., 2013; Tan and Tan, 1988; Thein et al., 2021). The strong hemolytic activity of T. erythrurus venom is most likely due to the presence of direct hemolytic factors like PLA2 enzymes which are also observed in the venom from other Trimeresurus spp. (Soogarun et al., 2008).
Histopathological observations indicated T. erythrurus venom-induced epidermal alterations in envenomed mice. Those pathophysiological conditions in skin, altogether, are more likely to contribute to ecchymosis, vesiculation, and severe secondary infection (Suankratay et al., 2002). This venom also induced severe myonecrosis and inflammatory infiltration of cells as envenoming in the muscles of treated mice groups, very similar to the modification in skeletal muscle observed with the envenomation of pit vipers, T. malabricus (Raghavendra Gowda et al., 2006). Such severe myonecrosis may cause rhabdomyolysis (Harris and Cullen, 1990), myopathy (Williams et al., 2019), and enhance secondary and/or opportunistic infections in the victims (Suankratay et al., 2002). Histopathological alterations were noticed as a consequence of T. erythrurus venom in pulmonary tissue in this study. Similar cellular pathology in the pulmonary tissue section has been turned out as a flagrant effect of pit viper venom i.e., B. jararacussu, Crotalus durissus, and C. durissus. Such pulmonary histopathology may cause dyspnea in snakebite victims due to interruption of the gaseous exchange mechanism leading to respiratory failure, and even death.
The nephrotoxic effect of experimented venom caused shrinkage of glomeruli, vascular congestion, vacuolation of the renal tubule, and hemorrhage were observed. Pit viper venom has also been testified as nephrotoxic (Sathish et al., 2021) and causes acute renal injury to renal failure, and even may be a cause of death of early surviving snakebite victims as a long-term effect of venom (Rodrigues Sgrignolli et al., 2011). Features of cardiotoxic episodes of T. erythrurus venom were similar to consequence of other documented crotalid venom such as vascular congestion, inflammatory infiltration of cells followed by hemorrhage, and degeneration of cardiac tissues leading to focal, multifocal, or occasional myonecrosis. Such cardiac involvement is a rare manifestations of snakebite envenoming, however, arrhythmias, bradycardia, and tachycardia are the common manifestations, and even the severity of cardiotoxic venom may cause ischemic stroke and cardiac arrest (Ongprakobkul et al., 2019).
We also found that T. erythrurus venom was responsible for remarkable alteration in intestinal tissue. Although the previous report on the effect of pit viper venom on intestinal tissues was limited except for extravasation of blood and acute ulcer from Crotalus sp. Venom. Those intestinal histopathology might cause vomiting which was a clinical manifestation of T. erythrurus envenoming bite (Tun-Pe et al., 2000). Although severe hepatotoxic effects such as degeneration and inflammatory infiltration of hepatocytes prompted by pit vipers, we did not observe any significant changes in hepatic tissue induced by T. erythrurus venom.
T. erythrurus is distributed over the large geographical ranges of the some densely populated countries like Bangladesh, India, Myanmar, and Nepal (Wallach et al., 2014). Therefore, most of the snakebite incidents by T. erythrurus occur in rural areas, where treatment facilities are wanting (Tun-Pe et al., 2000). The snake is already considered one of the medically important venomous species in Bangladesh (Haidar et al., 2023). In such circumstances, above mentioned effect of T. erythrurus venom may collectively cause a direct threat to the human lives in its localities. Clinical application of an appropriate antivenom can be a remedy to recover from its venom-induced consumption coagulopathy (Maduwage and Isbister, 2014), which causes internal hemorrhage of systemic organs of the snakebite victim. However, species-specific antivenom is yet to be designed and developed for treatment of T. erythrurus envenoming. This fundamental study on T. erythrurus venom profile and toxicity indices will provide basement for species-specific antivenom designing, and subsequently will contribute to reduce snakebite induced mortality and morbidity. Moreover, the insights provided by this study on the histopathological alteration potentiality of the venom components holds promises in reducing venom-induced health risks associated with T. erythrurus envenomation in rural tropics.
5 Conclusion
The venom of T. erythrurus is a cocktail of lethal toxins that is capable to induce clinical toxicity in envenoming victims. Besides, histological alteration potentiality of the venom may cause of systematic physiological failure leading to mortality. The species is abundant in its range in south Asian countries, shows highly aggressive behavior and responsible for frequent envenoming bite in its locality. Considering all the fact, this species has been considered as medically important snake in Bangladesh. In contrast, the symptomatic medication and management is the only possible treatment against T. erythrurus envenomation due to the unavailability of any specific antivenom. Thus, the biochemical properties and toxicological features of the venom that we report here may aid future targeted designing of antivenom and may pave the way for reducing severity and mortality from T. erythrurus envenomation. Additionally, documentation on histological alteration potentiality of the venom in mammalian organs provides complementary information for clinicians that can be supportive to symptomatic treatment of the envenoming patients and clinical snakebite management.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the first author on reasonable request.
Consent for publication
Not applicable.
Authors Contribution
Conceptualization: IKAH, MAWC, MMI and MAR; Funding acquisition: IKAH, MAWC, AAS and AG; Data curation: IKAH, MM and SR; Writing - original draft: IKAH, MAWC and MM; Writing - review & editing: AAS, AG, MMI and MAR; Visualization: IKAH, MAWC and MN; Investigation: IKAH, MM, MH, MSRS, MN, MMR, AA, SR, MRI and MAU; Validation: MAWC, MMI and MAR; Formal analysis: IKAH, MAWC, MM and SR; Methodology: IKAH, MAWC, MM and MAR; Supervision: MAWC, AAS, AG, MMI and MAR; Resources: IKAH, MM, MMR, MRI, MAU, AAS, AG and MAR. All authors have read and agreed to the published version of the manuscript.
Ethics approval
The animal research protocol is based on the International Council of medical organizations (CIOMS) guidelines for animal experiments and is approved by the Institute of Biological Sciences, University of Rajshahi, Bangladesh (Ethics license number: 225/ 320 IAMEBBC/ IBSc).
Funding
This research was funded by Venom Research Centre (VRC), a project of Non-Communicable Disease Control (NCDC) under Directorate General of Health Services (DGHS) by Ministry of Health and Family Welfare, Government of the People’s Republic of Bangladesh [Grant Ref: DGHS/LD/NCDC/Proc. Plan/RPA-GOB (Service)/2019-2020/SP-03/Negotiation/1654] to collect snakes and venom, and by Research and Publication Cell, University of Chittagong, Chattogram 4331, Bangladesh [Grant Ref: 331/CU/2021/Date:23/06/2021] to design experiment, and to collect and analyze experimental data.
Acknowledgements
We are grateful to Bangladesh Forest Department for their permission and support to capture snakes for venom collection. We would like to thank Non-Communicable Disease Control (NCDC) programme of the Directorate General of Health Services (DGHS), Ministry of Health and Family Welfare, Government of the People’s Republic of Bangladesh; and Research and Publication Cell, University of Chittagong, Chatoogram for financial support. We are also thankful to Mahfuz Russel and STS Sourav for their help during snake collection.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biological and Pathological Studies of Rosmarinic Acid as an Inhibitor of Hemorrhagic Trimeresurus flavoviridis (habu) Venom. Toxins (basel).. 2010;2:2478-2489.
- [CrossRef] [Google Scholar]
- CIOMS International Guiding Principles for Biomedical Research Involving Animals. Altern. Lab Anim. 1985:12.
- [Google Scholar]
- Recommendations for euthanasia of experimental animals: Part 2. Laboratory Animals 1997
- [CrossRef] [Google Scholar]
- Costa, S.K.P., Camargo, E.A., Antunes, E., 2017. Inflammatory Action of Secretory Phospholipases A2 from Snake Venoms 35–52. 10.1007/978-94-007-6452-1_10.
- Clinical and pathological observations of sixty-five cases of viper bite in Israel. Am. J. Trop. Med. Hyg.. 1953;2:1085-1108.
- [CrossRef] [Google Scholar]
- Finney, D.J., 1971. Probit Analysis, 3rd ed. Cambridge University Press, New York. 10.1002/jps.260060094.
- Ghose, A., Faiz, A., 2014. Snake Envenomation in Bangladesh Snakebite Epidemiology in Bangladesh 1–14. 10.1007/978-94-007-6288-6.
- An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26:411-413.
- [CrossRef] [Google Scholar]
- Mechanism of action of myotoxins isolated from snake venoms. Rev. Biol. Trop.. 1984;32:213-222.
- [Google Scholar]
- Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon. 2009;54:958-975.
- [CrossRef] [Google Scholar]
- Implementation of Ecological Distribution of Venomous Snakes for Clinical Management of Snakebite in Bangladesh. J. Med.. 2023;24:139-151.
- [CrossRef] [Google Scholar]
- Muscle necrosis caused by snake venoms and toxins. Electron Microsc. Rev.. 1990;3:183-211.
- [CrossRef] [Google Scholar]
- Amphibians and reptiles of Bangladesh: A Field Guide. Dhaka: Arannayk Foundation; 2014.
- Handbook of Haye’s principles and methods of toxicology (6th ed). CRC Press: Boca Raton, NY; 2014.
- Purification, Some properties and amino-acid sequences of two phospholipases A (CM-II and CM-III) from Naja naja kaouthia Venom. Eur. J. Biochem.. 1980;112:493-499.
- [CrossRef] [Google Scholar]
- Metalloproteases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: Definition and nomenclature of interaction sites. Toxins (basel).. 2016;8
- [CrossRef] [Google Scholar]
- Studies on the quantitative method for determination of hemorrhagic activity of habu snake venom. Japanese J. Med. Sci. Biol.. 1960;13:43-51.
- [CrossRef] [Google Scholar]
- Kumkate, S., Chanhome, L., Thiangtrongjit, T., Noiphrom, J., Laoungboa, P., Khow, O., Vasaruchapong, T., Sitprija, S., Chaiyabutr, N., Reamtong, O., 2020. Venomics and cellular toxicity of Thai pit vipers (Trimeresurus macrops and T. hageni). Toxins (Basel). 12. 10.3390/toxins12010054.
- Biochemical, biological and molecular characterization of an L-Amino acid oxidase (LAAO) purified from Bothrops pictus Peruvian snake venom. Toxicon. 2017;139:74-86.
- [CrossRef] [Google Scholar]
- Current Treatment for Venom-Induced Consumption Coagulopathy Resulting from Snakebite. PLoS Negl. Trop. Dis.. 2014;8
- [CrossRef] [Google Scholar]
- The action of phospholipase A on lipoproteins. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab.. 1965;98:554-565.
- [CrossRef] [Google Scholar]
- Clinical features and management of snake bite. Med. J. Armed Forces India. 2002;58:247-249.
- [CrossRef] [Google Scholar]
- Sudden cardiac arrest and cerebral thrombosis due to bites by Russell’s viper (Daboia siamensis) Toxicol. Commun.. 2019;3:40-42.
- [CrossRef] [Google Scholar]
- Strong myotoxic activity of Trimeresurus malabaricus venom: Role of metalloproteases. Mol. Cell. Biochem.. 2006;282:147-155.
- [CrossRef] [Google Scholar]
- Defibrination By Agkistrodon Rhodostoma Venom. Anim. Toxins. 1967;323–335
- [CrossRef] [Google Scholar]
- Acute kidney injury caused by bothrops snake venom. Nephron - Clin. Pract.. 2011;119
- [CrossRef] [Google Scholar]
- Clinical Aspects of Green Pit Viper Bites in Bangladesh : A Study on 40 Patients. Asia Pacific J. Med. Toxicol.. 2013;2(3):96-100.
- [Google Scholar]
- Histopathological profile of fatal snake bite autopsy cases in a tertiary care center in South India. Egypt. J. Forensic Sci.. 2021;11
- [CrossRef] [Google Scholar]
- Variations in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Mol. Cell. Biochem.. 2002;229:93-101.
- [CrossRef] [Google Scholar]
- Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol.. 2017;177:947-959.
- [CrossRef] [Google Scholar]
- Analysis of green pit viper (Trimeresurus alborabris) venom protein by LC/MS-MS. J. Biochem. Mol. Toxicol.. 2008;22:225-229.
- [CrossRef] [Google Scholar]
- Tetanus after white-lipped green pit viper (Trimeresurus albolabris) bite. Wilderness Environ. Med.. 2002;13:256-261.
- [CrossRef] [Google Scholar]
- Takahashi, M., Omori-Satoh, T., 2002. What is the rabbit skin method by Kondo et al. (1960) for determining hemorrhagic activities of snake venoms? Toxicon 40, 1061. 10.1016/S0041-0101(01)00261-6.
- Biological properties of Trimeresurus purpureomaculatus (shore pit viper) venom and its fractions. Toxicon. 1988;26:989-996.
- [CrossRef] [Google Scholar]
- Venomics of trimeresurus (Popeia) nebularis, the cameron highlands pit viper from Malaysia: Insights into venom proteome, toxicity and neutralization of antivenom. Toxins (basel).. 2019;11
- [CrossRef] [Google Scholar]
- A study of Snake Venom Yield by different Methods of Venom Extraction. Amphib. Reptil.. 1986;7:187-191.
- [CrossRef] [Google Scholar]
- Characteristics and significance of “green snake” bites in Myanmar, especially by the pit vipers Trimeresurus albolabris and Trimeresurus erythrurus. Toxicon. 2021;203:66-73.
- [CrossRef] [Google Scholar]
- Green pit viper (Trimeresurus erythrurus) bites in Myanmar: clinical features, venom antigen levels and development of natural venom antibodies. Mhsrj. 2000;12:1-6.
- [Google Scholar]
- Edema-inducing activity of phospholipase A2 purified from human synovial fluid and inhibition by aristolochic acid. Inflammation. 1988;12:549-561.
- [CrossRef] [Google Scholar]
- Wallach, V., Williams, K.L., Boundy, J., 2014. Snakes of the world: a catalogue of living and extinct species, CRC Press. Taylor and Francis Group, Boca Raton, Florida. 10.5860/choice.52-0600.
- Mechanisms underpinning the permanent muscle damage induced by snake venom metalloprotease. Toxicon. 2019;159:S13-S14.
- [CrossRef] [Google Scholar]
- Evaluation of the cross-neutralization capacity of Thai green pit viper antivenom against venom of Myanmar green pit viper. Toxicon. 2020;177:41-45.
- [CrossRef] [Google Scholar]







