Translate this page into:
Therapeutic potential of kaempferide against paraquat instigated cardiac toxicity in rats
⁎Corresponding author. asmaashraf@gcuf.edu.pk (Asma Ashraf)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Paraquat (PQ) is an effective herbicide however, due to its adverse effects on different organs including heart, it is considered as highly toxic to human beings. Kaempferide (KMF) is a plant-based flavonoid with conspicuous pharmacological properties. This experiment was executed to evaluate the palliative actions of KMF on PQ prompted cardiac toxicity in rats. Twenty-four rats were apportioned into 4 equal groups which were designated as control, toxicant (PQ treated), Co-treated (PQ + KMF) and KMF group. After 30 days of treatment, PQ intoxication resulted in a remarkable reduction in antioxidant enzymes activities which include, glutathione reductase (GSH), glutathione S-transferase (GST), catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD), and glutathione disulfide reductase (GSR), whereas an elevation was observed in reactive oxygen species (ROS), & malondialdehyde (MDA) as well as hydrogen peroxide (H2O2) level. Furthermore, concentrations of cardiac injury markers, creatinine phosphokinase (CPK), creatine kinase-myoglobin binding (CK-MB), & lactate dehydrogenase (LDH), as well as troponin I were increased in response to PQ treatment. Moreover, inflammatory cytokines such as tumour necrosis factor alpha (TNF-α), nuclear factor-kappa B (NF-κB), and interleukin-6 (IL-6), interleukin-1 beta (lL-1β), and cyclooxygenase-2 (COX-2) levels were augmented in PQ intoxicated group. PQ exposure reduced the gene expression of cardiac anti-apoptotic markers (Bcl-2), but the gene expression of apoptotic marker (caspase-9, caspase-3 and Bax) was increased. Histopathological damages were also observed in toxicant (PQ) exposed group. However, the administration of KMF significantly palliated PQ induced aforementioned disruptions. In the light of these findings, it is concluded that KMF is a promising bioactive compound that may be used as a curative agent against PQ instigated cardiac damage due to its marvelous antioxidant, anti-apoptotic, anti-inflammatory as well as cardioprotective potential.
Keywords
Kaempferide
Paraquat toxicity
Cardiac injury
Oxidative stress
Inflammation
1 Introduction
Paraquat is a nitrogen-based herbicide which is used to control the progression of different herbs by interrupting electron transport chain during the process of photosynthesis (Qian et al., 2009). According to Dinis-Oliveira et al. (2008), In poorly developed countries such as Asia, the intoxication of PQ considered as a major health threat to living organisms. The rate of fatalities due to intoxication of paraquat depends upon the duration of exposure and route (ingestion, intravenous, intraperitoneally) of exposure, for instance, PQ exposure through skin and mouth for 3.5 h can cause immediate death (Nikdad et al., 2020). Animals as well as humans are highly susceptible to both acute and chronic PQ intoxication.
Primary signs of PQ intoxication include respiratory failure, cardiac damages, hepato-renal injuries, adrenal gland malfunctioning, spleen inflammation as well as dysfunctioning of immune system (Dinis-Oliveira et al., 2008). It is documented that reactive oxygen species (ROS) are major culprit behind organ toxicities instigated by PQ. ROS are byproducts of cellular metabolism and responsible for proper modulation of cell signaling, cell survival, inflammatory responses as well as cell differentiation. However, excessive generation of free radical without proper control can result in serious injuries (Zeinvand-Lorestani et al., 2015). It is reported that paraquat produces large amounts of ROS which ultimately elevates level of oxidative stress and causes cellular damages (McCormack et al., 2005).
PQ consumption results in rapid systemic absorption which damages multiple organs (Onyon and Volans, 1987). Cardiovascular damage is one of the overt complications of PQ poisoning. Abovementioned complications lead toward various cardiogenic events such as arrhythmias, heart failure, and cardiac arrest (Fukushima et al., 2010). Numerous investigations documented that PQ intoxication surge up the level of inflammatory markers as well as ROS generation (Lin et al., 2021). Another study delineated that PQ prompted cardiac damages by intensifying vascular permeability which reduces the fractional shortening and cardiac remodeling in animals (Ahmad et al., 2021).
Flavonoids are known for their distinct ability to function as antioxidant as well as wide range of other pharmacological activities. KMF (methoxy flavone) is an emerging plant-based flavonoid which is extracted from the roots of Alpina officinarum, commonly known as lesser galangal (Matsuda et al., 2009). KMF exhibits excellent antioxidant properties and has been found to manifest other as well as pharmacological activities such as anti-inflammatory, anti-cancerous & antihypertensive (Maruyama et al., 2009). The current research was executed to assess the cardioprotective abilities of KMF against PQ instigated cardiac injury.
2 Materials and methods
2.1 Chemicals
Both PQ and KMF were procured from Sigma-Aldrich (Merch, Germany).
2.2 Animals
Twenty-four albino rats aged from 10 to 12 weeks having weight between 180 and 200 g were placed at the research station of University of Agriculture, Faisalabad. A light/dark cycle of 12 h was strictly maintained. Prior to the trials animals were underwent to acclimatization to the laboratory conditions for one week and provided them food pellets as well as tap water while temperature was adjusted at 20–26 °C. The animals were handled as per the guidelines of European Union of animal care and experimentation approved (CEE Council 86/ 609).
2.3 Experimental layout
Animals were apportioned into equal sized groups having six rats in each. All the groups were assigned treatment as follow: Group 1st was termed as untreated group. Group 2nd was provided with (5 mg/kg) of PQ. Group 3rd was given with combine treatment of PQ (5 mg/ kg) + KMF (20 mg/ kg) while the group 4th was given with KMF (20 mg/ kg) only for 30 days with the help of an oral gavage. On the 30th day of trial, rats were made unconscious with the help of ketamine (60 mg/kg) + xylazine (6 mg/kg) then decapitated and blood was collected in blood sample vial for further analysis. Single piece of heart was fixed in a buffer solution of 10 % formalin for histopathological evaluations while the other piece was kept in a zip-lock bag and freeze at −20 °C to access various biochemical parameters.
2.4 Evaluations of antioxidants enzymes
The protocol described by Chance and Maehly (1955) was utilized to assess the CAT activity. Biochemical activity of SOD was measured by using strategies described by Kakkar et al., (1984). Sedlak and Lindsay (1968) technique was employed to determine GSH activity. The GSR activity was ascertained by using the protocol elaborated by Carlberg and Mannervik (1975). The methodology of Couri and Abdel-Rahman (1979) was used for the analysis of GST activity. The protocol elaborated by Lawrence and Burk (1976) was used to evaluate GPx activity.
2.5 Evaluation of oxidative stress
Ohkawa et al. (1979) method was employed to determined MDA level. A technique described by Hayashi et al. (2007) was utilized to assess the level of ROS·H2O2 concentration was evaluated using Pick and Keisari's technique (1981).
2.6 Evaluation of cardiac injury markers
Bais and Philcox (1994) strategy was employed to assess the activity of lactate dehydrogenase (LDH) was measured in serum. With the help of fully automated analyzer Cobas-Integra 400, the activity of CPK and CK-MB was determined through enzymatic assay as well as immune inhibition assay respectively (Tietz et al., 1983). The protocol elaborated by Panteghini et al. (2004) was employed to evaluate the activity of troponin I.
2.7 Inflammatory markers analysis
The inflammatory markers level such as IL-6, NF-ƙB, TNF-α and IL-1β as well as COX-2 activity were assessed using rat ELISA kits (Shanghai-Biotech. Ltd).
2.8 Assessment of apoptotic proteins
The standard ELISA kits were used to assess the level of caspase-9, Bcl-2, caspase-3, and Bax according to the manufacturer’s instruction.
2.9 Histopathological observation
The cardiac tissues were fixed in 10 % formaldehyde following the process of dehydration with different alcoholic reagents. A rotatory microtome was used to cut the dehydrated pieces into 4–5 μm. The slices were stained using hematoxylin-eosin stain and then observed under light microscope while the photographs were captured with the help of MoticTM megapixel camera.
2.10 Statistical analysis
The findings of the current investigation were displayed in tables as Means ± SEM by using ONE way ANOVA. Minitab (Version 17) software was used to compare the treatments by applying Tukey’s test p < 0.05 was adjusted as the level of significance.
3 Results
3.1 Effect of PQ and KMF on antioxidant enzymes
PQ exposure considerably (p < 0.05) suppressed the activities of GST, GSR, CAT, GPx, SOD, & level of GSH when juxtaposed with untreated group. However, KMF treatment exhibited prominent (p < 0.05) escalation in enzymatic antioxidant capacity and GSH level in PQ + KMF treated group in contrast with PQ-intoxicated group, which shows the antioxidant ability of KMF. However, no remarkable change was noticed in the antioxidant enzymes activity in KMF (only) as well as untreated group (Table 1). Values displaying distinct letters (superscripts) differ from remaining groups.
Groups
CAT (U/mg protein)
GPx (μmole)
SOD (nmoL)
GSR (Nm NADPH oxidized/min/mg tissue
GST (mg/dL)
GSH (nM /min/mg protein)
Control
10.62 ± 0.43a
25.71 ± 0.82a
7.83 ± 0.06a
7.05 ± 0.11a
31.71 ± 0.89a
17.47 ± 0.19a
PQ
4.84 ± 0.07c
7.81 ± 0.16c
2.93 ± 0.11c
2.64 ± 0.05c
10.55 ± 0.43c
4.36 ± 0.38b
PQ + KMF
7.52 ± 0.09b
15.98 ± 1.02b
6.45 ± 0.16b
6.26 ± 0.09b
25.52 ± 0.64b
12.33 ± 0.65c
KMF
10.67 ± 0.42a
25.82 ± 0.87a
7.85 ± 0.07a
7.08 ± 0.12a
32.59 ± 0.79a
17.68 ± 0.32a
3.2 Effect of PQ and KMF on oxidative stress markers
The intoxication of PQ led to a considerable (p < 0.05) rise in H2O2, ROS & MDA levels when compared with untreated group. This indicates the role of PQ in the elevation of oxidative stress. Nevertheless, when rats were co-treated (PQ + KMF), there was a remarkable (p < 0.05) reduction in oxidative stress markers levels when compared with the values of group intoxicated with Paraquat alone. Furthermore, mean values of oxidative stress markers are nearly equal in both KMF (only) and untreated group (Table 2). Values displaying distinct letters (superscripts) differ from remaining groups.
Groups
ROS (μmoL/g)
MDA (nmoL/g)
H2O2 (mg/dL)
Control
0.33 ± 0.07c
0.66 ± 0.06c
1.72 ± 0.06c
PQ
8.21 ± 0.28a
6.59 ± 0.14a
6.98 ± 0.12a
PQ + KMF
2.82 ± 0.08b
1.92 ± 0.05b
2.37 ± 0.10b
KMF
0.32 ± 0.07c
0.64 ± 0.06c
1.69 ± 0.06c
3.3 Effect of PQ and KMF on cardiac injury markers
PQ exposure remarkably (p < 0.05) upregulated the level of cardiac injury markers (CPK, LDH, CK-MB & troponin I). However, Co-treatment (PQ + KMF) markedly (p < 0.05) lowered the concentration of aforementioned myocardial damage markers in the blood as compared to PQ treated group. Moreover, the concentrations of these markers in only KMF administrated rats were close to the values of untreated group (Table 3). Values displaying distinct letters (superscripts) differ from remaining groups.
Groups
LDH (mg/dL)
CPK (mcg/L)
CK-MB (ng/mL)
Troponin I (pg/mL)
Control
16.22 ± 1.23c
186.49 ± 2.54c
31.92 ± 0.82c
0.39 ± 0.04c
PQ
45.55 ± 1.26a
445.71 ± 5.60a
84.35 ± 1.87a
3.02 ± 0.06a
PQ + KMF
25.91 ± 1.63b
255.35 ± 5.32b
44.34 ± 1.93b
1.16 ± 0.03b
KMF
16.17 ± 1.24c
184.41 ± 2.64c
31.56 ± 0.79c
0.37 ± 0.04c
3.4 Effect of KMF against PQ on inflammatory markers
The supplementation of PQ resulted in a remarkable (p < 0.05) rise in IL-6, TNF-α, NF-κB & IL-1β as well as activity of COX-2 when matched with control animals. This signifies that PQ performs an integral function in unleashing inflammatory damage. In contrast, the treatment with (PQ + KMF) reduced inflammatory markers levels compared to the group treated with Paraquat alone, demonstrating the anti-inflammatory properties of KMF. Moreover, the group treated with KMF (only) and control showed approximately the same values (Table 4). Values displaying distinct letters (superscripts) differ from remaining groups.
Groups
NF-κB (ng/g)
IL-1β (ng/g)
TNF-α (ng/g)
COX-2 (ng/g)
IL-16 (ng/g)
Control
14.88 ± 0.96c
22.54 ± 0.93c
8.30 ± 0.70c
17.48 ± 1.22c
6.39 ± 0.64c
PQ
87.10 ± 1.44a
82.82 ± 0.89a
25.63 ± 1.56a
83.70 ± 1.31a
37.48 ± 0.76a
PQ + KMF
27.96 ± 1.81b
38.03 ± 1.74b
12.62 ± 0.87b
35.47 ± 1.63b
13.92 ± 0.78b
KMF
14.74 ± 0.97c
22.45 ± 0.90c
8.12 ± 0.58c
17.14 ± 0.99c
6.34 ± 0.63c
3.5 Effect of KMF against PQ on apoptotic markers
The intoxication of paraquat consequences in substantial (p < 0.05) rise in the levels of Bax, caspase-3, as well as caspase-9 while reduced the levels of bcl-2 when matched to the control rats. Nonetheless, treatment with PQ and KMF significantly reduced the level of caspase-9, Bax & caspase-3 & escalated the Bcl-2 level in contrast to the PQ-administrated rat, which reveals the anti-apoptotic potential of KMF. Nevertheless, the levels of apoptotic markers were similar in KMF supplemented and untreated group (Table 5). Values displaying distinct letters (superscripts) differ from remaining groups.
Groups
Caspase 3(pg/mL)
Bax (pg/mL)
Bcl-2 (ng/mL)
Caspase-9 (pg/mL)
Control
1.83 ± 0.05c
1.39 ± 0.09c
15.65 ± 0.55a
2.37 ± 0.08c
PQ
11.59 ± 0.28a
8.31 ± 0.12a
3.79 ± 0.17c
15.70 ± 1.19a
PQ + KMF
3.27 ± 0.15b
2.67 ± 0.17b
10.41 ± 0.70b
5.71 ± 0.29b
KMF
1.82 ± 0.05c
1.37 ± 0.09c
15.57 ± 0.58a
2.34 ± 0.08c
3.6 Effect of PQ and KMF on histopathological parameters
PQ intoxication resulted in inflammation, oedema as well as degeneration in the structure of myofibrils when juxtaposed with untreated group. Histopathology of cardiac tissues shows normal architecture of cardiac tissues in co-treated group in contrast to PQ-intoxicated rats. Moreover, no discrepancy was observed between the group treated with KMF and the untreated group (Fig. 1).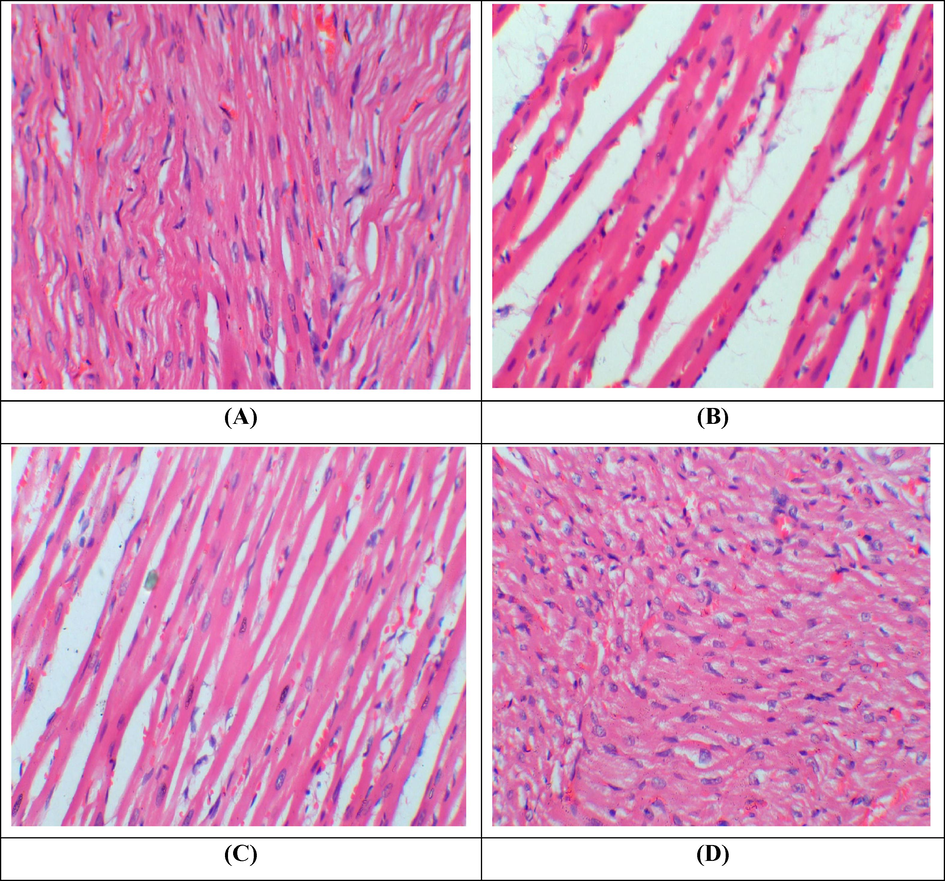
Histopathological examination of cardiac tissues. H&E stain; 40X (A) Group I; Normal showed normal architecture of cardiac muscles (B) Paraquat treated group; Showed cardiac interstitial edema, inflammation and fibrosis (C) Paraquat + KMF group: Co-administrated group; showed a significant recovery as compared to paraquat treated group (D) KMF administrated group; Demonstrated normal architecture of cardiac tissues.
4 Discussion
Antioxidant enzymes (GPx, CAT, SOD, GSH, GST,) are referred to as the first layer of defense against free radicals. GSH (non-enzymatic antioxidant) primarily protects the proteins which have sulfhydryl (-SH) group from oxidative stress produced by free radicals (Khan et al., 2018). The potential activity of CAT is crucial to cease the transformation of H2O2 into OH- (Vicente-Sánchez et al., 2008) while the activity of SOD prevents the accumulation of hydroxyl ions via cleavage of H2O2 into O2 (Kheradmand et al., 2010). GST primarily targets the oxidative stress by scavenging free radicals (Hayes et al., 2005). Recent investigations elucidated that paraquat exposure upregulated the levels of H2O2which ultimately increase the levels of oxidative stress (Men et al., 2022). Talas et al. (2014) elaborated that exaggerated production of ROS upsurge the oxidative stress levels which eventually escalates the MDA levels. In the current investigation, administration of PQ depreciated the level of antioxidant enzymes while upsurged the level of ROS, H2O2 & MDA. However, Co-treatment (PQ + KMF) escalated the antioxidant enzymes activities while down scaled ROS, H2O2 and MDA levels. The findings of our investigations are compatible with the study of Tang et al. (2021) who elucidated KMF demonstrated antioxidant activity by down regulating the level of oxidative stress in obese rats. Berroukeche et al. (2022) elaborated that natural compounds could scavenge reactive oxygen species which is attributed to the presence of their polyphenolic structural configuration. Therefore, ROS quenching ability of KMF may be attributed to the presence of multiple hydroxyls and conjugated double bond in its structure.
Different cardiac injury markers such as CPK, troponin I, cardiac LDH as well as CK-MB are released in response to cardiac muscle damage. CK-MB is used as diagnostic test marker during various sorts of coronary disorders such as acute myocardial infraction (Christenson et al., 1997). Furthermore, magnitude of these markers in the blood reflects the intensity of myocardia injury (Ibrahim and Abdel-Daim, 2015). In our investigation, the level of CPK, troponin I, cardiac LDH and CK-MB were increased in response to PQ treatment. However, the Co-treated group showed remarkable reduction in the levels of aforementioned markers of cardiac injury. These findings demonstrated the cardioprotective potential of KMF via regulating the markers of cardiac injury.
Oxidative stress is responsible for the activation of NF-κB which mediates the activation of TNF-α, IL-6, & IL-1β as well as COX-2 as elucidated by Wang et al. (2018). NF-κB regulates various phases of inflammation via eliciting the levels of abovementioned cytokines. COX-2 is a potent cytokine which evokes different cascade of inflammation (Lee et al., 2004). Our findings revealed that PQ administration elevated the level of inflammatory markers while KMF supplementation ameliorated inflammation via downregulating the level of inflammatory cytokines.
Apoptotic proteins (caspase-3, Bcl-2 & caspase-9 as well as Bax) play crucial roles during the process of apoptosis (Lopez-Neblina et al., 2005). Caspase-3 is considered as the central regulator of apoptosis which is activated by a series of transduction signals between Bcl-2 and Bax. Dong et al. (2003) documented that activation of caspase-9 is regulated by apoptotic pathway which is induced by the ratio of Bcl-2/Bax. Bcl-2 exerts significant effects during the regulation of myocadiac apoptosis by impeding the liberation of cytochrome c from mitochondrial membrane as well as reducing the activity of caspases (Cook et al., 1999). In the current study, PQ administration escalated caspase-9, caspase-3 and Bax whereas lowered the Bcl-2 level. However, supplementation of KMF abrogated the effects of PQ by reducing the level of pro-apoptotic markers as well as escalating the anti-apoptotic markers. These results evinced the anti-apoptotic abilities of KMF against paraquat induced apoptosis.
Normal cardiac histology is necessary for maintaining the structural as well as functional integrity of cardiac tissues. However, administration of PQ disrupted the normal histology of cardiac tissues. These disruptions include myocardial damage, inflammation, fibrosis as well as alterations in cellular morphology. These alterations impair myocardial perfusion and cardiac contractility which ultimately leads toward the onset of various cardiovascular diseases. PQ is reported to damage various organs, particularly myocardial injury by modulating oxidative stress which ultimately caused heart failures (Vinciguerra et al., 2012). It is reported that escalated levels of oxidative stress are major factors underlying various histopathological impairments (Rehman et al., 2021). Our analysis revealed that KMF is a potential candidate which restored aforementioned cardiac histopathological damages instigated by PQ.
5 Conclusion
Our investigation revealed that KMF exhibited marvelous palliative action against PQ induced cardiac damage. Results of the present study elucidated that KMF abrogated PQ prompted dysregulation via escalating level of antioxidant enzymes, cardiac function markers, apoptotic as well as inflammatory markers. Furthermore, the administration of KMF restored histopathological damage induced by PQ. The cardioprotective tendency of KMF may be because of its anti-inflammatory, antioxidative as well as anti-apoptotic activities. However, further clinical trials are indispensable to evaluate the efficacy and safety of KMF against cardiac disorders in humans.
Acknowledgments
This work was funded by Researchers Supporting Project number (RSP2023R191), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dilated cardiomyopathy associated with paraquat herbicide poisoning. Clin. Pract.. 2021;11:679-686.
- [Google Scholar]
- IFCC methods for the measurement of catalytic concentration of enzymes. Part 8. IFCC methods for lactate dehydrogenase (L-lactate: NAD oxidoreductase, EC 1.1.1.27) J. Autom. Chem.. 1994;16:167-182.
- [Google Scholar]
- Investigation of antioxidant and anti-hemolytic properties of Algerian Bunium incrassatum tubers and their effects as diet on histological and biochemical parameters of normal Wistar rats. Asian J. Agric. Biol 2021
- [CrossRef] [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [Google Scholar]
- Cardiac markers in the assessment of acute coronary syndromes. Md. Med. J.. 1997;1985:18-24.
- [Google Scholar]
- Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ. Res.. 1999;85:940-949.
- [Google Scholar]
- Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol.. 1979;3:451-460.
- [Google Scholar]
- Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol.. 2008;38:13-71.
- [Google Scholar]
- Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res.. 2003;13:385-391.
- [Google Scholar]
- Out-of-hospital cardiac arrest caused by acute intoxication. Chudoku Kenkyu. 2010;23:41-46.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. - Genet. Toxicol. Environ. Mutagen.. 2007;631:55-61.
- [Google Scholar]
- Modulating effects of spirulina platensis against tilmicosin-induced cardiotoxicity in mice. Cell J.. 2015;17:137-144.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Raspberry ketone protects against isoproterenol-induced myocardial infarction in rats. Life Sci.. 2018;194:205-212.
- [Google Scholar]
- Ghrelin promotes antioxidant enzyme activity and reduces lipid peroxidation in the rat ovary. Regul. Pept.. 2010;162:84-89.
- [Google Scholar]
- Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun.. 1976;71:952-958.
- [Google Scholar]
- Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci.. 2004;19:3375-3381.
- [Google Scholar]
- Hemodynamic and electromechanical effects of paraquat in rat heart. PLoS One. 2021;16:1-19.
- [Google Scholar]
- Molecular biology of apoptosis in ischemia and reperfusion. J. Invest. Surg.. 2005;18:335-350.
- [Google Scholar]
- Antihypertensive effects of flavonoids isolated from brazilian green propolis in spontaneously hypertensive rats. Biol. Pharm. Bull.. 2009;32:1244-1250.
- [Google Scholar]
- Melanogenesis inhibitors from the rhizomes of Alpinia officinarum in B16 melanoma cells. Bioorg. Med. Chem.. 2009;17:6048-6053.
- [Google Scholar]
- Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. J. Neurochem.. 2005;93:1030-1037.
- [Google Scholar]
- Phytochemical constituents and antioxidant activity of some medicinal plants collected from the Mekong Delta, Vietnam. Asian J. Agric. Biol.. 2022;202105230
- [CrossRef] [Google Scholar]
- Antioxidative effects of nano-curcumin on liver mitochondria function in paraquat-induced oxidative stress. Res. Mol. Med.. 2020;8:37-42.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- The epidemiology and prevention of paraquat poisoning. Hum. Toxicol.. 1987;6:19-29.
- [Google Scholar]
- Standardization of immunoassays for measurement of myoglobin in serum. Clinica Chimica Acta. 2004;341:65-72.
- [Google Scholar]
- Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages induction by multiple nonphagocytic stimuli. Cell. Immunol.. 1981;59:301-318.
- [Google Scholar]
- Inhibitory effects of paraquat on photosynthesis and the response to oxidative stress in Chlorella vulgaris. Ecotoxicol.. 2009;18:537-543.
- [Google Scholar]
- Exposure to heavy metals causes histopathological changes and alters antioxidant enzymes in freshwater fish (Oreochromis niloticus) Asian J. Agric. Biol. 2021
- [CrossRef] [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- Role of propolis on biochemical parameters in kidney and heart tissues against L-NAME induced oxidative injury in rats. Clin. Exp. Hypertens.. 2014;36:492-496.
- [Google Scholar]
- Kaempferide improves oxidative stress and inflammation by inhibiting the TLR4/IκBα/NF-κB pathway in obese mice. Iran. J. Basic Med. Sci.. 2021;24:493-498.
- [Google Scholar]
- IFCC methods for the measurement of catalytic concentration of enzymes. Part 5. IFCC method for alkaline phosphatase (orthophosphoric-monoester phosphohydrolase, alkaline optimum, EC 3.1 3.1). IFCC Document Stage 2, Draft 1, 1983-03 with a view to an IFCC Recommendation. Clin. Chim. Acta. 1983;135:339F.
- [Google Scholar]
- Effect of the flavonoid quercetin on cadmiuminduced hepatotoxicity. Food Chem. Toxicol.. 2008;46:2279-2287.
- [Google Scholar]
- mIGF-1/JNK1/SirT1 signaling confers protection against oxidative stress in the heart. Aging Cell. 2012;11:139-149.
- [Google Scholar]
- Alleviation of cadmium-induced oxidative stress by trehalose via inhibiting the Nrf2-Keap1 signaling pathway in primary rat proximal tubular cells. J. Biochem. Mol. Toxicol.. 2018;32:e22011.
- [Google Scholar]
- Comparative study of in vitro prooxidative properties and genotoxicity induced by aflatoxin B1 and its laccase-mediated detoxification products. Chemosphere. 2015;135:1-6.
- [Google Scholar]







