Translate this page into:
Therapeutic potential of Chemogenic Iron oxide Nanoparticles, its antioxidant and anti-Inflammatory effects with reduction of ankle joint swelling in BALB/c mice
⁎Corresponding author at: Shifa College of Medical Technology, Shifa Tameer-e-Millat University, Islamabad, Pakistan. mubin.scmt@stmu.edu.pk (Mubin Mustafa Kiyani),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background: Gout is a form of inflammatory arthritis caused by excessive deposition of uric acid in the body and joints. Prolonged administration of uric acid lowering drugs have multiple side effects. Objectives: To investigate the antioxidant and anti-gout properties of iron oxide nanoparticles in hyperuricemic gouty mice. Methodology: Sol-Gel precipitation technique was used for chemogenesis of Iron oxide nanoparticles by using Ferrous Chloride (FeCl2) and Ferric chloride (FeCl3). Characterization was carried out by X-ray diffraction, SQUID magnetization, scanning electron microscopy(SEM) and energy-dispersive X-ray spectroscopy(EDX). Monosodium Urate crystals (MSUC) induced gout into the ankle joint of the mice. Iron oxide nanoparticles were orally administrated to the mice. To study the impact of Iron oxide nanoparticles biochemical analysis like total protein, antioxidant activity, and ankle diameter were conducted. Results: Results showed that oral administration of iron oxide nanoparticles had a beneficiary impact on gouty mice. The antioxidant activity were significantly improved by increasing levels of thiobarbituric acid reactive substance and reactive oxygen species in treated group with 250 mg/kg of body weight of iron oxide nanoparticles. Ankle diameter and levels of total protein were also reduced in treated groups. Conclusion: Current research indicates that Iron oxide nanoparticles have antioxidant as well as anti-gout effects in gouty mice. These results provide credence to the possibility of using Iron oxide nanoparticles as a gout alternative treatment. The potential therapeutic uses of iron oxide nanoparticles in human patients require more investigation.
Keywords
Antioxidants
Allopurinol
Iron oxide nanoparticles
Gout
Inflammation
Mice
Data availability
Data will be made available on request.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
1 Introduction
Nanoparticles are extraordinary small size particles with size ranges between 1 and 100 nm either in two or three dimensional orientation (Oborny, 2007). This unique nature of the nanoparticles are incredibly useful in drug delivery (Mudshinge, Deore, Patil, & Bhalgat, 2011). Having said of their nano size, they can easily approaches the targeted location within the human body and bypass the biological barriers. Nanoparticles can be engineered for transportation and to prevent the degradation of drugs and therapeutic substances and it also allow slow and control release of these loaded substances. Moreover the surface of nanoparticles can also be modified for target drug delivery particularly to tissues and cells. The improved and enhance target drug delivery lowers the risk of drugs side effects (A. Kumar & Thakur, 2022).
Iron Oxide nanoparticles consisting of large variety of polymorphs in nanomaterials with unique physiochemical characteristics. They may have 4 standardize principal polymorphic forms, hematite (α-Fe2O3), β-Fe2O3, maghemite (γ-Fe2O3) and ε-Fe2O3; similarly the iron (III) oxy-hydroxide also comprises four polymorphic forms which are recognize as goethite, akaganeite, lepidocrocite and feroxyhyte as α, β, γ and δ-FeOOH respectively. Interestingly, α-Fe2O3 is a well-known n-type semiconductor having bandgap Eg = 2.1 eV, prominent for an excellent chemical stability and reduce toxicity under normal existing conditions. Out of theses mentioned oxides, α-FeOOH and α-Fe2O3 have been popular for magnetic devices, catalysts, gas sensors, effective UV-blocking (Havenga et al., 2022) and photoelectrodes applications (Matinise et al., 2018). Moreover the green synthesized iron oxide nanoparticles has gain popularity due to its ecological friendly approach and improved the biocompatiability (Hassan et al., 2018; Khalil et al., 2017; Köçkar, Karaagac, Özel, & Materials, 2019), antimicrobial activity (Aisida et al., 2020; Madubuonu et al., 2020).
Gout, a painful joint inflammation, affects approximately 1% of the population, predominantly males (Wu et al., 2022). It occurs because monosodium urate crystals (MSUC) are gathered in the joints and surrounding tissues. Gout patients often exhibit hyperuricemia, characterized by raised uric acid levels in the blood (>70 lg/ml). Acute gout attacks can be triggered by factors like injury (Abhishek, Valdes, Jenkins, Zhang, & Doherty, 2017), exposure to cold, high food intake, and obesity (Tan, Farrar, Gaucher, Miner, & evolution, 2016). Uric acid is a necessary intermediate in the breakdown of purines, and its serum levels can rise due to various factors, including genetic factors and lifestyle choices. In most organisms, uric acid is transformed into allantoin, a soluble material, by the enzyme uricase. However, in primates, including humans, uricase activity is diminished due to gene mutation (Parthasarathy, Vivekanandan, & systems, 2018), resulting in the accumulation of uric acid in the body. Uric acid has the lowest solubility among purine byproducts, and its crystallization into MSUC is a critical factor in uric acid-mediated inflammation seen in gout. The inflammatory response stimulated by MSUC can lead to the development of tophi, which accumulates urate crystals in the tissues and joints (Kosugi, Nakagawa, Kamath, & Johnson, 2009). Some studies have evident that raised levels of soluble uric acid may be linked with conditions such as hypertension and certain cardiovascular diseases (S. Kumar et al., 2019).
The broad spectrum utilization of iron oxide nanoparticles within different applied scientific fields (O Karaagac, Hasirci, & Köçkar, 2024), such as controlled drug delivery systems, enzyme immobilization, and medical diagnostics, is more interesting area of research. Iron oxide nanoparticles demonstrate remarkable qualities in medicine with their exceptional characteristics like high surface area, magnetic susceptibility, and biocompatibility (Bhatia, Bhatia, & algae, 2016). The combine impact of nanotechnology and drug delivery system holds great prospective for improving the cure of enormous diseases, including those related to bone health and uric acid metabolism (Yang & Webster, 2009). Ongoing research and developmental efforts in this field have potential to uncover cutting-edge and unique therapeutics that can improve patient outcomes and quality of life (Oznur Karaagac, Kockar, Tanrisever, & magnetism, 2011; Oznur Karaagac, Kockar, & magnetism, 2012).
In this current study, researchers have synthesized iron nanoparticles to investigate their effects on the reduction of ankle diameter, antioxidant and anti-gout properties in a gouty mice model.
2 Material and methods
2.1 Synthesis of Iron oxide nanoparticles
The iron oxide nanoparticles was obtained using the Sol-Gel precipitation method. A 1:2 ratio of the two iron salts FeCl2 and FeCl3 were dissolved in continuous stirring 50 ml of deionized water and then 50 ml of 8.5 M NaOH solution were added drop by drop. When precipitates were formed then the resulting mixture were undergone the filtration process, the precipitates obtained were then washed multiple time with deionized water. After the complete washing the precipitate is then subjected to calcination at 100°C for overnight which was the main step for iron oxide nanoparticles formation. Powder form Iron oxide nanoparticles were obtained after overnight calcination which were grinded using a mortar and pestle. This whole process of Iron oxide nanoparticles synthesis is represented in Fig. 1 with slight modifications of previously conducted study by Karaagac and Kockar (Oznur Karaagac et al., 2012).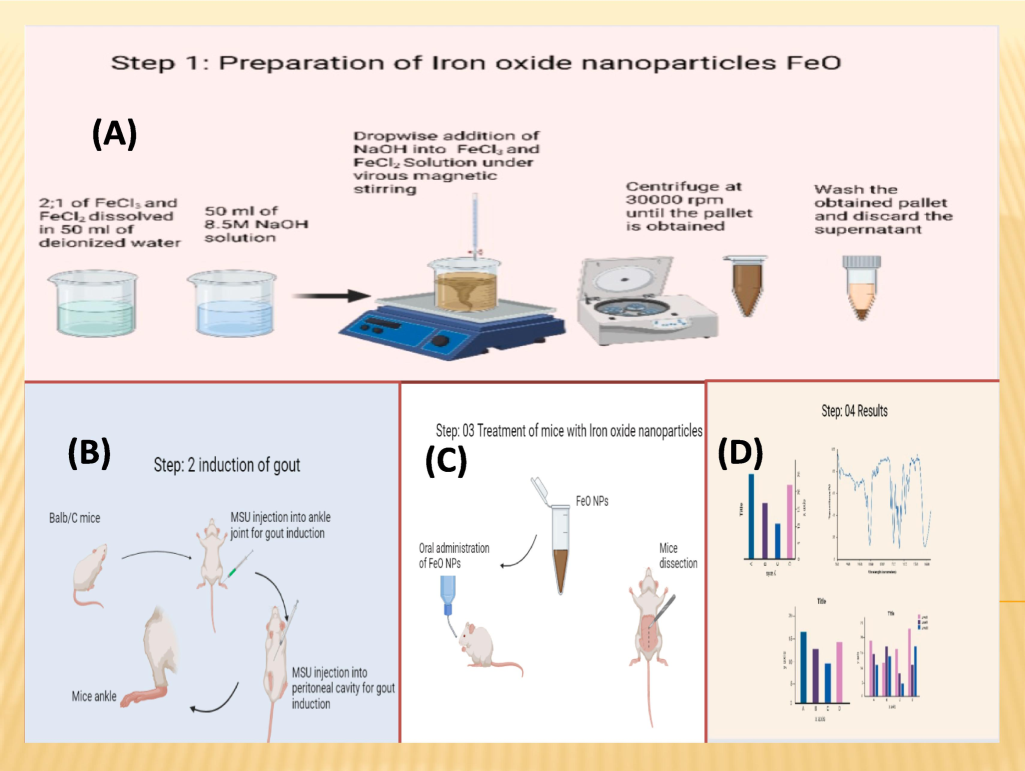
Graphical abstract; (A) Synthesis and characterization of iron oxide nanoparticles, (B) Induction of gout, (C) Treatment of mice with iron oxide nanoparticles, (D) Results.
2.2 Characterization of nanoparticles
Multiple analytical methods were applied for characterization of synthesized iron oxide nanoparticles. These methods includes X-ray diffraction (XRD) analysis which was carried out by using AXS D8 device. Whereas the surface structural morphology was examined by using the scanning electron microscopy (SEM) made up of Hitachi S-4800 device. While the elemental analysis of the nanoparticles was analyzed by energy-dispersive X-ray spectroscopy (EDS) Carried on a Horiba 6853-H system. The magnetic properties of the prepared iron oxide nanoparticle sample were measured for nanoscale particles using a superconducting quantum interference device (SQUID) magnetometer, MPMS-XL from Quantum Design Inc USA. The measurements were taken across the magnetic field, negative 20,000 Oe to a positive 20,000 Oe at room temperature, 300k and 4k to obtain the values of saturation magnetization (Ms) and coercivity (Hc) (Oznur Karaagac, Köçkar, & Materials, 2022).
2.3 In Vivo studies
The animal model experiment was carried out after the authorization of the protocols from the institutional animal ethics review committee having File No. XXXXXX of XXXXXX.
2.4 Preparation of monosodium urate crystals (MSUC)
MSCU ware obtained by heating 1.68 g of uric acid with 0.001 M sodium hydroxide solution, with meticulous control of the pH range maintained at 7.0 to 7.2 using hydrochloric acid (HCl). After pH adjustment, the mixture was allowed to settle down undisturbed at room temperature for 24 h to MSUC formed then the supernatant was carefully removed, and crystals was rinsed with double distilled water multiple times. Microscopic analysis was carried out to verify the presence of needle-shaped MSUC in the sample (Getting, Christian, Flower, Perretti, & Rheumatism, 2002; Kiyani, Moghul, Butt, et al., 2022).
2.5 Preparation of allopurinol solution
The allopurinol suspension was created out by dissolution of a powdered tablet in deionized water and was administered to mice with hyperuricemia through oral gavage. The suspension dosage was tailored according to the average weight of mice within group 6.
2.6 Experimental design
The MSUC induced hyperuricemia experimental mice were divided into six groups, all receiving MSUC, except for the control group. MSUC were injected intra-articularly in left ankle and intra-peritoneal over three weeks and two weeks respectively at one-day intervals. Blood samples were evacuated regularly to monitor the mice's uric acid concentration. After the initial three-week period, the mice were subjected to oral administration of various concentrations of iron oxide nanoparticles, as specified in Fig. 2. Upon completion of the experiment, the animal model mice were euthanized; blood and tissue samples of Kidney, liver, and muscle were collected and preserved within paraffin blocks for subsequent analysis of tissue iron levels using atomic absorption spectroscopy and for histopathology determination (Kakkar, Das, & Viswanathan, 1984).
Schematic diagram presenting dose administration in MSUC induced gouty mice.
2.7 Measurement of ankle swelling
The digital Vernier caliper was used to quantify the swelling of ankle diameter in millimeter regularly pre and post treatment to analyze the effectiveness of given treatment. Weekly measurements of ankle diameter were shown in Table 1.
Groups
(n = 6)
Measurements of the diameter (mm) of ankle joints
2nd Week
3rd Week
4th Week
5th week
Control group
2.49
2.54
2.65
2.56
MSUC group (−ve control)
5.85
6.84
6.87
6.95
Iron oxide nanoparticles (125 mg/kg of body weight)
5.29
5.97
3.34
2.94
Iron oxide nanoparticles (250 mg/kg of body weight)
5.13
6.23
3.25
2.66
Iron oxide nanoparticles (500 mg/kg of body weight)
5.76
6.21
3.20
2.53
Allopurinol (+ve control)
5.29
6.07
3.23
2.89
2.8 Total protein estimation
Total protein levels were estimated by purchasing commercially accessible kits from AMP Diagnostics AMEDA Labordiagnostik GmbH, Austria, as per manufacturer's instructions (Table 2). Expression of values are in Mean ± SD. aSignificantly different with comparison to the control group. bSignificantly different with comparison to the MSUC group. cSignificantly different with comparing to the allopurinol group.
Groups
(n = 6)
Parameters
Total protein
(mg/dl)
Control group
5.09 ± 0.04
MSUC group (−ve control)
11.73 ± 0.47a/b/c
Iron oxide nanoparticles (125 mg/kg of body weight)
6.61 ± 0.06a/b/c
Iron oxide nanoparticles (250 mg/kg of body weight)
6.20 ± 0.04a/b/c
Iron oxide nanoparticles (500 mg/kg of body weight)
3.74 ± 0.22a/b/c
Allopurinol (+ve control)
2.51 ± 0.03a/b/c
2.9 Antioxidant activity
The antioxidant enzyme levels in the hepatic tissue of both control as well as treated animals were assessed.
2.9.1 SOD, POD, and CAT analysis
Superoxide dismutase (SOD) levels were determined following the protocol previously used by (Chance et al., 1957). The assay included 0.1 m1 1M phenazine methosulphate and 1.2 ml of sodium pyrophosphate buffer (pH 8.3, 0.052 Al). Using a Unicam SP 500 spectrophotometer, the chromogen in the butanol was analyzed at 560 nm using 0.3 ml 300 1M nitroblue tetrazolium and 0.2 ml NADH (180 1). Catalase (CAT) and peroxidase (POD) levels were investigated with slight modifications to the method outlined in (Vickers, 2017). The concentration of peroxidase and catalase was measured by spectrophotometry utilizing the molecular extinction.
2.9.2 ROS and TBARS
ROS levels were quantified using the protocol employed from the previous study (Vickers, 2017). Thiobarbituric reactive acid substances (TBARS) levels were analyzed by using a SmartSpec plus spectrophotometer described by Wright et al (Wright, Sutherland, & Metabolism, 2008).
2.10 Complete blood picture
The complete blood picture was estimated with the help of AMP diagnostic kits (AMEDA Labordiagonstik GmbH). It includes the white blood cells, red blood cells, platelets and hemoglobin count.
2.11 Statistical analysis
The statistical inquiry was performed by utilizing a one way analysis of variance (ANOVA), which was then followed by the Tukey Karamer test to compare all groups. The data were represented as mean ± standard error of the mean (SEM) and were calculated by using GraphPad Prism software (San Diego, CA, USA). Statistically P < 0.05 was considered significant level.
3 Results
3.1 Characterization of nanoparticles
The X-ray diffraction analysis of the produced iron oxide nanoparticles corresponds to hematite indicates several Bragg reflections at different Bragg angles, as demonstrated in Fig. 3. Hematite exhibits a rhombohedral crystal structure with Fe3+ ions occupying octahedral coordination sites in a hexagonal close-packed array of oxygen ions. According to the previous study (Kiyani, Moghul, Butt, et al., 2022) the intense peak observed at around 2θ of 35.6748 corresponds to the (104) plane, characteristic of hematite α-Fe2O3 as per JCPDS card no. 33–0664. Moreover additional peaks further support its identification and is aligned with the electronic valence of Fe3+, which confirms trivalent oxidation state of iron within the hematite phase. The estimated particle size was to be 58.64 nm by using the Debye-Scherrer equation. The SEM images showed morphological spherical shape with minimal agglomeration of synthesized iron oxide nanoparticles with approximately 64 nm in size as depicted in Fig. 4 (a) Moreover, energy-dispersive X-ray (EDS) established the occurrence of iron in the desired quantity, along with traces of other elements like chloride and sodium, EDS showcases in Fig. 4 (b). The magnetic hysteresis curve for the iron oxide nanoparticle sample is given in Fig. 5. The results presented in the measurements show weak dependence on magnetic fields and negligible hysteresis at low magnetic fields. The saturation magnetization Ms was resolved to be approximately 0.700 emu g−1. Hc covering the magnetic field necessary to reduce the magnetization to zero after saturation was calculated to be −40.08 Oe. These results indicate the assumption made here that the nanoparticles are superparamagnetic as indicated by the fact that they have very low coercivity and zero remanent magnetization.
X-ray diffraction pattern (XRD) of synthesized Iron oxide nanoparticles.

(a) Scanning Electron Microscope (SEM) image of synthesized Iron oxide nanoparticles (b) Energy dispersive X-ray spectroscopy pattern of synthesized Iron oxide nanoparticles.

Hysterisis Plot for SQUID analysis showing M vs. H for iron oxide nanoparticle at 300 and 4 K.
3.2 Biochemical analysis
After the dissection of the animals, blood and tissue samples were evacuated for further biochemical analysis. These biochemical analysis includes antioxidant analysis and total protein estimation.
3.2.1 Total protein estimation
A significant raise levels of the total protein content was observed when compared with the control group. However, all groups which were treated with Iron oxide nanoparticles represents a significant drop levels of total proteins in comparison to the control, MSUC and allopurinol treated group Table 2.
3.2.2 SOD, POD, and CAT
While comparing with the control, the levels of SOD were significantly higher in MSUC group. Whereas, SOD levels in all treated groups were decreased significantly as compared to the MSUC group. Similarly, the groups which were treated with 125mg/kg and 250mg/kg showed significant low in SOD levels in comparison with the allopurinol treated group.
Similarly, the levels of POD decreased significantly in the MSUC group as compared to the control. While the group undergone treatment with 500mg of Iron oxide nanoparticles showed no significant change in POD levels as compared to the control, MSUC, and allopurinol treated groups, whereas Iron oxide nanoparticles treated groups have significant increase in POD levels as compared to the MSUC group. The 125mg/kg of Iron oxide nanoparticles treated group exhibited significant changes in POD levels in comparison to the allopurinol treated group.
The level of CAT were decreased significantly in MSUC group compared with the control group. Whereas, 125mg/kg of Iron oxide nanoparticles treated group showed significant increase in CAT levels with the comparison of MSUC and allopurinol treated groups. All other treated groups showed significant increase in CAT levels with comparison to the MSUC group. Table 3 represents the values of SOD, POD, and CAT. Expression of values are in Mean ± SD.
Groups (n = 6)
Parameters
SOD
POD
CAT
ROS
TBARS
(U/min)
(U/min)
(U/min)
(U/min)
(mmol/min/mg)
Control group
35.80 ± 0.43
3.05 ± 0.3
3.06 ± 0.17
1.58 ± 0.04
4.20 ± 0.07
MSUC group (−ve control)
21.51 ± 4.05a
1.10 ± 0.8a
1.56 ± 0.18a
3.56 ± 0.31a
15.38 ± 0.16a
Iron oxide nanoparticles (125 mg/kg of body weight)
27.01 ± 0.8a/b/c
1.36 ± 0.05c
2.33 ± 0.19a/b/c
1.78 ± 0.62a/b/c
2.25 ± 0.01a/b/c
Iron oxide nanoparticles (250 mg/kg of body weight)
28.46 ± 0.9a/b/c
3.15 ± 0.5a/b
3.17 ± 0.06b
1.37 ± 0.08a/.b/c
2.44 ± 0.02a/b/c
Iron oxide nanoparticles (500 mg/kg of body weight)
34.94 ± 0.3b
3.33 ± 0.2b
3.37 ± 0.09b
1.67 ± 0.15a/b/c
4.45 ± 0.03a/b/c
Allopurinol (+ve control)
34.87 ± 0.5
2.80 ± 0.76b
3.27 ± 0.61b
2.51 ± 0.27a/b/c
3.64 ± 0.06a/b/c
3.2.3 ROS and TBARS
The assessment of reactive oxygen species in MSUC group revealed a substantial raised in values with comparison to the control group. The Iron oxide nanoparticles treated groups exhibited a significant reduction in ROS levels with comparison to the control, MSUC, and allopurinol treated groups. Moreover, the TBARS evaluations of the MSUC group demonstrated a notable raised in values in comparison to control, while Iron oxide nanoparticles treated group exhibited a significant lowering in TBARS levels as compared to the control, MSUC, and allopurinol treated groups. The specific details can be observed in Table 3.
3.2.4 Complete blood picture
The WBCs count of the MSUC group was significantly raised (P <0.05) with comparison to other treatment groups. On the other hand the RBCs count of Allopurinol group presented a significant difference (P <0.001) with comparison to other groups. The detail of blood parameters is given in Table 4. Expression of values are in Mean ± SD.
Group (n = 6)
WBCs
103/ mm3
RBCs
ml/mm3
Hb
g/dl
Hematocrit
%
MCV
F1
Platelets
103/mm3
RDW CV
%
Normal Range
4–10
4.50–6.00
11–15
40–50
76–92
150–450
11.5–16
Control
7.21 ± 1.1c
7.28 ± 0.2b
12.1 ± 0.98bc
190.50 ± 2.6d
85.50 ± 2.46d
300.8 ± 94.6f
20.83 ± 2.33c
MSUC group
(−ve control)
37.63 ± 4.4g
8.13 ± 1.3c
9.83 ± 1.93a
186.33 ± 3.1d
49.83 ± 4.66a
187.6 ± 46.9g
20.83 ± 2.43c
Iron oxide nanoparticles
(125 mg/kg of body weight)
8.5 ± 0.41d
7.22 ± 0.9b
11.82 ± 2.12b
175.50 ± 2.0cd
59.00 ± 4.92b
855 ± 30.3c
24 ± 3.11d
Iron oxide nanoparticles
(250 mg/kg of body weight)
16.6 ± 2.0f
7.40 ± 1.4bc
14.13 ± 2.41d
106.50 ± 3.8b
63.50 ± 1.82bc
907.3 ± 25.5a
15.16 ± 1.21a
Iron oxide nanoparticles
(500 mg/kg of body weight)
5.23 ± 0.53b
6.29 ± 0.9a
13.17 ± 1.83c
89.50 ± 2.1a
73.17 ± 2.11c
350.8 ± 101.5e
15 ± 1.44a
Allopurinol
(+ve control)
7.48 ± 0.20e
7.73 ± 0.8bc
11.43 ± 1.09b
147.00 ± 4.2c
60.00 ± 4.2ab
890 ± 141.4b
24 ± 3.23d
4 Discussion
The findings of the current study demonstrated that Iron oxide nanoparticles show promise in the treatment of gout in mice resulting from hyperuricemia, even at lower concentrations, while minimizing excessive damage to cells. Additionally, various biochemical tests were performed in this research work to assess the impact of Iron oxide nanoparticles on mice. Dextran or starch is commonly employed as a stabilizing agent in forming iron oxide nanoparticles to create a system that can disperse in water (LaConte, Nitin, & Bao, 2005). In an earlier study, the impact of injected Iron oxide nanoparticles on oxidative stress in various tissues and monitoring changes in hepatic enzymes as a measuring parameter of liver function was assessed. During investigations, a gradual rise in serum iron levels over one week was followed by a slow drop down, indicating that the elimination of magnetic NPs from the body takes longer than three weeks. The increase in serum iron levels can be attributed to several concurrent processes, including the breakdown of injected iron particles, subsequent binding of iron to various type of serum proteins like transferrin and re-dispersion of bound iron to body cells and tissues, and eventual elimination. The study suggests that most body iron exists in the bound state due to its slow clearing kinetics (Jain, Reddy, Morales, Leslie-Pelecky, & Labhasetwar, 2008).
One of the previous research observed that administration of silver oxide nanoparticles resulted in a notable elevation in protein estimation and lipid profile values in a group of gouty mice induced by MSUC. The raised protein levels indicated the magnitude of the response of inflammation, which is closely linked to oxidative stress (Kiyani, Moghul, Javed, et al., 2022). A separate study concluded that administration of ZnO-NPs to hyperuricemia mice model with gouty arthritis resulted in a considerable reduction in levels of blood uric acid. Moreover, the lipid profile tests demonstrated that the treatment with nanoparticles effectively lowered cholesterol and LDL levels with comparison to the control group. However, both the nanoparticles-treated and allopurinol-treated groups exhibited increased in hepatic enzymes concentrations.
Nonetheless, histopathological examination of kidney, liver and muscle tissues revealed no noticeable variations. This research provides evidence supporting the potential of orally given iron oxide nanoparticles in reducing the concentration of uric acid, cholesterol as well as LDL of the blood (Kiyani et al., 2019). In a previous conducted research, it was reported that CuO nanoparticle administration at different concentrations reduced serum ankle swelling in gouty mice with comparison to the negative control group (Kiyani et al., 2020), and these results are similar to our findings that Iron oxide nanoparticles administration reduced swelling in all treated groups.
5 Conclusion
This research results represents that low concentrations of Iron oxide nanoparticles hold potential as a prospective remedy for gouty arthritis in mice. Administering gout-afflicted mice with Iron oxide nanoparticles at 125, 250 and 500 mg/kg of body weight showcased antioxidative effects, signifying their possible therapeutic application in gout treatment. Moreover, Iron oxide nanoparticles demonstrated the capacity to lower total protein levels, suggesting reduced inflammation associated with gout.
CRediT authorship contribution statement
Mubin Mustafa Kiyani: Writing – original draft, Methodology, Conceptualization. Sarah Sadiq: Formal analysis, Data curation, Conceptualization. Maria Sarfraz: Investigation, Funding acquisition, Formal analysis. Chanda Javed: Resources, Methodology, Investigation. Maisra Azhar Butt: Software, Resources, Project administration. Hassan Burair Abbas: Project administration, Investigation, Funding acquisition. Hamza Rehman: Validation, Software, Project administration. Syed Ali Imran Bokhari: Writing – review & editing, Visualization, Validation, Supervision.
Funding
This research was conducted without any external funding.
Acknowledgments
This manuscript constitutes a segment of Mubin Mustafa's doctoral dissertation. The authors thank the International Islamic University Islamabad, Pakistan and the National Institute of Health, Islamabad, Pakistan, for providing animal housing facilities.
Ethics Approval
Ethical approval letter has been taken from IRB of International Islamic University Islamabad Pakistan under File No. IBBC-IIUI-03.
Consent for Publication
All authors are mutually agreed for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Triggers of acute attacks of gout, does age of gout onset matter? A primary care based cross-sectional study. PLoS One. 2017;12(10):e0186096
- [Google Scholar]
- Biogenic synthesis of iron oxide nanorods using Moringa oleifera leaf extract for antibacterial applications. Appl. Nanosci.. 2020;10:305-315.
- [Google Scholar]
- Bhatia, S., & Bhatia, S. (2016). Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. Natural polymer drug delivery systems: Nanoparticles, plants, and algae, 33–93.
- Chance, B., Maehly, A., Colowick, S., Kaplan, N. J. S. C., Kaplan, N., & Academic Press, I. N. Y., NY, USA. (1957). Methods in enzymol. 4, 273.
- Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum.. 2002;46(10):2765-2775.
- [Google Scholar]
- Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol.. 2018;46(sup1):693-707.
- [Google Scholar]
- From Himba indigenous knowledge to engineered Fe2O3 UV-blocking green nanocosmetics. Sci. Rep.. 2022;12(1):2259.
- [Google Scholar]
- Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm.. 2008;5(2):316-327.
- [Google Scholar]
- Kakkar, P., Das, B., & Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase.
- Properties of iron oxide nanoparticles synthesized at different temperatures. J. Supercond. Nov. Magn.. 2011;24:675-678.
- [Google Scholar]
- Optimum saturation magnetization of superparamagnetic iron oxide nanoparticles for versatile applications. Acta Phys. Pol. A. 2024;146(2):154-164.
- [Google Scholar]
- Effect of synthesis parameters on the properties of superparamagnetic iron oxide nanoparticles. J. Supercond. Nov. Magn.. 2012;25:2777-2781.
- [Google Scholar]
- Improvement of the saturation magnetization of PEG coated superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater.. 2022;551:169140
- [Google Scholar]
- Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev.. 2017;10(4):186-201.
- [Google Scholar]
- Antioxidant and anti-gout effects of orally administered zinc oxide nanoparticles in gouty mice. J. Trace Elem. Med Biol.. 2019;56:169-177.
- [Google Scholar]
- Inhibition of hyperuricemia and gouty arthritis in BALB/c mice using copper oxide nanoparticles. Biol. Trace Elem. Res.. 2020;193:494-501.
- [Google Scholar]
- Effects of biocompatible surfactants on structural and corresponding magnetic properties of iron oxide nanoparticles coated by hydrothermal process. J. Magn. Magn. Mater.. 2019;474:332-336.
- [Google Scholar]
- Uric acid and hypertension: an age-related relationship? J. Hum. Hypertens.. 2009;23(2):75-76.
- [Google Scholar]
- Kumar, A., & Thakur, A. (2022). Use of Nanoparticles to Combat COVID-19. In Handbook of Research on Green Synthesis and Applications of Nanomaterials (pp. 412-440). IGI Global.
- Development of uric acid biosensor using gold nanoparticles and graphene oxide functionalized micro-ball fiber sensor probe. IEEE Trans. NanoBiosci.. 2019;19(2):173-182.
- [Google Scholar]
- Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A. 2020;126:1-8.
- [Google Scholar]
- Green synthesis of novel zinc iron oxide (ZnFe2O4) nanocomposite via Moringa Oleifera natural extract for electrochemical applications. Appl. Surf. Sci.. 2018;446:66-73.
- [Google Scholar]
- Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J.. 2011;19(3):129-141.
- [Google Scholar]
- Oborny, M. C. (2007). Nanotechnology: Industrial Hygiene Challenges and Recommended Practices (No. SAND2007-7100C). Sandia National Lab.(SNL-NM), Albuquerque, NM (United States).
- Urate crystal deposition, prevention and various diagnosis techniques of GOUT arthritis disease: a comprehensive review. Health Inf. Sci. Syst.. 2018;6:1-13.
- [Google Scholar]
- Coevolution of URAT1 and uricase during primate evolution: implications for serum urate homeostasis and gout. Mol. Biol. Evol.. 2016;33(9):2193-2200.
- [Google Scholar]
- Animal communication: when i’m calling you, will you answer too? Curr. Biol.. 2017;27(14):R713-R715.
- [Google Scholar]
- Antioxidant supplemention in the treatment of skeletal muscle insulin resistance: potential mechanisms and clinical relevance. Appl. Physiol. Nutr. Metab.. 2008;33(1):21-31.
- [Google Scholar]
- Nanotechnology controlled drug delivery for treating bone diseases. Expert Opin. Drug Deliv.. 2009;6(8):851-864.
- [Google Scholar]
Further reading
- Anti-hyperuricemic effect of iron oxide nanoparticles against monosodium urate crystals induced gouty arthritis in BALB/c mice. Biol. Trace Elem. Res.. 2022;200(4):1659-1666.
- [Google Scholar]
- In vivo effects of orally administered different concentrations of silver oxide nanoparticles in hyperuricemic mice. Biol. Trace Elem. Res. 2022:1-11.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103556.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







