Translate this page into:
Therapeutic effect of Berberis vulgaris fruit extract on histopathological changes and oxidative stress markers of ovarian ischemia and reperfusion injury in rats
⁎Corresponding author at: Department of Histology and Embryology, Faculty of Medicine, Kafkas University, Kars, Turkey. yigitserdar85@gmail.com (Serdar Yigit),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Aim
This study aimed to investigate the possible protective effect of berberine, the active compound of Berberis vulgaris plant extract, which has anti-inflammatory and antioxidant properties, in an ovarian ischemia–reperfusion model by utilizing molecular, biochemical, and histopathological methods.
Methods
In this experimental study, 42 adolescent female Sprague Dawley rats (6 weeks old) were divided into 7 equal groups. Under anesthesia, ischemia was induced by ligating the bilateral adnexal tissues, and after 3 h of ischemia, reperfusion was achieved by removing the adnexal ligation. Berberis vulgaris extract was administered by oral gavage 1 h after ischemia for the ischemia groups and 1 h before reperfusion for the ischemia + reperfusion groups. After 3 h of reperfusion, the experiment was terminated.
Results
The administration of Berberis vulgaris extract decreased the hemorrhagic areas, reduced the number of TUNEL-positive cells, and decreased CYC1 immunoreactivity levels in histopathological analysis compared to the groups subjected to ischemia or ischemia + reperfusion without the plant extract. CAT, SOD, and GSH increased due to Berberis vulgaris administration while MDA levels decreased. Berberis vulgaris also downregulated TNF-α levels compared to the ischemia and ischemia + reperfusion groups.
Conclusion
Berberis vulgaris extract may have a protective effect against ovarian ischemia–reperfusion injury. Further molecular studies are needed to clarify this protective effect.
Keywords
Berberis vulgaris
TNF-α
Ischemia
Reperfusion
Ovarian
- IR
-
ischemia–reperfusion
- ROS
-
reactive oxygen species
- HIF-1α
-
Hypoxia inducible factor-1α
- Bcl-2
-
B-cell lymphoma 2
- Bax
-
Bcl-2-associated X protein
- I
-
ischemia
- I + BV200
-
Berberis vulgaris 200 mg/kg + ischemia
- I + BV400
-
Berberis vulgaris 400 mg/kg + ischemia
- CNT
-
control
- TUNEL
-
terminal deoxynucleotidyl transferase dUTP nick end labeling
- SOD
-
superoxide dismutase
- CAT
-
catalase
- GSH
-
glutathione
- MDA
-
malondialdehyde
- RT-PCR
-
reverse-transcription polymerase chain reaction
- TNF-α
-
tumor necrosis factor-alpha
- CYC1
-
cytochrome c1
Abbreviations
1 Introduction
Ovarian torsion is common in adolescents, accounting for 3 % of gynecological emergencies (Soyman et al., 2017). It is primarily caused by rotation of the infundibulopelvic ligament, resulting in the compression of the vascular system supplying the ovaries and interruption of the blood flow (Colak et al., 2020). The adverse effects of ovarian torsion on ovarian reserves necessitate rapid intervention to maintain fertility (Yeral et al., 2019). Delayed diagnosis of ovarian torsion may lead to infertility and organ failure (Dayangan Sayan et al., 2018). Although detorsion of the rotated ovaries is necessary to salvage the organs, reperfusion may cause more damage than the ischemia itself, and this process is known as ischemia–reperfusion (IR) injury (Nayki et al., 2018). IR causes damage by interrupting the cellular aerobic metabolism. This damage increases the levels of reactive oxygen species (ROS) (Kaplan & Türk, 2022). While ROS increase rapidly in IR, the antioxidants that protect against ROS are also impaired (Valko et al., 2007). Disruption of the balance between ROS and antioxidants in favor of ROS increases the oxidative stress in the tissues (Surekha et al., 2007).
Berberis vulgaris is a plant from the family Berberidaceae used as a food supplement. Berberine alkaloids have been shown to inhibit ROS production that causes IR-induced damage, as well as decreasing oxidative stress and improving the release of proapoptotic factor cytochrome c and apoptosis-inducing factors in mitochondria. It has been reported that alkaloids found in the root parts of Berberis vulgaris exert antiapoptotic activity in response to ischemia by reducing HIF-1α, caspase-9, and caspase-3 and increasing the Bcl-2/Bax ratio (Imenshahidi & Hosseinzadeh, 2016). Berberis vulgaris contains various phenolic compounds, organic acids, flavonoids (anthocyanins), and alkaloids, and these alkaloids have antioxidant, hypoglycemic, anti-inflammatory, hypotensive, and hypolipidemic effects (Rahimi-Madiseh, Lorigoini, Zamani-Gharaghoshi, & Rafieian-Kopaei, 2017). The present study evaluated the potential protective effect of Berberis vulgaris against IR injury after ovarian torsion/detorsion using histopathological, molecular, and biochemical methods.
2 Materials and methods
Ethical clearance was provided by the Kafkas University Animal Experiments Local Ethics Committee with approval number KAU-HADYEK/2020–91. Forty-two adolescent female Sprague Dawley rats (6 weeks old, with a mean weight of 180 g) were used in the study. The experimental animals were housed with a temperature of 20 ± 2°C, relative humidity of 50 ± 10 %, and light/dark cycle of 12 h/12 h. Rats were fasted for 12 h before the surgical procedures. After they were anesthetized (30 mg/kg intraperitoneal thiopental sodium), an incision was made in the abdominal region. Bilateral adnexal structures supplying the ovaries and fallopian tubes were identified through the incision made in the midline area of the lower abdomen. Ischemia was induced by ligating the bilateral adnexal tissues with sutures of 4.0 Vicryl and the abdomen was closed. Following 3 h of ischemia, the abdomen was reopened and reperfusion was achieved by removing the adnexal ligation. Berberis vulgaris extract was administered by oral gavage to the ischemia groups 1 h after ischemia and to the IR groups 1 h before reperfusion, after the animals had recovered from the effects of anesthesia. After 3 h of reperfusion, the experiment was terminated with euthanasia and ovarian tissues were collected. Right ovaries were used for histological and immunoreactive examinations and left ovary tissues were used for biochemical and molecular studies (Table 1).
Name
Description
Abbreviation
N
Group 1
Control
CNT
6
Group 2
Ovarian ischemia (3 h)
I
6
Group 3
Ovarian ischemia (3 h) + Berberis vulgaris 200 mg/kg
I + BV200
6
Group 4
Ovarian ischemia (3 h) + Berberis vulgaris 400 mg/kg
I + BV400
6
Group 5
Ovarian ischemia (3 h) + reperfusion (3 h)
IR
6
Group 6
Ovarian ischemia (3 h) + Berberis vulgaris 200 mg/kg + reperfusion (3 h)
IR + BV200
6
Group 7
Ovarian ischemia (3 h) + Berberis vulgaris 400 mg/kg + reperfusion (3 h)
IR + BV400
6
2.1 Histological and immunohistochemical examinations
Ovarian tissues were fixed in 10 % formalin for 48 h (Sahin et al., 2021). Serial sections of 5 µm in thickness were taken from each tissue block with a Leica RM2125RTS microtome. Hematoxylin and eosin (H&E), TUNEL, and periodic acid-Schiff (PAS) staining were performed for histological examinations. Immunohistochemical (CYC1: Elabscience, E-AB-40271) and TUNEL (Elabscience-Cat No: E-CK-A331) staining were respectively performed according to the protocols specified by the manufacturer. Calculations were performed and the average immunoreactivity intensity was reported. Semi-quantitative scoring was carried out by light microscopy. The immunoreactivity of samples from each animal was scored as follows: none (0), mild (1), moderate (2), severe (3), or very severe (4). Findings were photographed with an Olympus BX43 microscope using the cellSens software program.
2.2 Biochemical examinations
2.2.1 Biochemical analysis
After being placed on ice, all tissues were homogenized in phosphate buffer (50 mM, pH 7.4). Following the centrifugation of tissue homogenates in Eppendorf tubes for 10 min at 4 °C and 4000 rpm, the supernatants were collected for microcentrifuge examination of biochemical parameters. Stored serum samples were gradually defrosted at −20 °C, then at 4 °C, and finally at 25 °C on the day of analysis and all tests were completed immediately. A microplate reader was used for all spectrophotometric tests. No standard was used for SOD and calculations were performed blindly. For CAT, serial concentrations of 940–14.7 U/mL were prepared. For GSH, concentrations of 2 mM and 0.035 nM were prepared. For MDA, 1,1,3,3-tetraethoxypropane was used to create a standard stock solution with a concentration of 200 µmol/L. Standard solutions were then created by serial dilution at various concentrations from the stock solution.
-
Determination of superoxide dismutase (SOD) enzyme activity
The SOD enzyme activity measurement method is based on the fact that the SOD enzyme inhibits free radicals in samples containing nitro blue tetrazolium (NBT) during the reduction of free oxygen radicals. The reaction’s color change was observed at 560 nm by spectrophotometry (Sun, Oberley, & Li, 1988). Pure SOD enzyme was used in the analysis of SOD enzyme activity.
-
Determination of catalase (CAT) enzyme activity
Using the procedure described by Campo et al. (2004), CAT activities in the homogenate supernatants were evaluated. The reaction was followed with a spectrophotometer at 405 nm. Activities of the samples were determined using the equation for the curve after standards were made from pure CAT enzyme at serial values ranging from 940 to 14.7 U/mL. Tissue sample results were reported as U/mL.
-
Determination of total glutathione (GSH)
Using the technique proposed by Sedlak and Lindsay (1968), total GSH was determined. After 30 min of incubation at 37 °C, the absorbance was measured at 412 nm. The following reduced glutathione concentrations were used as standards: 2 mM, 1 mM, 0.5 mM, 0.250 mM, 0.125 mM, 0.0625 mM, and 0.035 mM. The values of absorbance obtained from conventional measurements and their equivalents were used to calculate the total quantity of GSH.
-
Determination of malondialdehyde (MDA)
Using spectrophotometry, the absorbance of a pink mixture comprising thiobarbituric acid and MDA was measured at a wavelength of 532 nm after 60 min of incubation time at 95 °C (Ohkawa, Ohishi, & Yagi, 1979). A standard stock solution was created using 200 µmol/L 1,1,3,3-tetraethoxypropane. By serial dilution of the standard stock solution, standard solutions at various concentrations were prepared and results were recorded in micromoles.
2.3 Plant extraction
The Berberis vulgaris plants used in this study were collected from the Şenkaya district of Erzurum province. The drying of the plant samples was carried out in a dark laboratory environment with dry air flow, avoiding direct sunlight. The dried plant stems were ground in a grinder. Ethanol was used as the extraction solvent, 650 mL of which was placed in a boiling flask. The Soxhlet extraction method was used. The solvent was extracted for about 10 h until it became transparent (10–15 siphons). The obtained liquid extract was filtered using blue-band filter paper and all particles were separated. The filtered extract was vaporized at 35–45 °C using a rotary evaporator. The extract was then held in a desiccator for 12 h. The plant extract was stored in an extract box at 4 °C. Extraction experiments were performed in triplicate (Uluman & Kilicle, 2020; Wang & Weller, 2006).
2.4 RNA isolation, reverse transcription, and amplification
Total RNA was extracted from ovary tissue using the ECO-TECH Total RNA Kit. The RNA was extracted with 50 µL of nuclease-free water (NFW) and kept at −80 °C. Using the NanoDrop One/OneC Microvolume UV–Vis spectrophotometer (Thermo Fisher Scientific), the concentrations of the RNA samples and their quality (wavelengths: 260 and 280 nm) were verified.
RNA was reverse-transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) The following primer pairs were used on a custom plate: TNF-alpha: forward: 5-TGCCTCAGCCTCTTCTCATT-3, reverse: 5- CCCATTTGGGAACTTCTCCT-3; ACTB: forward: 5-ATCGCTGACAGGATGCAGAAG-3, reverse: 5-AGAGCCACCAATCCACACAGA-3. Data collection was performed using a 7500 Fast Real-Time PCR instrument and SYBR Master Mix (Applied Biosystems). The expression level of β-actin (ACTB) as a reference gene was used to normalize the expression level of each gene and the relative expression levels of the genes were evaluated according to the 2-ΔΔCt method.
2.5 Statistical analysis
One-way ANOVA was utilized with the Tukey post hoc test for the comparison of normally distributed groups and the Kruskal-Wallis test and Dunn post hoc test were utilized for the analysis of non-normally distributed groups. Analyses were performed with IBM SPPS Statistics 20.0 (IBM Corp.). Values of p < 0.05 were considered to reflect statistical differences between the groups. One-way ANOVA was performed with Origin Pro 2024 software to determine differences in gene expression between the groups.
3 Results
3.1 Extraction yield
The yield of Berberis vulgaris fruit extract was found to be 29.15 ± 0.20 %. Previous studies reported that chlorogenic acid and syringic acid were the most abundant phenolic compounds in samples of Berberis vulgaris collected in Turkey. The biological activity of the ethanolic extracts of the fruits is attributed to anthocyanins as phenolic components (Eroğlu, Çakır, Sağdıç, & Dertli, 2020; Gundogdu, 2013).
3.2 Histopathological findings
In the control group, H&E staining demonstrated normal structures in both the cortex and medulla. In addition, no abnormalities were detected in the vascular structures. In the group subjected to only ischemia, the borders of both the corpus luteum and follicle cells were not regular. Notably, hemorrhagic areas (Fig. 1) were observed in this group. In the groups for which ischemia was induced and the plant extract was administered, hemorrhagic regions decreased in a dose-dependent manner (p < 0.001). Furthermore, in comparison to the ischemia group, there was a decline in vascular occlusion in these groups. The group subjected to IR without injection of the plant extract incurred the most damage. The hemorrhagic areas were significant. In the groups in which IR was induced and the plant extract was administered, this damage decreased with increasing doses (p < 0.001). The 400 mg/kg plant extract dose was found to be significantly more effective than the 200 mg/kg dose (p = 0.0012). Findings from the high-dose group were histologically close to those of the control group.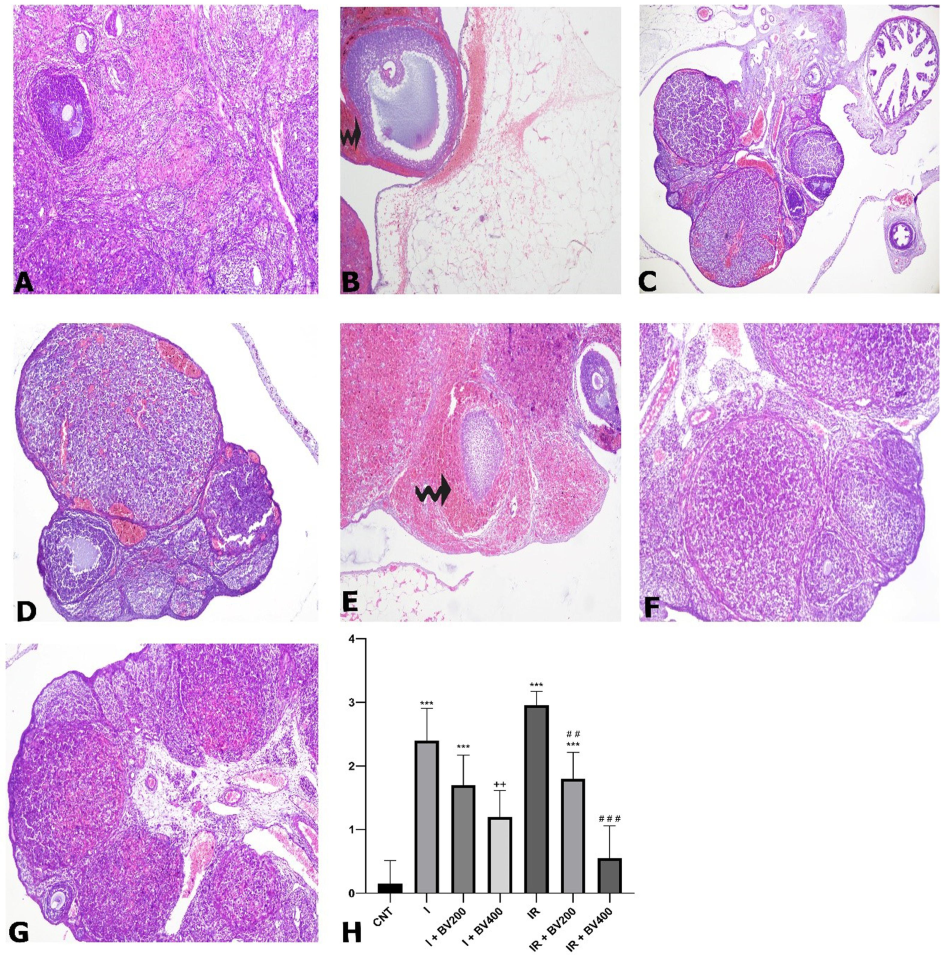
Hematoxylin and eosin (H&E) staining photomicrographs of all experimental groups. A) CNT, 10X; B) I, 10X; C) I + BV200, 10X; D) I + BV400, 10X; E) IR, 10X; F) IR + BV200, 10X; G) IR + BV400, 10X; curved arrows signify hemorrhagic areas. H) Histopathological scoring results for hemorrhagic areas. *: Compared to CNT; +: comparing I + BV groups with I; #: comparing IR + BV groups with IR. CNT: Control; I: ischemia, IR: ischemia + reperfusion; *, +, #: p<0.05; **, ++, ##: p<0.01; ***, +++, ###: p<0.001.
3.3 TUNEL staining findings
We observed TUNEL-positive cells, especially among granulosa cells and corpus luteum cells, after TUNEL staining. While TUNEL positivity was increased in the group subjected to ischemia without plant extract administration, it was decreased in the groups with ischemia + 200 mg/kg Berberis vulgaris plant extract and ischemia + 400 mg/kg Berberis vulgaris plant extract (Fig. 2). No significant difference was found between these two doses. While TUNEL positivity increased in the group subjected to IR without administration of the plant extract, 200 and 400 mg/kg doses of the extract significantly reduced the numbers of positive cells. This decreasing effect was considerably higher at 400 mg/kg than at 200 mg/kg (p < 0.001).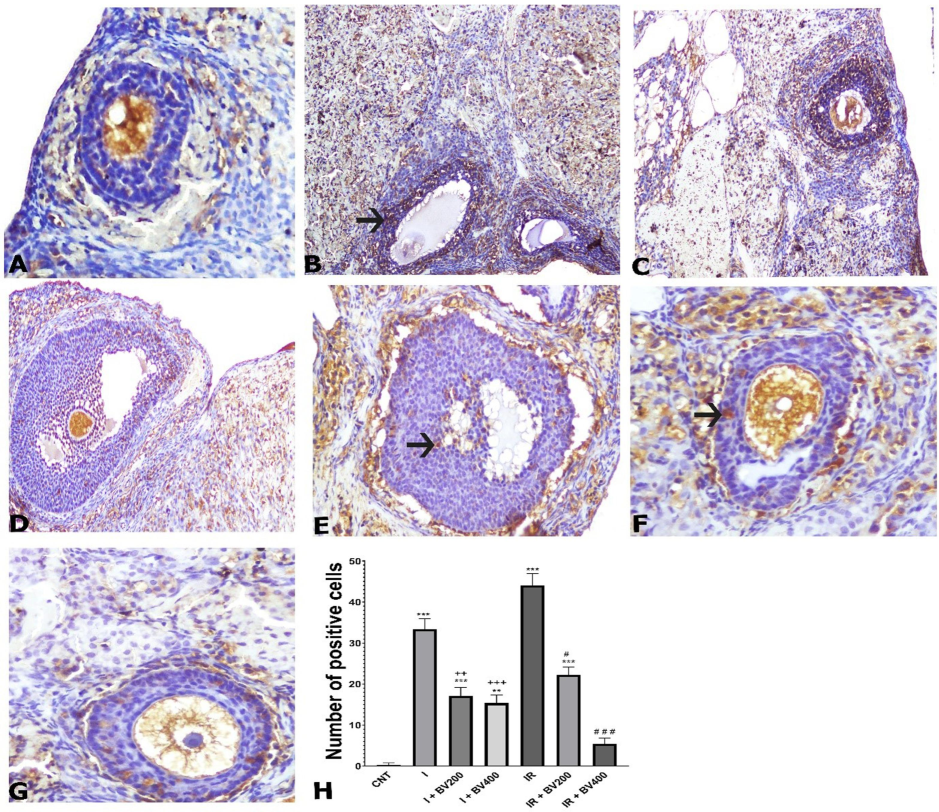
TUNEL staining photomicrographs of all experimental groups. A) CNT, 10X; B) I, 10X; C) I + BV200, 10X; D) I + BV400, 20X; E) IR, 20X; F) IR + BV200, 20X; G) IR + BV400, 20X; arrows signify apoptotic cells. H) Numbers of positive cells as seen by TUNEL staining. *: Compared to CNT; +: comparing I + BV groups with I; #: comparing IR + BV groups with IR. CNT: Control; I: ischemia, IR: ischemia + reperfusion; *, +, #: p<0.05; **, ++, ##: p<0.01; ***, +++, ###: p<0.001.
3.4 Immunohistochemistry findings
In the control group, no cytochrome c1 (CYC1) immunoreactivity was observed. In the ischemia groups, we observed a highly significant increase in CYC1 immunoreactivity in both granulosa and theca layers (Fig. 3) (p < 0.0001). However, this effect of ischemia was significantly attenuated with 200 and 400 mg/kg doses of Berberis vulgaris. At 400 mg/kg, the immunoreactivity level was reversed to that of the control group. IR increased the immunoreactivity level compared to ischemia alone, but this increase was not statistically significant. In the IR groups that received 200 mg/kg and 400 mg/kg Berberis vulgaris extract, immunoreactivity levels were decreased compared to the group subjected to IR without the administration of the plant extract. The 400 mg/kg dose returned the immunoreactivity level to that of the control group.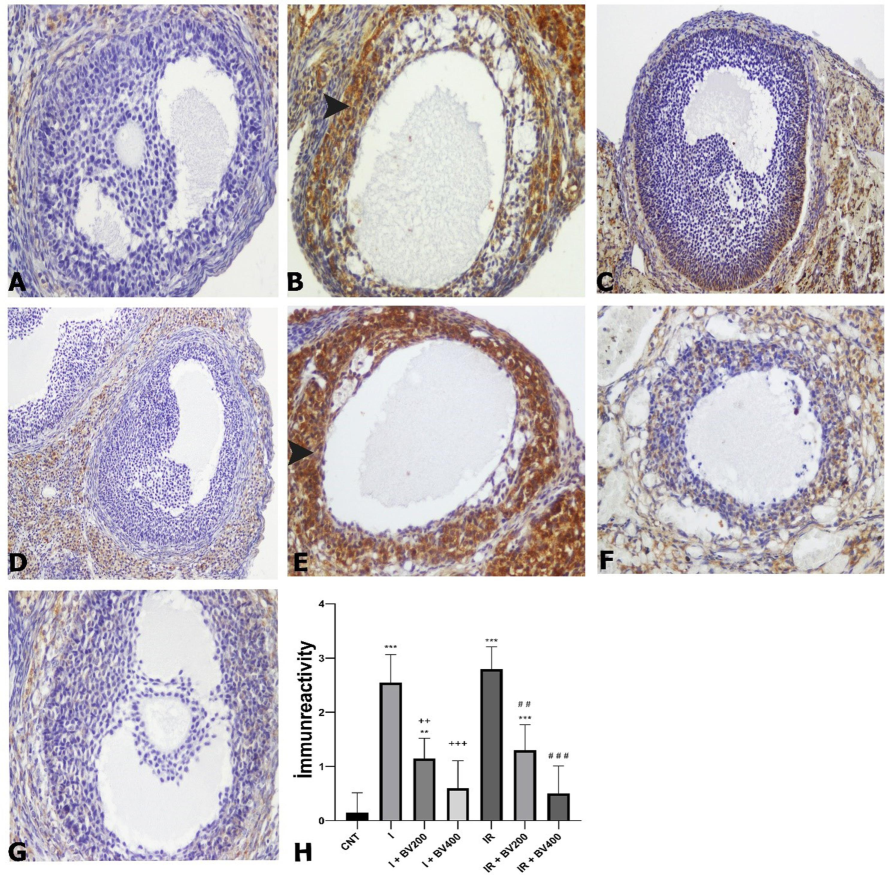
CYC1 immunoreactivity photomicrographs of all experimental groups. A) CNT, magnification 20X; B) I, magnification 20X; C) I + BV200, magnification 20X; D) I + BV400, magnification 20X; E) IR, magnification 20X; F) IR + BV200, magnification 20X; G) IR + BV400, magnification 20X; arrowheads signify immunopositive cells. H) CYC1 immunoreactivity according to immunohistochemical findings. *: Compared to CNT; +: comparing I + BV groups with I; #: comparing IR + BV groups with IR. CNT: Control; I: ischemia, IR: ischemia + reperfusion; *, +, #: p<0.05; **, ++, ##: p<0.01; ***, +++, ###: p<0.001.
3.5 Biochemical findings
While MDA levels were high in the ischemia and IR groups, they decreased in line with the administered dose of plant extract (p < 0.05). While GSH levels were lowest in the IR group, they increased dose-dependently with administration of the extract (p < 0.05). It was especially noteworthy that the I + BV200 group had levels similar to those of the control group. While SOD levels were lowest in the ischemia and IR groups, they increased dose-dependently with administration of the extract (p < 0.05). While CAT levels were lowest in the ischemia and IR groups, they increased with administration of the extract (p < 0.05) (Fig. 4).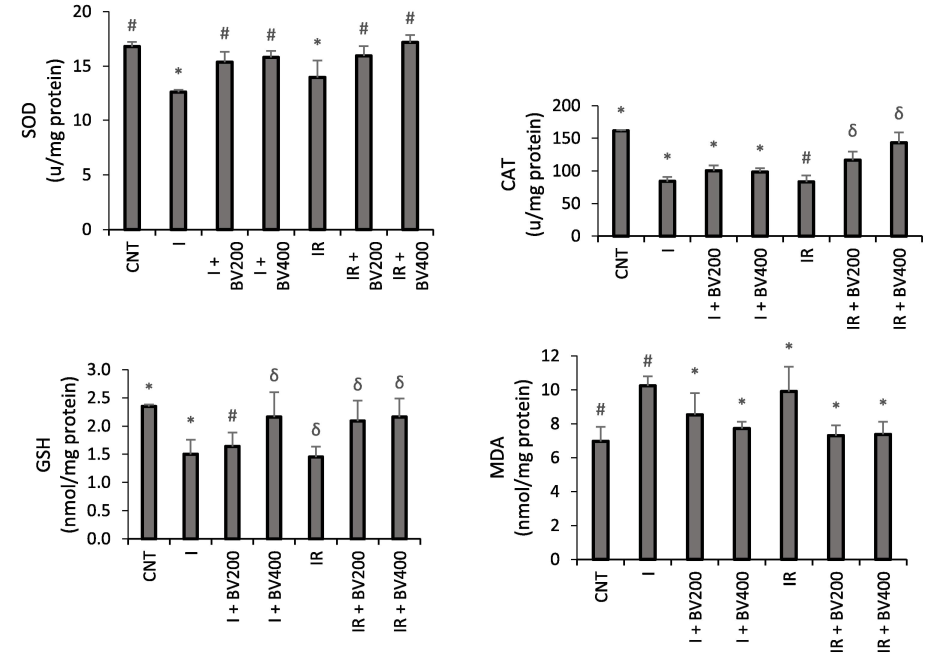
Effects of Berberis vulgaris plant extract on SOD, CAT, GSH, and MDA enzyme activity (mmol/min/mg tissue ± SD) in rats. *, #, δ: Bars marked with the same symbols do not differ statistically, while different symbols indicate that there is a significant difference (p < 0.05).
3.6 RT-PCR results
RT-PCR was used to quantify the changes in the expression levels of two genes and the results are displayed in Fig. 5. TNF-α expression levels were significantly higher in the IR group compared to the CNT group according to RT-PCR analysis (p < 0.01). The levels of the I + BV400 and IR + BV200 groups decreased in comparison to the ischemia and IR groups (p < 0.01).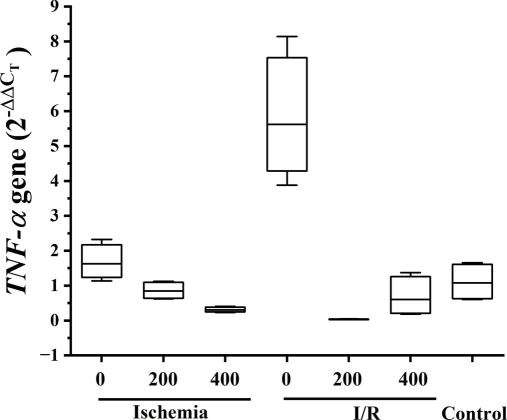
Relative expression of TNF-α gene analyzed in the ovary.
4 Discussion
Rapid resolution of ischemic injury arising from adnexal torsion is critical to preserve fertility. However, detorsion, the emergency surgical intervention applied for the treatment of ischemic injury, causes IR injury. Oxidative stress resulting from IR also leads to tissue injury (Bostancı et al., 2016). Without effective antioxidant and anti-inflammatory treatment in combination with surgical intervention, the prospects for maintaining fertility are reduced (Ozler et al., 2013). Numerous plants contain berberine, an isoquinoline alkaloid (Zhao, Xing, Zhang, & He, 2021). Although berberine has been reported to have antioxidant and anti-inflammatory effects in many different tissues, there are few studies on its therapeutic effects in the treatment of adnexal torsion-related injuries. Berberine improves liver function by decreasing oxidative stress levels and apoptosis via increased SOD activity while preventing the increase of MDA (Sheng et al., 2015). Berberine also exhibited protective effects in renal tissue by elevating SOD and GSH levels and reducing MDA levels in cases of IR injury (Visnagri, Kandhare, & Bodhankar, 2015), while another study revealed that berberine had similar protective effects against IR injury in cardiac tissue (Yu et al., 2016). Berberine was found to protect brain tissue against IR injury by increasing the SOD activity and lowering the MDA and nitric oxide levels (Chu, Zhou, Zhang, Xue, & Zhao, 2017). A previous study conducted in testes by applying testicular torsion showed that early treatment with berberine provided protective effects against tissue damage due to oxidative stress by increasing the levels of antioxidant activity (Kazaz et al., 2020). The findings of these previous studies are consistent with our results. A previous study of diabetic animals revealed that the administration of an extract of Berberis vulgaris at 200 mg/kg had significant effects (Rahimi-Madiseh, Karimian, Kafeshani, & Rafieian-Kopaei, 2017). Taking the amount of plant extract used in that study as a reference, we chose 200 mg/kg as our first dose and doubled that concentration for the high dose.
The administration of Berberis vulgaris extract to rats with ovarian ischemia and IR produced antioxidant effects by increasing GSH, SOD, and CAT enzyme levels with improved ovarian functions. In addition, Berberis vulgaris decreased the levels of MDA, a biomarker found in the ovaries with increased synthesis in response to ovarian injury, and brought those levels back to those of the control group.
The mitochondrial electron transport chain’s third proton pump is formed in part by the mitochondrial complex III subunit CYC1 (Duncan, Ozawa, Suzuki, & Rozen, 1994). Increased oxidative stress has been shown to activate different caspase enzymes, which releases CYC1 into the cytoplasm and initiates apoptosis (Haider et al., 2009). In our study, while CYC1 immunoreactivity was not observed in the control group, significant levels of CYC1 immunoreactivity were found after ovarian ischemia. Berberine treatment dramatically decreased those levels. It is thought that the increase in CYC1 immunoreactivity in ovaries subjected to ischemia or IR was due to cellular disruption, while berberine exerted a protective effect by decreasing the release of CYC1.
Ovarian IR injury is a highly complicated pathological condition that leads to excessive free radical formation in the affected tissues (Nayki et al., 2016). IR-induced ROS production increases the transcription of proinflammatory cytokines, including TNF-α (Prince, Rodríguez Lanzi, Fraga, & Galleano, 2019). TNF-α can exacerbate tissue and organ damage by inducing the synthesis of other inflammatory markers (Zhang et al., 2013). Yilmaz et al. showed that a single berberine dose of 200 mg/kg had a weaker effect on inflammation, but long-term berberine treatment at a dose of 150 mg/kg for 15 consecutive days caused a significant decrease in inflammation. In the same study, it was found that increased follicular cell degeneration, vascular congestion, and bleeding due to IR were reduced with berberine administration. These results are consistent with our findings. (Yilmaz, Ilgen, Mankan, Yilmaz, & Kurt, 2023). Berberine has been reported to alleviate ovarian tissue damage by reducing the expression of overproduced TNF-α and other inflammatory factors in patients with PCOS (Uri-Belapolsky et al., 2017). In contrast to the control group, we observed that the groups subjected to ovarian ischemia or IR had higher levels of TNF-α expression. These results support the findings of earlier research demonstrating elevated TNF-α levels following ovarian IR injury (Yuksel et al., 2023).
TNF-α induces inflammation and leads cells to programmed cell death (Lee et al., 2019). Although many studies have shown that IR injury in the ovaries triggers apoptosis, there are no studies on the protective effects of Berberis vulgaris extract against IR injury. In one study of ovaries subjected to IR injury, the expression of Bax, a biomarker of apoptosis, was found to be increased in the expanded vascular endothelium and particularly in the granulosa cells of the corpus luteum (Toprak, Akalın, Öcal, Çavuş, & Deveci, 2023). Another study of ovarian IR injury demonstrated a statistically significant increase in TUNEL-positive cells in the torsion/detorsion group; furthermore, IR injury increased the apoptosis index in preantral, antral, and Graafian follicles (Mohammadi et al., 2022). In our study, the H&E staining results obtained from histopathological evaluations showed that hemorrhagic areas were significantly more prevalent following ovarian ischemia and IR. However, administration of Berberis vulgaris extract reduced the damage following both ischemia and IR, providing results closer to those of the control group. With the TUNEL staining method that we utilized to evaluate apoptosis, it was observed that the numbers of TUNEL-positive cells, which were increased in the groups with ischemia and IR, decreased significantly after Berberis vulgaris administration, especially in the IR groups.
5 Conclusion
The results obtained in this study confirmed our hypothesis that berberine administration may attenuate ovarian injury induced by IR. The protective effects of berberine on ovarian tissues contributed to the suppression of inflammation and oxidative stress in ovarian tissues by decreasing TNF-α expression, decreasing MDA levels, and increasing SOD, CAT, and GSH enzyme activities. Berberine also exerted an antiapoptotic effect by reducing the number of TUNEL-positive apoptotic cells and CYC1 immunoreactivity. The findings of this study suggest the role of antioxidant and anti-inflammatory mechanisms in the protective effects of berberine against IR injury.
5.1 Limitations
This study has some limitations. As in the literature, we administered the therapeutic agent before IR injury. Clinically, however, medication is given in cases of ovarian torsion only after ischemia occurs. The pre-administration of Berberis vulgaris to patients at risk of ovarian torsion during surgical interventions may have the potential to provide protection. Although our study has provided valuable information to clarify the functions of berberine, our conclusions are preliminary because compounds other than berberine also play roles in the effects of Berberis vulgaris extract. Our experimental results showing that ovarian injury was reduced by Berberis vulgaris should be supported by clinical studies
6 Ethics committee approval
The Local Ethics Committee of Animal Experiments of Kafkas University approved this study (Permission Number:2020-91).
CRediT authorship contribution statement
Serdar Yigit: Methodology, Data curation, Conceptualization. Isa Yesilyurt: Formal analysis. Soner Bitiktas: Formal analysis. Pınar Aksu Kilicle: Methodology. Lale Duysak: Methodology. Muhammed Yayla: Methodology, Data curation, Conceptualization. Erdem Toktay: Methodology. Nilnur Eyerci: Methodology, Formal analysis, Data curation. Seyit Ali Bingol: Conceptualization. Arzu Gezer: Resources, Funding acquisition. Fatma Necmiye Kaci: Methodology. Ergin Taskin: Methodology. Gül Esma Akdogan: Methodology, Investigation. Hasan Cilgin: Methodology. Ali Alper Kahraman: Methodology.
Informed consent
Written informed consent was obtained from patients who participated in this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bostancı, M. S., Bakacak, M., İnanc, F., Yaylalı, A., Serin, S., Attar, R., Yildirim Ö, K., Yildirim, G. 2016. The protective effect of G-CSF on experimental ischemia/reperfusion injury in rat ovary. Arch Gynecol Obstet, 293(4), 789-795. doi:10.1007/s00404-015-3878-8.
- Campo, G. M., Avenoso, A., Campo, S., Ferlazzo, A. M., Micali, C., Zanghı̀, L., Calatroni, A. 2004. Hyaluronic acid and chondroitin-4-sulphate treatment reduces damage in carbon tetrachloride-induced acute rat liver injury. Life sciences, 74(10), 1289-1305.
- Berberine attenuates cerebral ischemia-reperfusion injury via activating PI3K-Akt signaling in a rat model of type 2 diabetes. Int. J. Clin. Exp. Med.. 2017;10(12):16196-16202.
- [Google Scholar]
- Protective effects of nebivolol on ovarian ischemia-reperfusion injury in rat. J. Obstet. Gynaecol. Res.. 2020;46(11):2407-2416.
- [CrossRef] [Google Scholar]
- What is the protective effect of metformin on rat ovary against ischemia-reperfusion injury? J. Obstet. Gynaecol. Res.. 2018;44(2):278-285.
- [CrossRef] [Google Scholar]
- Assignment of the gene for the cytochrome c1 subunit of the mitochondrial cytochrome bc1 complex (CYC1) to human chromosome 8q24.3. Genomics. 1994;19(2):400-401.
- [CrossRef] [Google Scholar]
- Bioactive characteristics of wild Berberis vulgaris and Berberis crataegina fruits. J. Chem.. 2020;2020(1):8908301
- [Google Scholar]
- Determination of antioxidant capacities and biochemical compounds of Berberis vulgaris L. fruits. Adv. Environ. Biol.. 2013;7(2):344-348.
- [Google Scholar]
- Concurrent upregulation of endogenous proapoptotic and antiapoptotic factors in failing human hearts. Nat. Clin. Pract. Cardiovasc. Med.. 2009;6(3):250-261.
- [CrossRef] [Google Scholar]
- Berberis Vulgaris and Berberine: An update review. Phytother. Res.. 2016;30(11):1745-1764.
- [CrossRef] [Google Scholar]
- Effects of vitamin B12 on rat ovary with ischemia-reperfusion injury. Biotech. Histochem.. 2022;97(4):284-289.
- [CrossRef] [Google Scholar]
- Berberine inhibits the ischemia-reperfusion induced testicular injury through decreasing oxidative stress. Am. J. Emerg. Med.. 2020;38(1):33-37.
- [CrossRef] [Google Scholar]
- Absence of cytosolic 2-Cys Prx subtypes I and II exacerbates TNF-α-induced apoptosis via different routes. Cell Rep.. 2019;26(8):2194-2211.e2196.
- [CrossRef] [Google Scholar]
- The impact of Chrysin on the Folliculogenesis and ovarian apoptosis in ischemia-reperfusion injury in the rat model. Int. J. Fertil. Steril.. 2022;16(4):299-305.
- [CrossRef] [Google Scholar]
- Effect of Kineret® on ovarian ischemia reperfusion injury in a rat model. J. Obstet. Gynaecol. Res.. 2016;42(11):1525-1533.
- [Google Scholar]
- The effect of rutin on ovarian ischemia-reperfusion injury in a rat model. Gynecol. Endocrinol.. 2018;34(9):809-814.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [Google Scholar]
- The biochemical and histologic effects of adnexal torsion and early surgical intervention to unwind detorsion on ovarian reserve: An experimental study. Reprod. Sci.. 2013;20(11):1349-1355.
- [CrossRef] [Google Scholar]
- Dietary (-)-epicatechin affects NF-κB activation and NADPH oxidases in the kidney cortex of high-fructose-fed rats. Food Funct.. 2019;10(1):26-32.
- [CrossRef] [Google Scholar]
- The effects of ethanol extract of Berberis vulgaris fruit on histopathological changes and biochemical markers of the liver damage in diabetic rats. Iran. J. Basic Med. Sci.. 2017;20(5):552-556.
- [CrossRef] [Google Scholar]
- Berberis vulgaris: Specifications and traditional uses. Iran. J. Basic Med. Sci.. 2017;20(5):569-587.
- [CrossRef] [Google Scholar]
- The effect of dragon fruit extract on experimental mesentery arterial ischemia-reperfusion in rats. Kafkas Üniversitesi Veteriner Fakültesi Dergisi. 2021;27(5)
- [Google Scholar]
- Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem.. 1968;25:192-205.
- [Google Scholar]
- Protective effect of Berberine pretreatment in hepatic ischemia/reperfusion injury of rat. Transpl. Proc.. 2015;47(2):275-282.
- [CrossRef] [Google Scholar]
- Effects of apigenin on experimental ischemia/reperfusion injury in the rat ovary. Balkan Med. J.. 2017;34(5):444-449.
- [CrossRef] [Google Scholar]
- A simple method for clinical assay of superoxide dismutase. Clin. Chem.. 1988;34(3):497-500.
- [Google Scholar]
- Oxidative stress and total antioxidant status in myocardial infarction. Singapore Med. J.. 2007;48(2):137-142.
- [Google Scholar]
- Effects of daidzein on rat ovary against ischemia-reperfusion. Acta Cir. Bras.. 2023;38:e384423
- [CrossRef] [Google Scholar]
- The investigation of the possible antigenotoxic in vivo effects of pomegranate (Punica granatum L.) peel extract on mitomycin-C genotoxicity. Turk. J. Vet. Anim. Sci.. 2020;44(2):382-390.
- [Google Scholar]
- Interleukin 1-alpha deficiency increases the expression of Follicle-stimulating hormone receptors in granulosa cells. Mol. Reprod. Dev.. 2017;84(6):460-467.
- [CrossRef] [Google Scholar]
- Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol.. 2007;39(1):44-84.
- [CrossRef] [Google Scholar]
- Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren. Fail.. 2015;37(3):482-493.
- [CrossRef] [Google Scholar]
- Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol.. 2006;17(6):300-312.
- [Google Scholar]
- What is the protective effect of krill oil on rat ovary against ischemia-reperfusion injury? J. Obstet. Gynaecol. Res.. 2019;45(3):592-599.
- [CrossRef] [Google Scholar]
- The effects of berberine on ischemia-reperfusion injuries in an experimental model of ovarian torsion. Clin. Exp. Reprod. Med.. 2023;50(4):292-298.
- [CrossRef] [Google Scholar]
- Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: Role of silent information regulator 1. Oxid. Med. Cell. Longev.. 2016;2016:1689602
- [CrossRef] [Google Scholar]
- Effect of trimetazidine against ovarian ischemia/reperfusion injury in rat model: A new pathway: JAK2/STAT3. Iran. J. Basic Med. Sci.. 2023;26(11):1370-1379.
- [CrossRef] [Google Scholar]
- Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose. Food Chem. Toxicol.. 2013;58:50-55.
- [CrossRef] [Google Scholar]
- Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod. Health. 2021;18(1):171.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103578.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







