Translate this page into:
The effect of sour cherry consumption on blood pressure, IL-6, CRP, and TNF-α levels: A systematic review and meta-analysis of randomized controlled trials sour cherry consumption and blood pressure
⁎Corresponding author at: School of Public Health and Health Management, Chongqing Medical University, No. 1 Yixueyuan road, Yuzhong district, Chongqing, China. zhangyongcq@live.cn (Yong Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Cherries are rich in polyphenols and anthocyanins and are believed to possess strong anti-inflammatory activity and cardio-protective effects. This study aim was to summarize the overall effect of sour cherry consumption on blood pressure and biomarkers associated with the inflammation.
Methods
MEDLINE/PubMed, Cochrane, and Scopus were searched to find relevant papers up to April 2019. There were no time and language restrictions. This systematic review and meta-analysis was performed in accordance with the Preferred Items for Reporting of Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Eleven studies with 275 participants were included in this meta-analysis. Mean dose of the sour cherry was 170 mL/day, whilst mean duration of interventions was six weeks. Combined the results using random-effects model showed significant reductions in diastolic blood pressure levels following sour cherry consumption (WMD: −2.32 mmHg, 95% CI: −4.45, −0.19, I2 = 39%), but there were no significant changes in systolic blood pressure (WMD: −2.64 mmHg, 95% CI: −5.84, 0.56, I2 = 33%), IL-6 levels (WMD: 0.12 pg/mL, 95% CI: −0.13, 0.36, I2 = 49%), hs-CRP (WMD: −0.13 mg/mL, 95% CI: −0.47, 0.22, I2 = 49%) or TNF-α (WMD: −0.07 pg/mL, 95% CI: −0.18, 0.05, I2 = 00%).
Conclusion
Sour cherry supplementation results a decrease in diastolic blood pressure, but elicits no significant effect on systolic blood pressure, IL-6 levels, CRP levels and TNF-α.
Keywords
Sour cherry
Blood pressure
Inflammation
- WMD
-
weighted mean difference
- IL
-
Interleukin
- CRP
-
C-Reactive Protein
- TNF
-
Tumor necrosis factor alpha
- CVD
-
cardiovascular diseases
Abbreviations
1 Introduction
Globally, cardiovascular diseases (CVDs) persist as one of the primary causes of mortality and morbidity among non-communicable diseases (Roth et al., 2017). Annually, 17.9 million deaths are attributed to CVDs, approximating 31% of the world’s deaths. Furthermore, CVDs are estimated to directly cause 80% of deaths in Americans who are over 64 years of the age (Benjamin et al., 2019), and whilst in 2015, USA health care expenditure for CVDs was around 656 billion dollars, it is expected to be double by 2030, and reach 1208 billion dollars (Benjamin et al., 2019). High blood pressure, or hypertension, and inflammation are the two major factors in the development of CVDs and atherosclerosis (Gupta et al., 2013).
Typically, the treatment of high blood pressure and inflammation consists of medications, primarily consisting of calcium channel blockers, angiotensin converting enzyme inhibitors, lipid-lowering agents, diuretics, beta-blockers, and non-steroid anti-inflammatory drugs (NSAIDs) for inflammation (Chai et al., 2018). These medications are shown to be somewhat beneficial in reducing cardio-vascular risks. Although these agents lower the risk of CVDs, they are associated with side effects, such as angiotensin converting enzyme inhibitors-induced dry cough, NSAIDs induced hepato-toxicity and nephrotoxicity, and lipid-lowering agents induced muscle damage(rhabdomyolysis), which cause harm and, in some cases, are fatal (Banach et al., 2015).
Concomitant to traditional pharmacotherapies, there is a growing evidence base to suggest that natural resources, such as phytochemicals within fruits and vegetables, may act to reduce risk CVDs, and conceivably reduce health care burdens (Miller et al., 2017). However, there is equivocality with regard to whether natural resources are sufficient to treat and prevent cardiovascular risk factors. Indeed, various epidemiological studies state that a diet rich in fruits and vegetables serves as a protective agent against CVDs. Furthermore, fruits, which are rich, source of polyphenolic compounds, especially, anthocyanin, possess potentially cardio-protective properties (Aune et al., 2017). Cherries are fruits which are rich in polyphenols and anthocyanin, and are believed to possess strong anti-inflammatory activity and cardio-protective effects. Moreover, cherries increase the activity of nitric oxide synthase, which serves to be efficient in arterial stiffness, and thus, they may be potentially beneficial in preventing atherosclerosis, by helping to maintain proper endothelial functions (Chai et al., 2018). In addition, cherries possess anti-oxidant properties as well as lipid reducing power. Furthermore, there are no known adverse side effects associated with cherries, and their consumption can ameliorate post-exercise induced inflammation, improve muscle recovery, and enhance circulatory melatonin levels (Lynn et al., 2014b). Therapeutic application of cherries has also yielded beneficial responses in inflammation-associated diseases, especially in osteoarthritis and gout (Schumacher et al., 2013b). A number of observational studies exploring the effect of raw cherries on blood pressure, C-reactive protein (CRP), Tumor Necrotic Factor (TNF), and Interleukin-6 (IL-6) (Chai et al., 2018; Lynn et al., 2014b) have recently been conducted, however, to date, there exists no summative assessment available. Therefore, the aim of the present study was to summarize the overall effect of sour cherry consumption on blood pressure and the biomarkers associated with inflammation.
2 Methods
PRISMA statement used to conduct present meta-analysis (Moher et al., 2015).
2.1 Search strategy
A search was performed in PubMed/MEDLINE, Cochrane and SCOPUS by two reviewers, from database inception until April 2019. Details of the comprehensive search strategy are presented in supplementary Table 1.
Author
Location
year
Participants (n)
Gender(1women, 2men, and 3 both
Age (year)
dose (mg or ml/ day)
Duration of study (week)
Sheau C. Chai
USA
2019
37
3
65–80
480
12
Brown, M. A.
UK
2019
20
2
19
30
1
Bakkar, Z. A.
UK
2019
12
1
54
1.7
4
Martin, K. R.
USA
2018
10
3
38
240
10
Jackman, S. R.
UK
2018
16
1
60–75
30
3
Desai, T.
UK
2018
11
3
30
30
10
Chai, S. C.
USA
2018
37
3
65–80
480
12
Bell, P. G.
UK
2016
16
1
25
30
1
Levers, K.
USA
2015
23
1
20
480
2
Lynn, A.
UK
2014
47
3
30–50
30
6
Naruszewicz, M.
Poland
2007
44
3
56
255
6
2.2 Selection criteria and data extraction
The PICOS criteria was used to conduct this studies. Two authors evaluated the title and abstract of all articles, independently. The inclusion criteria for including were: Intervention using Sour Cherry, RCT studies reporting our outcome measures in the form of mean differences (MD) with the confidence intervals. Studies that did not report relevant outcomes, were animal-based or review studies and non-randomized studies were excluded. Furthermore, studies without a control group, conference, commentaries, and case-reports studies were also excluded.
Data extraction from the included studies as performed by two reviewers, independently.
2.3 Quality assessment
Cochrane tool was used for the assessment of quality of the included studies (Higgins et al., 2011).
2.4 Statistical analysis
All statistical analyses were conducted using the STATA 14 (StataCorp LP, College Station, USA). Weighted mean difference (WMD) with the 95% CI was used to measure effects of Sour Cherry on outcome measures. DerSimonian and Laird method random effects model was used to calculate the pooled effects. Heterogeneity across the included studies was evaluated by the I-squared and an alpha of 0.05 for p heterogeneity. Sensitivity analysis was run to examin effect of each studies. The publication bias evaluated by funnel plot, Egger’s and begg’s weighted tests.
3 Results
Following comprehensive systematic searching, 63 studies were retrieved (Fig. 1). After removing duplicates and title and abstract screening, 16 articles remained for full text examination. In full text screening, 5 studies were excluded because they did not meet our inclusion criteria such as they was not RCTs or was not on human, and finally, 11 studies were included in this meta-analysis(Bakkar et al., 2019; Bell et al., 2016; Brown et al., 2019; Chai et al., 2018; Chai et al., 2019a,b; Desai et al., 2018; Jackman et al., 2018; Levers et al., 2015; Lynn et al., 2014a; Martin et al., 2018; Schumacher et al., 2013a).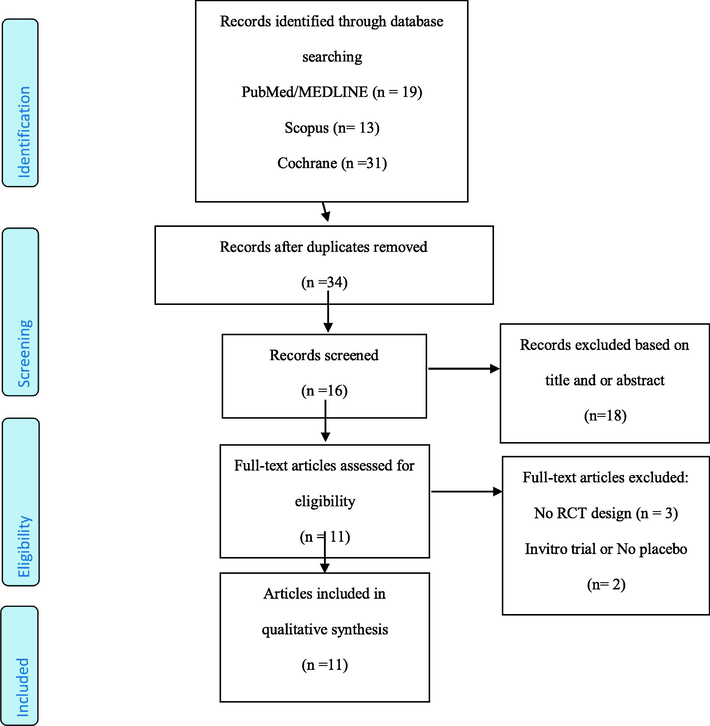
Flow chart of included studies.
3.1 Study characteristics
Table 1 details characteristics of the included studies, which were published between 2013 and 2019 and conducted in the US (Chai et al., 2018; Chai et al., 2019a,b; Levers et al., 2015; Martin et al., 2018; Schumacher et al., 2013a) or UK (Bakkar et al., 2019; Bell et al., 2016; Brown et al., 2019; Desai et al., 2018; Jackman et al., 2018; Lynn et al., 2014a). The total sample size was 275 participants with a men age of 45 years. Mean dose of the sour cherry was 170 mL/day, whilst mean duration of interventions was six weeks, ranging from one to 12 weeks. The risk of bias in included randomized control trials was low (Table 2).
Study name
Year
Selection bias Random sequence generation
Selection bias Allocation concealment
Performance bias Blinding
Detection bias Blinding
Attrition bias Incomplete outcome data
Reporting bias Selective reporting
Other bias Other sources of bias
Chai, S.C.
2019
Low
Unclear
Unclear
Low
Low
Low
Low
Brown, M. A.
2019
Low
Unclear
Low
Low
Low
Low
Low
Bakkar, Z.A
2019
Low
Low
Low
Low
Low
Low
Low
Martin, K.R.
2018
Low
Unclear
Low
Low
Low
Low
Low
Jackman S.R.
2018
Low
Unclear
Low
Low
unclear
Low
Low
Desai, T
2018
Low
Unclear
Low
Low
Low
Low
Low
Chai, S.C.
2018
Low
Unclear
Low
Low
Low
Low
Low
Bell, P.G
2016
Low
Unclear
Low
Low
Low
Low
Low
Levers, K.
2015
Low
Unclear
Low
Low
Low
Low
Low
Lynn A
2014
Low
Low
Low
Low
Low
Low
Low
Schumacher, H.
2013
Low
Unclear
Low
Low
Low
Low
Low
3.2 Results of meta-analysis
Three studies with 102 participants reported blood pressure as an outcome measure (Chai et al., 2018; Desai et al., 2018; Lynn et al., 2014a). The random-effects model demonstrated significant reductions in diastolic blood pressure levels following sour cherry consumption (WMD: −2.32 mmHg, 95% CI: −4.45, −0.19, I2 = 39%) (Fig. 1), but there was not any significant change in systolic blood pressure (WMD: −2.64 mmHg, 95% CI: −5.84, 0.56, I2 = 33%).
Three studies, providing 60 participants, reported IL-6 as an outcome measure (Bakkar et al., 2019; Jackman et al., 2018; Levers et al., 2015). Pooled data showed not reduction in IL-6 levels in the intervention group compared with the placebo group (WMD: 0.12 pg/mL, 95% CI: −0.13, 0.36, I2 = 49%).
Results from the random-effects model on seven studies (Bakkar et al., 2019; Bell et al., 2016; Brown et al., 2019; Chai et al., 2019a,b; Jackman et al., 2018; Lynn et al., 2014a; Schumacher et al., 2013a) containing 195 participants (104 participants in intervention group and 91 participants in placebo groups) indicated that sour cherry did not have significant reduction in hs-CRP levels (WMD:-0.13 mg/mL, 95% CI: −0.47, 0.22, I2 = 49%) (Fig. 2.).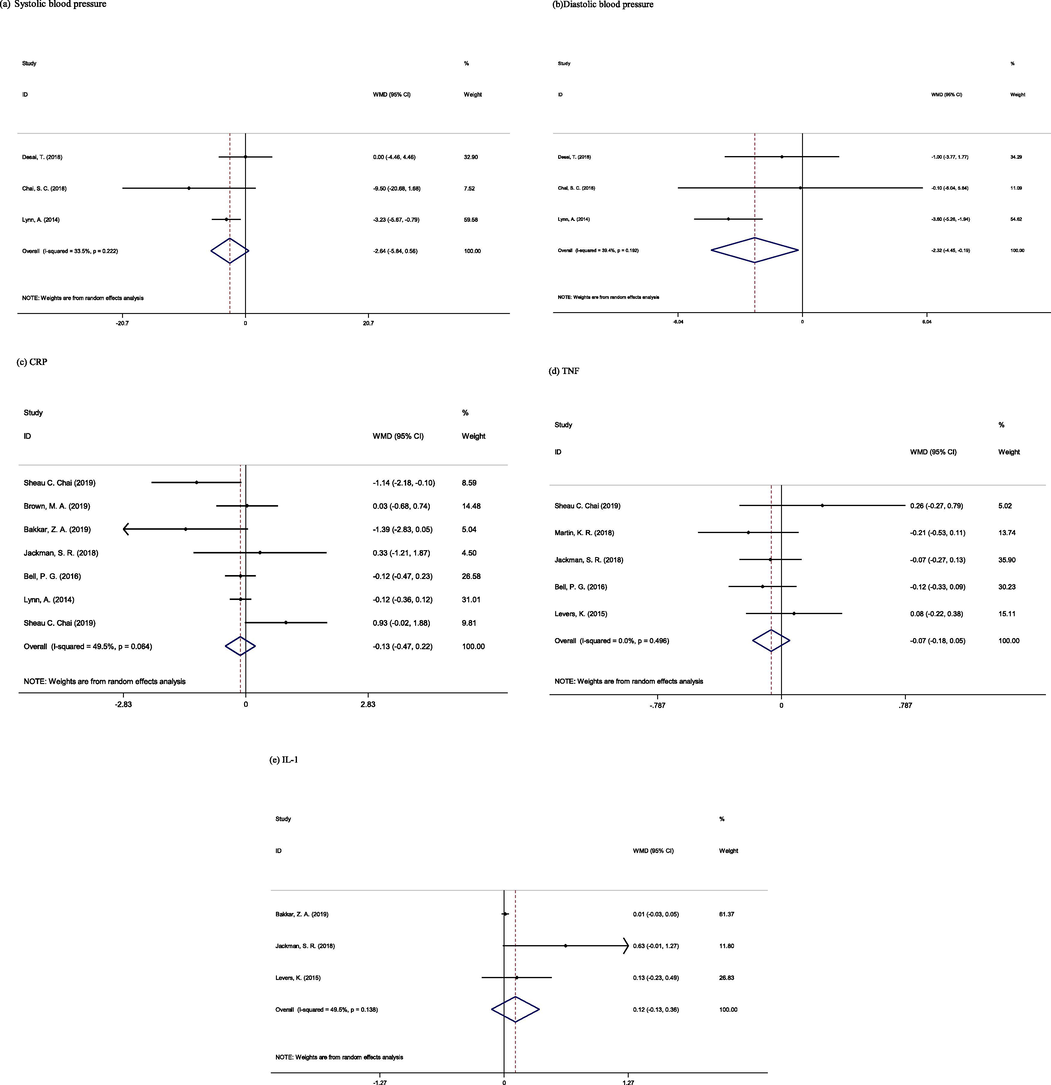
Meta-analysis of effect of Sour Cherry on (a) Systolic blood pressure, (b) Diastolic blood pressure, (c) CRP, (d) TNF, and (e) IL-1.
We analyzed the results from five studies containing 106 participants that reported TNF-α as an outcome measures (Bell et al., 2016; Chai et al., 2019a,b; Jackman et al., 2018; Levers et al., 2015; Martin et al., 2018) using a random-effects model. The results did not show any significant change in the intervention group compared to the placebo group (WMD: −0.07 pg/mL, 95% CI: −0.18, 0.05,I2 = 00%).
3.3 Publication bias and sensitivity analysis
Publication bias is illustrated in Supplementary Fig. 1. The Egger’s and Begg’s tests show not publication bias for systolic blood pressure (p = 0.82, p = 0.60), diastolic blood pressure (p = 0.35, p = 0.52), CRP (p = 0.81, p = 0.65), TNF-α (p = 0.31, p = 0.49), and IL-6 (p = 0.27, p = 0.11). The sensitivity analysis shows not significant differences beyond the limits of 95% CI of calculated pooled results.
4 Discussion
Sour cherry is the result of the hybridization of sweet cherry and the European dwarf cherry, and belongs to the Rosaceae family. Sour cherry (Prunus cerasus L.) is shown to possess anti-oxidant, anti-inflammatory and anti-mutagenic activity, which is attributable to a high flavonoid content. Moreover, sour cherry is considered to have the capacity to control hypertension because of the presence of components like anthocyanins and some flavone and flavan-3-ol compounds, although it is difference between hemodynamic process and inflammatory/oxidative processes (Nemes et al., 2018; Solak et al., 2016).
Anthocyanins are natural water-soluble flavonoids, and chemically they are polydroxy and polymethoxylated glycosides of anthocyanidins bound to several moieties. Anthocyanins exhibit a wide variety of biological effects, including vasodilators, and attributed to act as free radical scavenging and antioxidant activity (Soobrattee et al., 2005). However, the major correlation was found with vasodilatory capacity (Nile et al., 2015). Evidence from the reviews showed that anthocyanins condensed tannin-containing fractions that showed to have vasodilation activity compared to other polyphenols (Reis et al., 2016). In a rat model study, hypertensive stroke-prone rats are treated with anthocyanins rich blueberries for 8 weeks demonstrated significant reduction in the systolic blood pressure. Anthocyanins showed nitric oxide-dependent vasodilation via endothelium induced by acetylcholine through the nitric oxide metabolism pathway — furthermore, the other vasodilator effect of anthocyanins in the endothelium-dependent relaxation (Kalea et al., 2009).
Clinical trials have yielded evidence that sour cherry can significantly reduce hypertension, were such effects are likely manifest due to the circulating phenolic content of the cherries (Keane et al., 2016). Moreover, Chai et al. suggested that the anti-hypertensive ability of sour cherry can be due to its anti-oxidant and anti-inflammatory activity (Chai et al., 2019a,b).
We analyzed eleven studies and found that sour cherry consumption can significantly reduce diastolic blood pressure, but elicited no significant effect on systolic blood pressure, IL-1 levels, hs-CRP levels, and TNF-α. We observed that sour cherry consumption can significantly reduce diastolic blood pressure but the reduction in the systolic blood pressure, was found to be non-significant. Sour cherry has a high flavonoid content (Nemes et al., 2018), which are well linked with the prevention of CVDs (He & Giusti, 2010). Moreover, a previous meta-analysis of seven prospective cohort studies revealed that flavonol intake can significantly reduce the cardiovascular mortality risk (Huxley & Neil, 2003), whilst a significant reduction in blood pressure, HbA1c and body weight was observed among diabetic woman (Ataie-Jafari et al., 2008). However, our study revealed that there is no significant reduction in systolic BP, which has previously been reported (Anthony Lynn et al., 2014). Thus, there is a need to assess the exact chemical constituency of sour cherries, to ascertain what is contributing to its cardioprotective effect. However, evidence from seven studies included in the present meta-analysis demonstrated that there was no significant reduction in CRP level after consumption of sour cherry, when compared to the placebo group. CRP can inhibit nitric oxide (NO) production and bioavailability, which, in turn, leads to the process of inflammation, atherogenesis and sclerosis (Chai et al., 2019a,b). CRP is strongly recommended as an inflammatory response mediator, and a rise in CRP level can indicate an increased cardiovascular risk (Willerson & Ridker, 2004). Previously, it has been reported that patients who have low CRP levels have better clinical outcomes than those with higher CRP levels, and a 75% higher risk in those in the highest quartile of CRP,when compared to those who are in the lowest quartile range of CRP (Ridker et al., 1998). However, our analysis revealed that sour cherry consumption did not reduce the levels of CRP, and thus, may not represent a viable strategy in reducing CRP levels.
In the present study, pooled analysis of studies revealed that sour cherry juice consumption was not significantly associated with IL-6 and TNF-α reduction, when compared to the placebo group. It is evident that an increase in IL-1β and IL-18 levels will be observed in cases of hypertension, and subsequently lead to renal and vascular inflammation (Krishnan et al., 2014). TNF-α and IL-1β are heavily involved in the pathogenesis of inflammatory bowel disease, and empirical studies have shown that anthocyanin extract of pure sour cherry can significantly reduce the production of TNF-α and IL-1β. Moreover, sour cherry extract can significantly inhibit the production of IL-6 and IL-8, and shown to be beneficial in controlling inflammatory bowel disease monolayers (Le Phuong Nguyen et al., 2018).
A recent review by Ahmed et al. highlighted that sour cherries are widely used in the Unani and Ayurvedic system of medicine, and it is quoted as essential in the treatment of urinary tract infections and other urinary diseases. Furthermore, Amhed et al asserted that sour cherry contains heptatonic, lithotriptic, diuretic, antipyretic, astringent, detergent, demulcent, spermatogenic, aphrodisiac, brain-tonic, anti-inflammatory, sedative and antioxidant properties (Shamsi, 2017). Constituents, like cyanidin-3- glucosyl-rutinoside and cyanidin-3-rutinoside, have been shown to exhibit an anti-oxidant and anti-inflammatory effect, which can be attributed to the cardioprotective effect of the sour cherry fruit and its byproducts (Yılmaz et al., 2018).
The strength of the current meta-analysis that we have used the highest level of evidence available, randomized controlled trials, to assess the effect of sour cherry on hypertension and inflammatory biomarkers, whilst there was no heterogeneity observed among all the studies. However, there was some limitation to the study. Even though we have performed a comprehensive electronic database literature search, we elected to omit grey literature, which may conceivably contain some relevant findings.
5 Conclusion
Sour cherry decrease diastolic blood pressure, but elicits no significant effect on systolic blood pressure, IL-6 levels, hs-CRP levels and TNF-α. Although, results show reducing effect of cherry but use from it in practice dependent to individual and country price and conditions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effects of sour cherry juice on blood glucose and some cardiovascular risk factors improvements in diabetic women: a pilot study. Nutrition Food Sci.. 2008;38(4):355-360.
- [Google Scholar]
- Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol.. 2017;46(3):1029-1056.
- [CrossRef] [Google Scholar]
- Montmorency cherry supplementation attenuates vascular dysfunction induced by prolonged forearm occlusion in overweight, middle-aged men. J. Appl. Physiol.. 2019;126(1):246-254.
- [CrossRef] [Google Scholar]
- Lipids, blood pressure and kidney update 2014. Pharmacol Res. 2015;95–96:111-125.
- [CrossRef] [Google Scholar]
- The effects of montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients. 2016;8(7)
- [CrossRef] [Google Scholar]
- Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. 2019;139(10):e56-e528.
- [CrossRef] [Google Scholar]
- Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur. J. Sport Sci.. 2019;19(1):95-102.
- [CrossRef] [Google Scholar]
- Impact of tart cherry juice on systolic blood pressure and low-density lipoprotein cholesterol in older adults: a randomized controlled trial. Food Funct.. 2018;9(6):3185-3194.
- [CrossRef] [Google Scholar]
- Effects of tart cherry juice on biomarkers of inflammation and oxidative stress in older adults. Nutrients. 2019;11(2)
- [CrossRef] [Google Scholar]
- Effects of tart cherry juice on biomarkers of inflammation and oxidative stress in older adults. Nutrients. 2019;11(2):228.
- [Google Scholar]
- The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Eur. J. Appl. Physiol.. 2018;118(12):2523-2539.
- [CrossRef] [Google Scholar]
- Emerging risk factors for cardiovascular diseases: Indian context. Indian J. Endocrinol. Metab.. 2013;17(5):806-814.
- [CrossRef] [Google Scholar]
- Anthocyanins: natural colorants with health-promoting properties. Ann. Rev. Food Sci. Technol.. 2010;1:163-187.
- [Google Scholar]
- The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928
- [Google Scholar]
- The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr.. 2003;57(8):904.
- [Google Scholar]
- Tart cherry concentrate does not enhance muscle protein synthesis response to exercise and protein in healthy older men. Exp. Gerontol.. 2018;110:202-208.
- [CrossRef] [Google Scholar]
- Vascular reactivity is affected by dietary consumption of wild blueberries in the Sprague-Dawley rat. J. Med. Food. 2009;12(1):21-28.
- [Google Scholar]
- Effects of Montmorency tart cherry (Prunus Cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr.. 2016;103(6):1531-1539.
- [Google Scholar]
- IL-1β and IL-18: inflammatory markers or mediators of hypertension? Br. J. Pharmacol.. 2014;171(24):5589-5602.
- [Google Scholar]
- Protective effect of pure sour cherry anthocyanin extract on cytokine-induced inflammatory caco-2 monolayers. Nutrients. 2018;10(7):861.
- [Google Scholar]
- Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J. Int. Soc. Sports Nutr.. 2015;12:41.
- [CrossRef] [Google Scholar]
- Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Hum. Nutr.. 2014;69(2):122-127.
- [CrossRef] [Google Scholar]
- Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: a randomized, crossover pilot study. Food Funct.. 2018;9(10):5290-5300.
- [CrossRef] [Google Scholar]
- Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390(10107):2037-2049.
- [CrossRef] [Google Scholar]
- Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Rev.. 2015;4(1):1.
- [Google Scholar]
- Determination of flavonoid and proanthocyanidin profile of hungarian sour cherry. Molecules. 2018;23(12):3278.
- [Google Scholar]
- Determination of anthocyanin content and antioxidant capacity of different grape varieties. Ciência e Técnica Vitivinícola. 2015;30(2):60-68.
- [Google Scholar]
- Action mechanism and cardiovascular effect of anthocyanins: a systematic review of animal and human studies. J. Transl. Med.. 2016;14(1):315.
- [Google Scholar]
- Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998;98(9):839-844.
- [Google Scholar]
- Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol.. 2017;70(1):1-25.
- [CrossRef] [Google Scholar]
- Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthritis Cartilage. 2013;21(8):1035-1041.
- [CrossRef] [Google Scholar]
- A review on sour cherry (Prunus cerasus): A high value Unani medicinal fruit. Int. J. Green Pharmacy (IJGP). 2017;11(01)
- [Google Scholar]
- Hypertension as an autoimmune and inflammatory disease. Hypertens. Res.. 2016;39(8):567.
- [Google Scholar]
- Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutation Res./Fund. Mol. Mech. Mutagenesis. 2005;579(1–2):200-213.
- [Google Scholar]
- Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21_suppl_1):II-2-II-10.
- [Google Scholar]
- Sour cherry by-products: compositions, functional properties and recovery potentials–A review. Crit. Rev. Food Sci. Nutr. 2018:1-15.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.01.002.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







