Translate this page into:
The effect of electrodeposited copper sulfide on the cathodic mechanism of copper immersed in synthetic seawater
⁎Corresponding author. khadija.elmouaden@gmail.com (Khadija EL Mouaden) khadija.elmouaden@edu.uiz.ac.ma (Khadija EL Mouaden)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The corrosion behavior of copper electrode in unpolluted and sulfide polluted synthetic seawater (SSW) was carried out. It was demonstrated that the coexistence of sulfide and dissolved dioxygen in seawater leads to an increase in the anodic and cathodic current densities. However, the addition of chitosan polymer as corrosion inhibitor resulted in a significant decrease in the anodic as well as cathodic current densities. The immersion of copper sample in the sulfide-polluted seawater resulted in the formation of a copper sulfide layer on the metal surface. It was observed that the copper sulfide formed on copper foam immersed in sulfide-polluted seawater affects the oxygen reduction reaction (ORR) and, therefore, the entire corrosion mechanism. The copper sulfide film was also obtained on the copper electrodes following electrodeposition method (chronoamperometry). The deposited film increased the diffusion-limiting current suggesting the diffusion-controlled mechanism of the ORR. The presence of chitosan decreased the current density and the rate of oxygen reduction in the synthetic seawater solution.
Keywords
Catalytic activity
Corrosion inhibition
Oxygen reduction
Cathodic reaction
Sulfide polluted synthetic seawater
Electrodeposition
1 Introduction
The sulfide corrosion of copper is considered as the most dangerous attack of copper immersed in the sulfide-polluted seawater. This corrosion is due to the deterioration of the copper oxide film formed on the copper surface, which prevents the metal dissolution. In the presence of sulfide, the formation of copper sulfide takes place, which modifies the surface structure and the corrosion resistance of the copper. Copper sulfide is a p-type semiconductor with a band gap (Eg) of 1.2 eV. The morphology of Cu2S strongly depends on the preparation method and nanowires, nanocages and nanoparticles have been reported earlier (Kuo et al., 2011; Li et al., 2011; Liu et al., 2005). For this purpose, the recent efforts have been devoted on studying the mechanism for the modification of the copper surface by the sulfide films.
One of the methods for the preparation of copper sulfide is the electro-deposition of the layer on the metallic surface. The is deposited on the layers by an exchange reaction of by and the resulting films obtained have an hexagonal structure (Mínguez-Bacho et al., 2015). In solar energy applications, it is obtained by cathodic electro-deposition on a thin film using EDTA and an electrolyte solution composed by and . The deposition parameters (potential, concentration and deposition time) were optimized. The band gap energy calculated was about for obtained copper sulfide layers (Anuar et al., 2002). In another study, the copper sulfide was fabricated through an electro-catalytic reduction of to formic acid and acetonitrile (Qing-Gong et al., 2016). In addition, it can be similarly obtained indirectly by electrodepositing and sulfurization of using a polysulfide electrolyte (Xi et al., 2015).
Herein, this present work consists to clarify the effect of sulfide on the corrosion mechanism of copper immersed in polluted seawater in the presence and in absence of chitosan biopolymer.
For this purpose, we herein present the first report on the electro-deposition of copper sulfide on copper foam for testing the effect of several depot conditions. The deposited film was used to study its influence on oxygen reduction reaction (ORR). The effect of chitosan polymer on the mechanism of cathodic ORR is also studied for immersion of copper sample in synthetic seawater.
2 Materials and methods
2.1 Chemicals
The used sodium sulfide nanohydrate and chitosan powder were purchased from SIGMA-ALDRICH®. The synthetic seawater synthetic seawater (SSW) solution used was prepared according to the ASTM D 1141–90 (1992) with a slight modification, it was composed by: NaCl (24.53 g), KCl (0.695 g), MgCl2 (5.20 g), Na2SO4 (4.09 g), CaCl2 (1.16 g) and NaHCO3 (0.201 g) for one liter of SSW. The pH value of the solution was adjusted at 8.2 using NaOH solution (5 M). All the chemicals used were of analytical grade purity and used without any further purifications.
The Fig. 1 shows the absorbance spectrum of chitosan in the range of 4000–400 cm−1 recorded in KBr disks. The spectrum shows the characteristic absorbance bands of chitosan viz. 3429 cm−1 (O—H and N—H stretch), 2870 cm−1 (C—H elongation), between 450 cm−1 and 1750 cm−1 (N—H vibrations).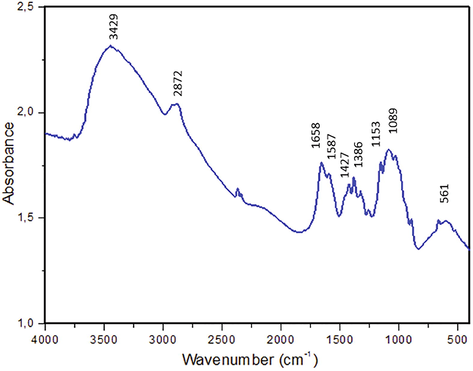
FT-IR spectra of tested chitosan biopolymer.
2.2 Electrochemical method
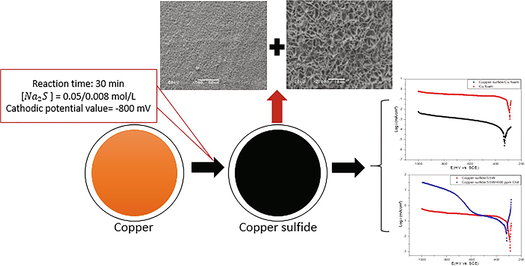
The Cu-foam was firstly pretreated by degreasing in acetone and rinsing with distilled water. The film on copper surface was prepared via the immersion of the copper sample (working electrode) in the electrolyte solution using a thermostatic electrochemical cell with a platinum electrode and a saturated calomel electrode (SCE) as the reference electrode. Na2S solution having concentration of 0.008 and 0.05 mol L−1 was used as the electrolyte. The anodic electro-deposition was performed using chronoamperometry method by applying a constant voltage of +0.8 V/SCE to the copper electrode for 30 min at the temperature of 30 °C with slow stirring. During this period, a black film was formed on the copper surface. After that, the copper foam was washed with distilled water and dried. The morphologies of the deposited films are characterized using a JEOL JSM-6480 LV scanning electron microscopy (SEM). The films were also characterized by X-ray diffraction using X-ray diffractometer (Bruker D8 Advance) for the 2ϴ (range = 20°–80°) with Cu Kα radiation of (K = 1.5418) and scan speed of 1 (°).s−1.
An electrochemical workstation Versastat 3 was used for all electrochemical experiments. The potentiodynamic polarization measurements were carried out in three-electrode cell as described in the deposition part. The working electrode consisted of Cu2S/Cu-foam or Cu-foam. Tafel plots were recorded in aerated synthetic seawater at a sweep rate of mV s−1. In order to elucidate the copper sulfide effect, the cathodic polarization curves were investigated for Cu2S/Cu-foam and Cu-foam from −0.28 to −1 V/SCE in the synthetic seawater solution at a scan rate of 1 mV s−1 with and without stirring.
3 Results and discussion
3.1 Effect of sulfide polluted SSW on the copper corrosion behavior
Fig. 2 shows the effect of addition 20 ppm of sulfides on the anodic and cathodic curves of copper in SSW recorded from −600 to +600 mV/SCE. The obtained electrochemical parameters are summarized in Table 1.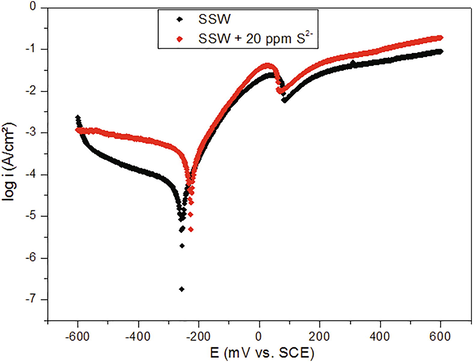
Anodic and cathodic polarization curves for copper immersed in unpolluted and sulfide polluted SSW.
−Ecorr
(mV)jcorr
(µA/cm2)ba
(mV)Rp
(ohm.cm2)Ej
(%)
SSW
256.3
39.8
70.5
505.7
–
SSW + 20 ppm
227.3
94.4
86.0
201.4
–
SSW + 20 ppm S2− + 600 ppm Chit
220.5
16.8
65.3
909.0
82
The shifted towards more positive potential values. In fact, both the anodic and the cathodic reactions were catalyzed by the HS− addition considering the increase of the anodic and cathodic current density. It can be summarized that the sulfides accelerate the copper corrosion in aerated sulfide polluted seawater. These results can be compared to that reported in studies which confirm that the presence of both sulfide and oxygen in seawater, raises the corrosion rate of copper metal (Jacobs and Edwards, 2000; Syrett and Wing, 1980).
We observe that the anodic current density was increased by addition of sulfides (20 ppm) to the SSW. The oxidation peak recorded at +100 mV is attributed to Cu oxidation followed by a current rise due to the conversion of Cu to Cu2S. It appears that the anodic mechanism was also affected. This behavior was supported by the electrochemical impedance spectroscopy recorded for copper sample in SSW without and with sulfide ions which shows that these ions alter the mechanism of the anodic and cathodic polarization curves as reported earlier (El Mouaden et al., 2018).
In addition, it is observed that the addition of sulfide ions leads to the appearance of a limiting current density region in the cathodic part. This plateau (diffusion-limited current) was assigned to the reduction of diffusing only through the boundary layer just, or through the boundary layer and a surface film as a partially reduced film. However, in the both cases, the plateau is controlled by a mass transfer in the presence of sulfide (Quevedo and Genesca, 2009).
3.2 Effect of chitosan-biopolymer
The chitosan biopolymer was added as a corrosion inhibitor to the sulfide containing SSW to study its influence on the corrosion and dissolution of the metal induced by the sulfide ions. Fig. 3 presents the polarization curves for copper obtained in sulfide polluted seawater in the absence and the presence of chitosan. The results reveal that the addition chitosan at 600 ppm reduced considerably the current density values of both the anodic and the cathodic Tafel curves. The introduction of chitosan to the electrolyte solution brought a drastic decrease in the corrosion current density from 94.4 to 16.8 µA cm−2. The corrosive effect of the sulfide species was thereby controlled in the presence of the biopolymer suggesting its effective corrosion inhibition behavior with the inhibition efficiency reaching 82% (Table 1) at a concentration of 600 ppm. The region of the limiting current density was found to be clearly reduced in the presence of chitosan which suggests the formation of a protective barrier on the copper surface which limits at the diffusion of the sulfide ions to the metal surface. It can also be understood that the observed decrease in the cathodic current density takes place due to the non-availability of the reactant (oxygen) (Jacobs and Edwards, 2000) at the electrode surface in the in the presence of chitosan.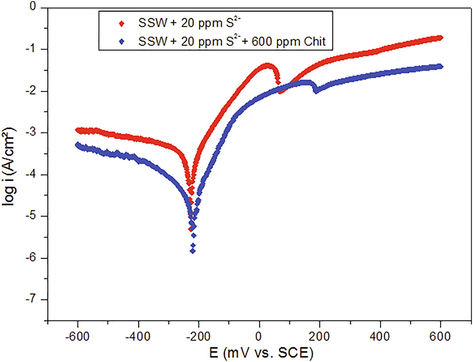
Anodic and cathodic polarization curves for copper immersed in sulfide polluted SSW without and with addition of chitosan at 600 ppm.
In general, the corrosion of metals and their alloys in different media involves at least three necessary steps: (i) transport of the reactant to the metal surface, (ii) electron exchange reaction resulted as a metal loss and (iii) the transport of the corrosion products to the bulk solution (Quevedo and Genesca, 2009). These steps occur in series, so, it is important to consider the two mechanisms (charge and mass transfer) for describing the whole process of corrosion in aqueous solutions.
In the present work, the focus is to obtain an understanding on the relationship of the availability of sulfide and the cathodic reaction as an oxygen reduction mechanism via electro-deposition of copper sulfide.
3.3 Morphology of the copper sulfide film
Fig. 4 shows the SEM and EDS results of copper surfaces after copper sulfide deposition at sulfide concentrations of 0.008 and 0.05 M. It can be observed that the deposited film at 0.008 M of Na2S is composed by agglomerated irregular particles and its growth scan is very low which does not cover the entire copper surface. Regarding to the first image, the second one corresponds to the rinsing of Na2S concentration until 0.05 M. This concentration is enough to observe the growth of the film through irregular particles which, appear more uniform than the first one. On the other hand, the EDS patterns reveal a major intensity for the peaks of copper and culfur in both the electrolytes.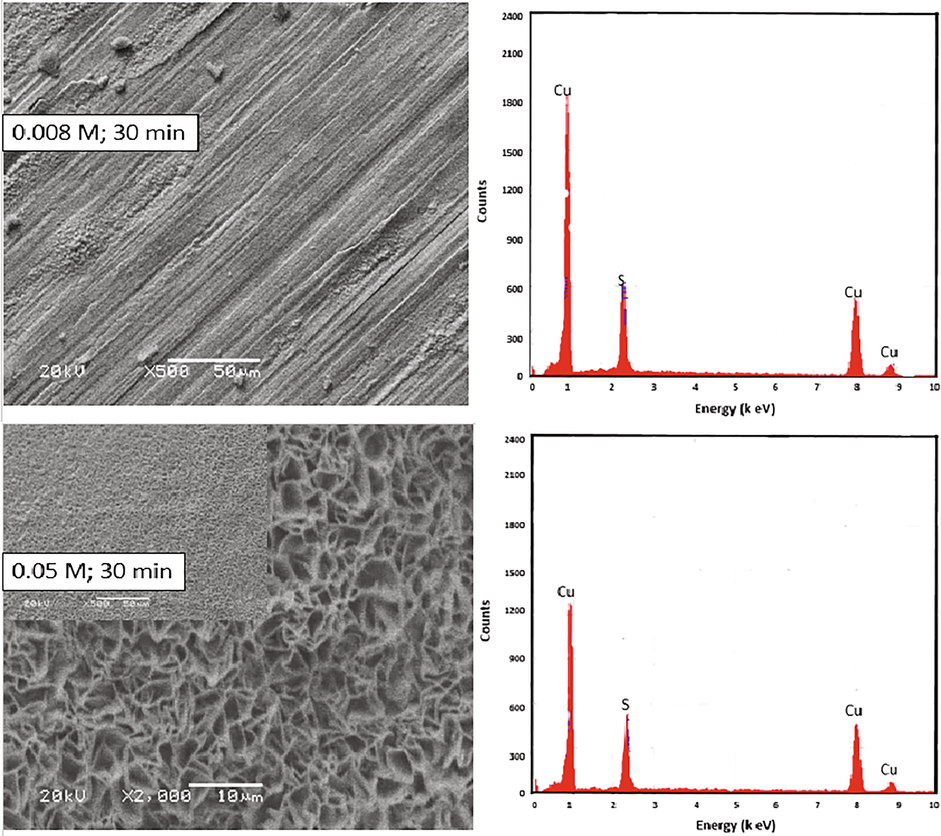
SEM micrographs and EDS patterns of deposited Cu2S film on the copper surface using Na2S solutions at 0.008 and 0.05 M for 30 min electrodeposition time.
The XRD pattern of the deposited film on the copper surface was shown in Fig. 5. The diffraction patterns of the samples (a) and (b) are in accordance with the standard data of the cubic Cu2S (00-053-0522). The relative intensity of the (2 2 0) plane of the Cu2S (a and b) presents the biggest rise compared with the intensity given in the Cu2S PDF card. The increase in the sodium sulfide concentration induced a rise in the intensity of the copper sulfide peaks. On the other hand, the two peaks of copper were detected for the copper foam sample under XRD analysis.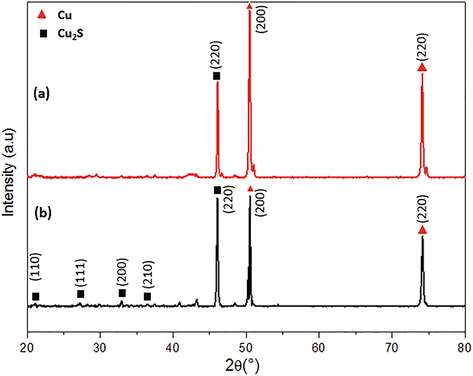
X-ray diffraction patterns of deposited Cu2S on the copper foam in (a) 0.008 and (b) 0.05 M Na2S solutions.
3.4 Application of the deposited copper sulfide film
Fig. 6 presents the cathodic and anodic polarization curves recorded for copper sample in the aerated synthetic seawater at a scan rate of 1 mV s−1 at 30 °C. In the cathodic region, at higher negative potentials values (region I.0 on Fig.67), the current density rises due to the hydrogen evolution reaction. In the Region I.1 on Fig. 6, the oxygen reduction occurs, and it is controlled by the mass transport mechanism resulting in a limiting current density. At less negative potentials values (region I.2 on Fig. 6), the reaction was found to take place under the control of a charger-transfer mechanism by a 4e- process as suggested earlier (Tammeveski et al., 1997; Tammeveski et al., 1999):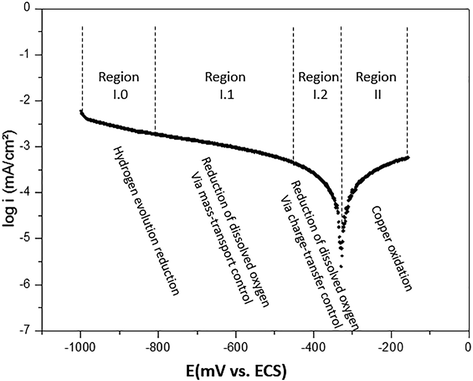
Potentiodynamic polarization curve for copper in SSW solution.
While the anodic branch of the polarization region (region II on Fig. 6) is associated to the oxidation of copper in two continuous steps according to following equations (El-Sayed Sherif et al., 2007; Hu et al., 2010; Mansfeld et al., 1994):
In order to explore the interfacial charge-transfer property of copper sulfide deposited on the copper foam with stirring, the cathodic polarization is recorded and shown in Fig. 7. In the seawater solution, the cathodic reaction is dominated by an oxygen reduction. As can be seen in Fig. 7, the cathodic current of copper is much lower than that of Cu2S/Cu.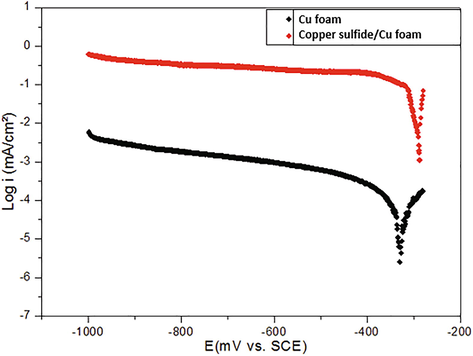
Cathodic polarization curves for copper foam and Cu2S/Cu foam in SSW solution.
In the case of a deposited film, the current density is related to the electro-catalytic activity. Consequently, the electro-catalytic activity was changed considerably for the film formation on copper surface. But the copper sulfide formation shifts the corrosion potential towards anodic values. In addition, the film formation extends the diffusion-limiting current on the cathodic part, confirming that the oxygen reduction is controlled by a diffusion controlled mechanism at the copper sulfide film formed on the electrode surface.
It is commonly agreed that the copper sulfide film exhibits an efficient electro-catalytic activity on the reduction reactions due to its high current density as compared to copper foam electrodes (Qing-Gong et al., 2016). In the current study, this property was verified with the deposited films and it can be concluded that the presence of sulfide affects the corrosion of copper by accelerating the cathodic reduction of oxygen with an activation of metallic oxidation.
Similar observations by Sanchezis and Schiffrin (1982), affirmed that the effect of the formed copper sulfide film is the acceleration of a charge transfer mechanism of the oxygen reduction, resulting in a reduction current density controlled by the diffusive transport. The effect of chitosan biopolymer on the cathodic behavior of copper sulfide film was studied. Fig. 8 shows the cathodic polarization of the deposited copper sulfide in the synthetic seawater without and with the chitosan.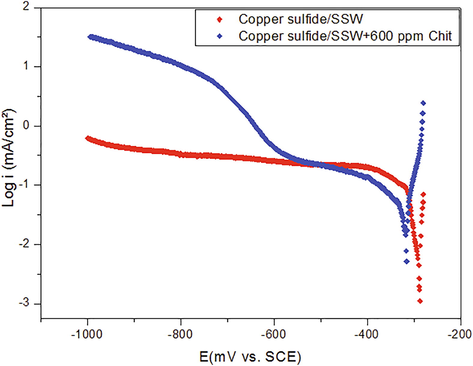
Cathodic polarization curves for copper covered by Cu2S immersed in SSW without and with addition of 600 ppm chitosan biopolymer.
It can be observed that the cathodic diffusion-limiting current disappeared on the addition of chitosan. Also, the current density value was found to be lowered in the presence of chitosan in the Region I.2 of a charge transfer mechanism during the oxygen reduction. This result suggests that the chitosan polymer reacts with the sulfide film for reducing its corrosive effect by dissolution of the layer or by limiting the diffusion of oxygen and electrolytic elements to the metal by the formation of a protective barrier around its surface.
In addition, it can be noted that the presence of chitosan was involved the apparition of a hydrogen-evolution region with a higher cathodic current density. This suggests that ion reduction can occur partially in the cathodic reaction of copper sulfide deposited electrodes immersed in seawater. Besides, this behavior is interpreted in the literature as a boundary reaction between the oxide-type and the sulfide films formed at the electrode surface (Syrett, 1981). It is proposed that the protection efficiency of chitosan biopolymer arises by the reinforcement of the oxide copper film previously deposited underneath the sulfide film as suggested by Mor and Beccaria (Mor and Beccaria, 1975).
It is reported that the hydrodynamic strongly affect conditions the interfacial mechanism of the formation, growth and the stability of the layers. For this purpose, the polarization curves of Cu2S/Cu foam are studied without and with electrolyte stirring (Fig. 9).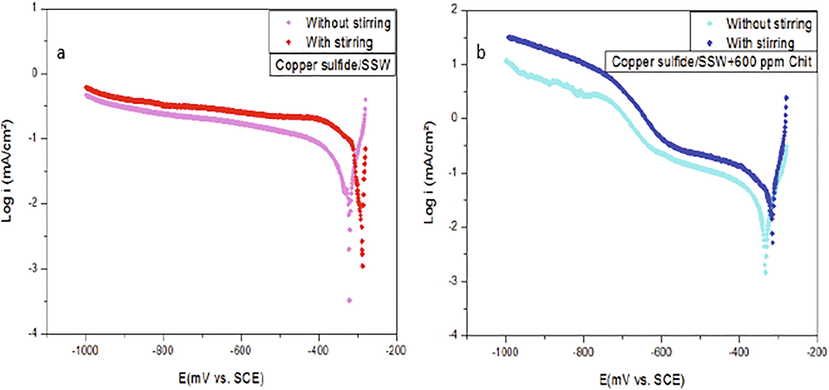
Cathodic polarization curves for copper covered by Cu2S immersed in SSW solution (a) in the absence and (b) in presence of chitosan polymer without and with stirring.
It can be observed that the stirring-parameter has a considerable influence on the shape of polarization plots. A lower current density and cathodic potential of Cu2S/Cu foam were obtained in the static system as compared to the stirring one. This result can be related to an increasing of the catalytic activity of deposited film by the electrolyte stirring process. However, the cathodic diffusion-limiting current was found to be greater for the dynamic systems. Consequently, the electro-catalytic activity of the film was found to be better with the stirring of the electrolyte. Stirring an electrolyte containing chitosan, leads to an increase in the current density of the electrode and the evolution of hydrogen reduction without affecting the shape of the polarization curve shape.
These results can be explained by the variation in the phase and structure of copper sulfide during the potential scan. This is in a good agreement with the reported results in Zhao et al. (2009), which were pointed out that the compositions of nanocrystals were changed from (covellite) to (djurleite) by the adjusting of reduction potential value.
4 Conclusion
A direct electro-deposition of Cu2S/Cu on the copper substrate was performed using chronoamperometry technique by applying a potential of +0.8 V/SCE for 30 min, in 0.008 and 0.05 M Na2S solutions This electrocatalytically active copper sulfide is obtained using a low cost and environmentally friendly electrodeposition method on the copper foam. The variation of Na2S concentration has affected the formed film morphology and its growth. The deposited films are used for studying their effect on the mechanism of oxygen reduction reaction (ORR). The results show that the simultaneous presence of sulfide and dissolved dioxygen is responsible for increase in the rate of ORR and the cathodic current density and the global corrosion process of copper. However, the presence of chitosan biopolymer was reinforced the protective copper oxide growth in order to limit the accelerated attack of the copper sulfide SSW solution.
Acknowledgements
One of the authors (Khadija El Mouaden) gratefully acknowledges the support of Morocco CNRST (National Center for Scientific & Technical Research) through the excellence scholarship for searching program (2014 edition).
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- Cathodic electrodeposition of Cu2S this film dor solar energy conversion. Sol. Energy Mater. Sol. Cells.. 2002;73:351-365.
- [Google Scholar]
- Corrosion of copper in aerated synthetic sea water solutions and its inhibition by 3-amino-1,2,4-triazole. J. Colloid Interface Sci.. 2007;309:470-477.
- [Google Scholar]
- Chitosan polymer as a green corrosion inhibitor for copper in sulfide-containing synthetic seawater. Int. J. Biol. Macromol.. 2018;119:1311-1323.
- [Google Scholar]
- Electrochemical and thermodynamic investigation of diniconazole and triadimefon as corrosion inhibitors for copper in synthetic seawater. Corros. Sci.. 2010;52:2891-2896.
- [Google Scholar]
- Cu2O nanocrystal-templated growth of Cu2S nanocages with encapsulated Au nanoparticles and in-situ transmission x-ray microscopy study. Adv. Funct. Mater.. 2011;21:792-797.
- [Google Scholar]
- Morphology evolution od Cu2-xS nanoparticles: from spheres to dodecahedrons. Chem. Commun.. 2011;47:10332-10334.
- [Google Scholar]
- Growth of Cu2S ultrathin nanowires in a binary surfactant solvant. J. Phys. Chem. B. 2005;109:10699-10704.
- [Google Scholar]
- The corrosion behavior of copper alloys, stainless steels and titanium in seawater. Corros. Sci.. 1994;36:2063-2095.
- [Google Scholar]
- Controlling the nanomorphology of thin conformal Cu2S overlayers grown on Cu2O compact layers and nanowires. Mater. Lett.. 2015;159:47-50.
- [Google Scholar]
- Behavior of copper in artificial sea water containing sulphides. J. British Corrosion.. 1975;10:33-38.
- [Google Scholar]
- Cu2S on Cu foam as highly efficient electrocatalyst for reduction of CO2 to formic acid. Acta Phys.-Chim. Sin.. 2016;32:261-266.
- [Google Scholar]
- Influence of turbulent flow on the corrosion of Al-Zn-Mg galvanic anode in artificial seawater media. Mater. Corros.. 2009;60:424-430.
- [Google Scholar]
- The flow corrosion mechanism of copper base alloys in sea water in the presence of sulphide contamination. Corros. Sci.. 1982;22:585-602.
- [Google Scholar]
- The mechanism of accelerated corrosion of coppernickel alloys in sulphide polluted seawater. Corros. Sci.. 1981;21:187-209.
- [Google Scholar]
- Effect of flow on corrosion of copper-nickel alloys in aerated seawater and in sulphide-polluted seawater. Corrosion. 1980;36:73-85.
- [Google Scholar]
- Oxygen electroreduction on titanium-supported thin Pt films in alkaline solution. Electrochim. Acta. 1997;42:2961-2967.
- [Google Scholar]
- The reduction of oxygen on Pt-TiO2 coated Ti electrodes in alkaline solution. J. Electrochem. Soc.. 1999;146:669-676.
- [Google Scholar]
- Fabricating CuS counter electrode for quantum dots-sensitized solarcells via electro-deposition and sulfurization of Cu2O. Electrochim. Acta. 2015;178:329-335.
- [Google Scholar]
- Plasmonic Cu2−xS nanocrystals: optical and structural properties of copper-deficient copper(I) sulfides. J. Am. Chem. Soc.. 2009;131:4253-4261.
- [Google Scholar]







