Translate this page into:
The effect of Brazil nuts on selenium levels, Glutathione peroxidase, and thyroid hormones: A systematic review and meta-analysis of randomized controlled trials
⁎Corresponding author at: School of Public Health and Health Management, Chongqing Medical University, No.1 Yixueyuan Road, Yuzhong District, Chongqing 400016, China. zhangyongcq@live.cn (Yong Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Brazil nuts or Bertholletia excelsa provide a rich natural source of magnesium, phosphorus, and manganese. Furthermore, it is rich of anti-oxidants such as selenium, vitamin E, and phenols like gallic acid and ellagic acid and have improvement effects on plasma selenium levels, Glutathione peroxidase (GPx), and thyroid hormones but the results have not been summarized in a meta-analysis. The purpose of this study is to investigate the effect of brazil nut on plasma selenium levels, GPx, and thyroid hormones. Literature search was done in MEDLINE/PubMed, Scopus and web of sciences databases up to October 2019. Studies included that had RCTs design, use brazil nut as intervention, and reported selenium levels, Glutathione peroxidase, or thyroid hormones as outcome. PRISMA guidelines followed to perform this meta-analysis and results combined using DerSimonian and Laird random effect model. Seven studies with 315 participant’s included and analyzed in this meta-analysis. Mean duration of intervention was 11 weeks and mean dosage of brazil nut was 9.42 g/day in included studies. Our study found brazil nut have significant increasing effect on plasma selenium levels (WMD: 87.63 microg/l, 95% CI: 36.02, 139.24, I2 = 98%). Furthermore, Brazil nut had increasing effect on GPx levels too (WMD: 8.05U/gHb, 95% CI:0.65, 15.45, I2 = 96%) but brazil nut had no significant effect on T3 (WMD: 0.06 pg/ml, 95% CI: −0.50, 0.39, I2 = 74%), T4 (WMD: −0.01 pg/ml, 95% CI: −0.46, 0.44, I2 = 82%), and TSH levels (WMD: 0.01 ng/ml, 95% CI: −0.03, 0.05, I2 = 0.67%).The findings of this meta-analysis indicates brazil nut increase plasma selenium and GPx levels.
Keywords
Brazil nut
Selenium
Glutathione peroxidase
- GPx
Glutathione peroxidase
- TSH
thyroid stimulating hormone
- T3
Triiodothyronine
- T4
Thyroxine
Abbreviations
1 Introduction
Nuts are regarded as high energy foods that containing high unsaturated fatty acids (Venkatachalam and Sathe, 2006). In addition to their fat content, nuts are abundant sources of fibre, folate, and antioxidants (Kornsteiner et al., 2006; Ros, 2010). There has been extensive research carried out regarding nut consumption and health outcomes, primarily attributable to their desirable nutritional composition, and are purportedly capable of reducing the risk of cardiovascular disease (Cardoso et al., 2017; Mozaffarian, 2016), and its risk factors such as oxidative and inflammation factors (López-Uriarte et al., 2009), and hypercholesterolemia (Cardoso et al., 2017; Del Gobbo et al., 2015).
Selenium supports various physiological processes and regulations (Roman et al., 2014). Moreover, consumption of this micronutrient has been associated with many health benefits, for instance, decreased cancer risk (Méplan & Hesketh, 2014), decreased risk of various neurodegenerative diseases (Cardoso et al., 2015), and modulation of the thyroid function (Ahren et al., 2019; De Farias et al., 2015). Although considered an essential micronutrient, insufficient selenium intake has been reported globally, including Europe, the Middle East, Australasia, South America, and Asia (Abdul-Aziz et al., 2015; Cardoso et al., 2010; Thomson, 2004; Thomson, 2004). This lack of selenium intake represents a global issue with potentially injurious health ramifications, and because of this, brazil nuts have been asserted to be an effective alternative to improve the selenium levels, because they are the most abundant food source of selenium (Thomson et al., 2008). In fact, according to Thomson et al., daily consumption of two nuts that equal to 53 μg of selenium was effective as 100 μg selenium selenomethionine supplementation in improving plasma selenium levels (Thomson et al., 2008).
Furthermore, a recent critical review of brazil nut aetiology found that the nuts possess a high nutritional value (Yang, 2009). It was also discerned that 160% of the US Recommended Daily Allowance (RDA) of selenium provides with one single Brazil nut (Yang, 2009). In Yang et al, the authors surmised that these compounds elicit beneficial effects due to their antioxidant and antiproliferative activities, which are linked to a reduced risk for various non-communicable diseases (Yang, 2009). However, whilst brazil nuts have been well reviewed, to the author’s knowledge, there exists no quantitative analysis elucidating the overall effect of brazil nuts on selenium levels and thyroid hormones. Therefore, the aim of this the study was to investigate the effect of brazil nuts on selenium levels and thyroid hormones, by conducting a systematic review and meta-analysis of randomized controlled trials.
2 Methods
2.1 Literature search
PRISMA guidelines were followed to perform this systematic-review and meta-analysis. Literature search with MeSH terms and keywords conducted in MEDLINE/PubMed, Scopus and Web of sciences (WOS) databases without time and language limitation by two reviewers, independently from inception up to October 2019 (Supplementary Table 1). References cited of relative articles (review and original) were examined too. All disagreements resolved by discussion with senior author.
2.2 Selection criteria
Inclusion criteria were: 1- RCTs design (parallel or cross-over), 2-Intervention with brazil nut, 3- Age ≥ 18 years, 4- Reported selenium levels, Glutathione peroxidase, and thyroid hormones as outcome. We excluded Animal, in vivo, or in-vitro studies, and non-original, conference, and review papers. Studies that examined multiple-component interventions too. In missing data, Authors were contacted with correspond of included studies.
2.3 Statistical analyses
Risk of bias assessment was done by RevMan 5.3, and references managed by End-Note X9 software. Furthermore, statistical analyses performed by STATA software (version 12, College119 station, Texas). Mean difference and SD in intervention and control group used to calculated combined results. Following formula (SD2change = SD2baseline + SD2final − (2 × Corr × SDbaseline × SDfinal)) used to calculate SD of the mean difference, if the studies did not report it (Higgins and Green, 2011). Pooled weighted mean difference (WMD) calculated by Random-effects model. Heterogeneity among results assessed using the I-squared (I2) statistics test. Meta-regression performed to find source of heterogeneity. Publication bias assessed using funnel plots, Begg’s test and Egger’s regression test (Egger et al., 1997). The leave-one-out method conducted to determine the impact of each study on pooled results in sensitivity analysis. Cochrane collaboration’s tool followed for risk of bias assessment (Higgins et al., 2011).
3 Results
3.1 Study selection
Fig. 1 presented the flow diagram of systematic search in databases. In primary search in three databases 205 articles were identified that 86 articles removing because duplication. In title and/or abstract screening 99 articles excluded and in final step of screening full text of 20 articles evaluated that 13 of them did not meet included criteria. Finally, 7 studies selected for inclusion in this meta-analysis (Carvalho et al., 2015; Duarte et al., 2019; Hu et al., 2016; Huguenin et al., 2015; Reis et al., 2019; Rita Cardoso et al., 2016; Thomson et al., 2008).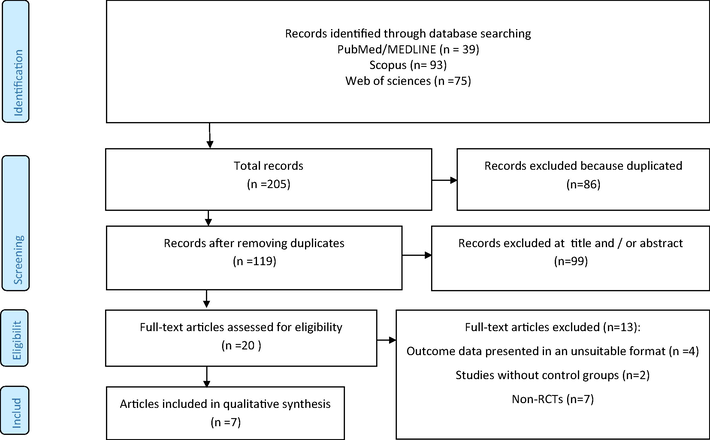
Flow chart of included studies.
3.2 Study characteristics and quality assessment
Table 1 presented details on the characteristics of the included studies. Studies were publishing between 2008 and 2019. Five studies were conducted in Brazil (Carvalho et al., 2015; Duarte et al., 2019; Huguenin et al., 2015; Reis et al., 2019; Rita Cardoso et al., 2016), one in New Zealand (Thomson et al., 2008), and one in Australia (Hu et al., 2016). Five studies conducted in both gender (Carvalho et al., 2015; Hu et al., 2016; Huguenin et al., 2015; Rita Cardoso et al., 2016; C. D. Thomson et al., 2008) and two in women (Duarte et al., 2019; Reis et al., 2019). One study had cross over design (Huguenin et al., 2015) and six had parallel (Carvalho et al., 2015; Duarte et al., 2019; Hu et al., 2016; Reis et al., 2019; Rita Cardoso et al., 2016; Thomson et al., 2008). Mean duration of intervention was 11 weeks from 8 to 24 weeks and mean dosage of brazil nut was 9.42 g per day from 5 to 15 g per day. Quality of studies presented in Fig. 2. Most of included studies had good quality.
Author
Location
year
Participants (n)
Gender
Age (year)
dose (g/day)
Duration of study (week)
Rreis
Brazil
2019
54
Female
18–55
5
8
Duarte
Brazil
2019
49
Female
18–55
5
8
Rita Cardoso
Brazil
2016
20
Both
77.7
5
24
Hu
Australia
2016
21
Both
52–75
15
6
Huguenin
Brazil
2015
91
Both
>20
13
12
carvallo
Brazil
2015
77
Both
40–80
13
12
Thomson
New zeland
2008
40
Both
18–60
10
12
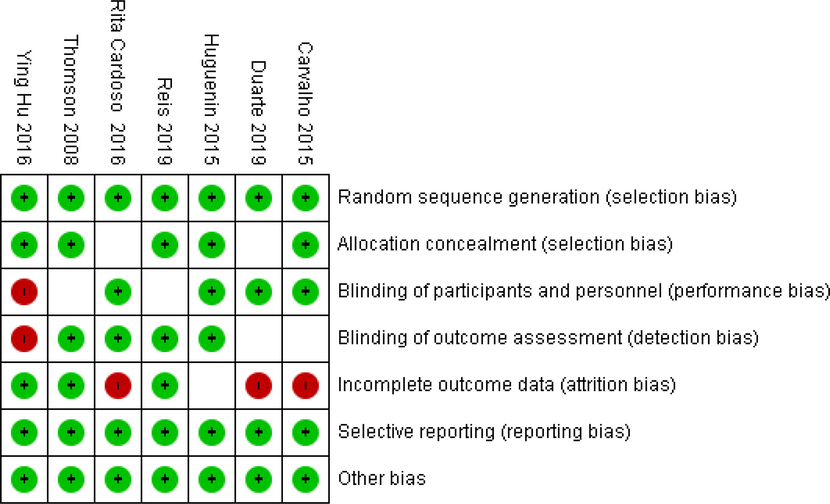
Cochrane risk of bias assessment.
3.3 Meta-analysis
Three studies reported TSH as an outcome measure (Carvalho et al., 2015; Hu et al., 2016; Reis et al., 2019). Combined result by random effect model show not any significant effect from brazil nut on TSH levels (WMD: 0.01 ng/ml, 95% CI: −0.03, 0.05, I2 = 67%) (Fig. 3a). Meta-regression based on dose of brazil nut showed that there is an inverse relation between Dose of brazil nut and TSH levels (Coef = −0.0069) but this relation is not statistically significant (p = 0.26).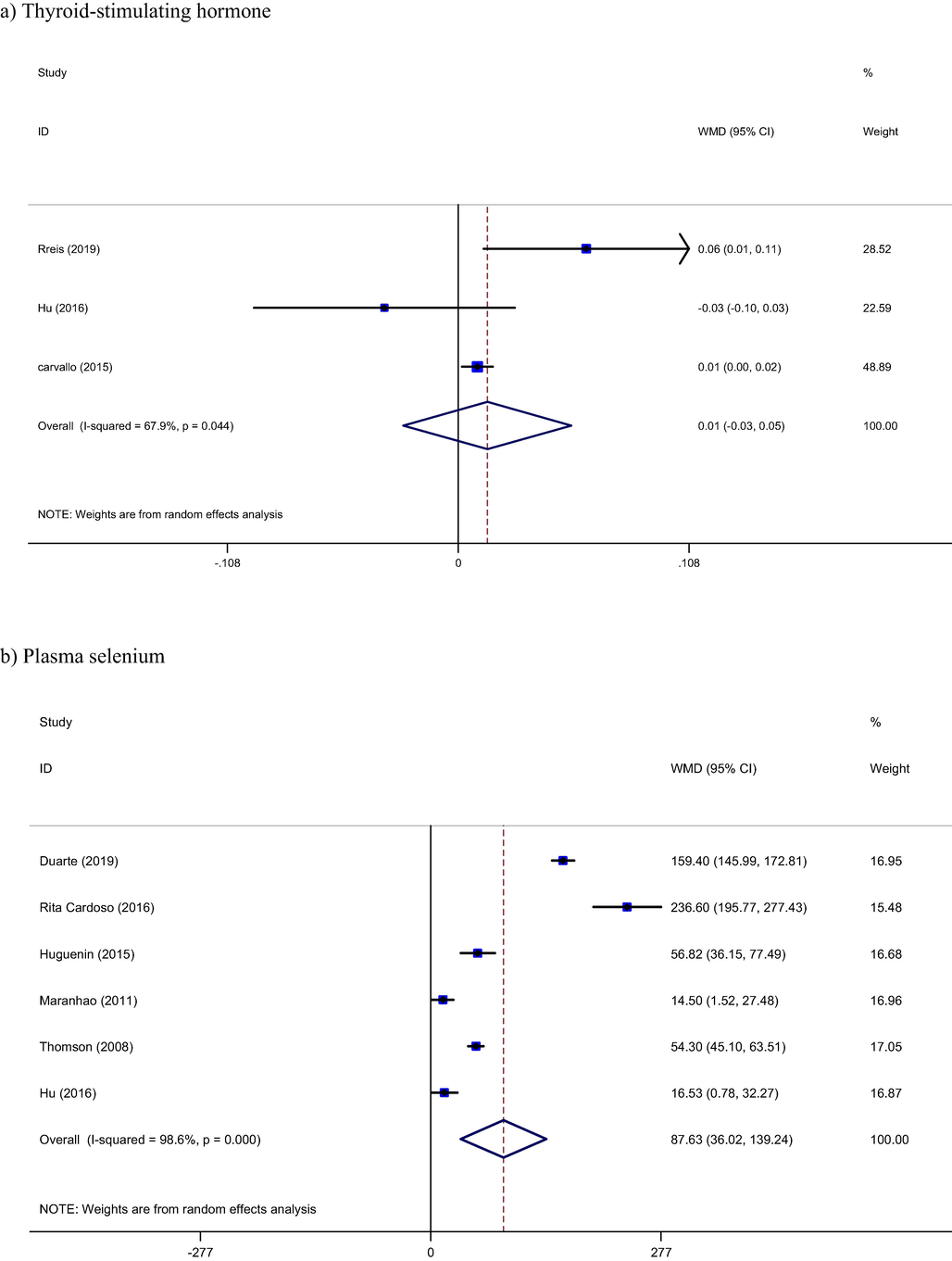
Meta-analysis of effect of brazil nut consumption on.
Meta-analysis of effect of brazil nut consumption on.
Meta-analysis of effect of brazil nut consumption on.
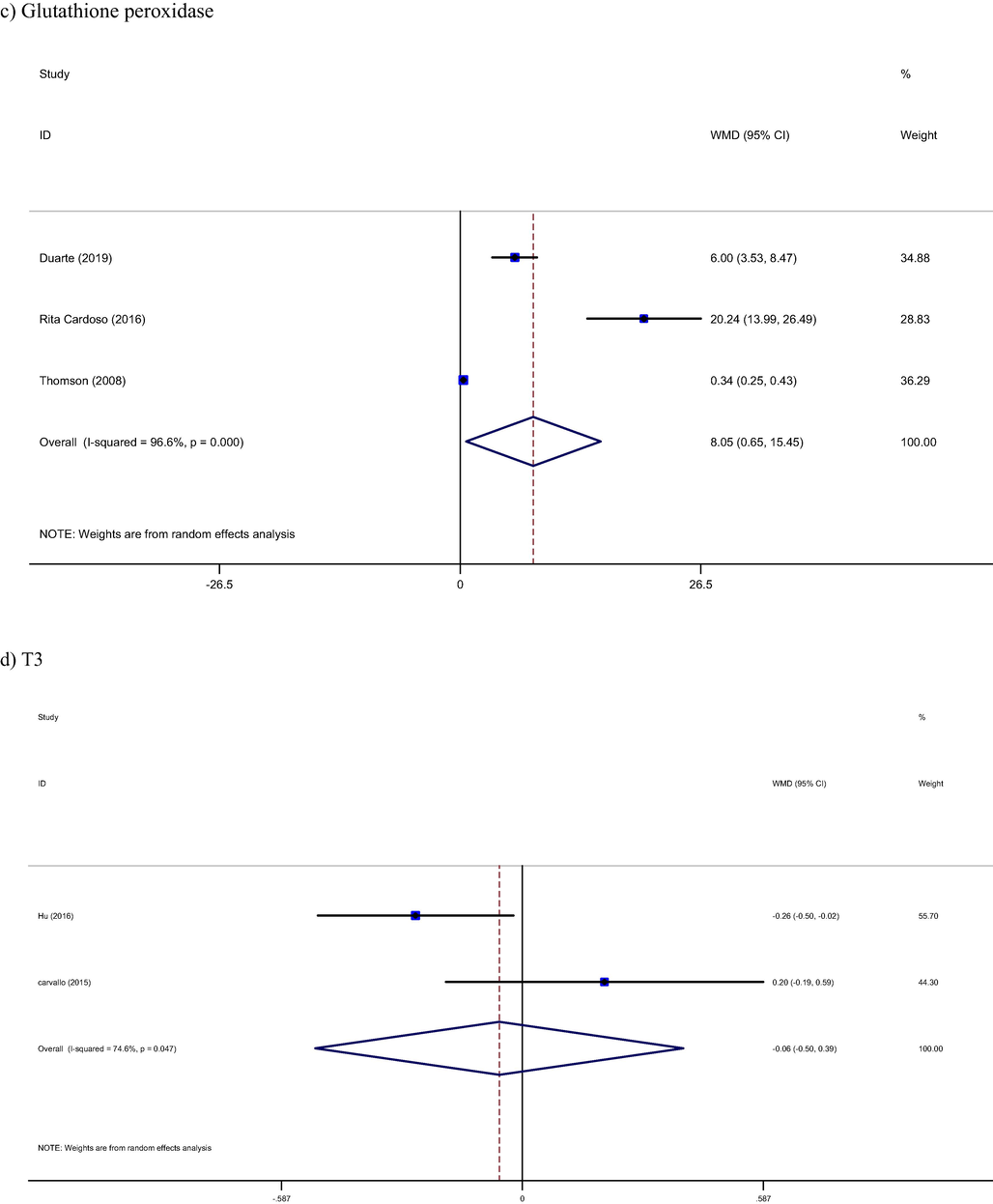
Seven studies with 315 participants reported plasma selenium levels as an outcome measures (Carvalho et al., 2015; Duarte et al., 2019; Hu et al., 2016; Huguenin et al., 2015; Reis et al., 2019; Rita Cardoso et al., 2016; Thomson et al., 2008). Brazil nut had a significant increase effect on plasma selenium levels (WMD: 87.63 microg/l, 95% CI: 36.02, 139.24, I2= 98%). Furthermore, Brazil nut had increasing effect on GPx levels too (WMD: 8.05 U/gHb, 95% CI: 0.65, 15.45, I2 = 96%) (Duarte et al., 2019; Rita Cardoso et al., 2016; Thomson et al., 2008) (Fig. 3b, 3c).
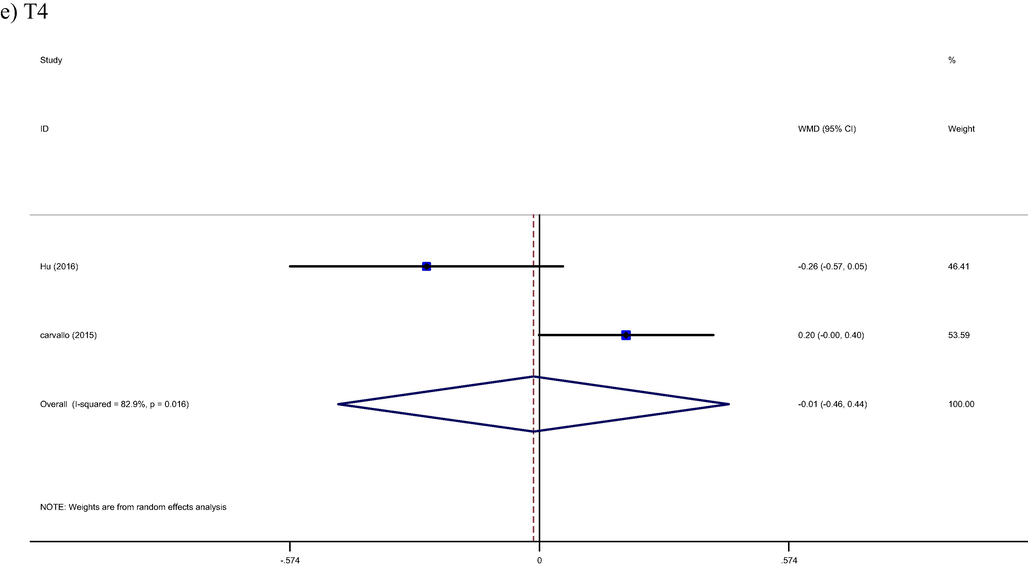
Pooled result from included studies show not significant effect from brazil nut intervention on T3 (WMD: 0.06 pg/ml, 95% CI: −0.50, 0.39, I2= 74%) and T4 (WMD: −0.01 pg/ml, 95% CI: −0.46, 0.44, I2 = 82%) hormones, respectively (Carvalho et al., 2015; Hu et al., 2016) (Fig. 3d, 3e).
3.4 Publication bias and sensitivity analysis
Funnel plots of included studies showed that there is not any asymmetric among distribution of studies. Furthermore, the Egger’s and Begg’s test show not publication bias between included studies for TSH (p = 0.89, p = 0.60), Plasma selenium (p = 0.51, p = 0.34), GPx (p = 0.09, p = 0.11), T3 (p = −, p = 0.31) and T4 (p = −, p = 0.31) hormones, respectively.
The effect sizes for the influence of brazil nut on TSH, Plasma selenium, GPx, T3 and T4 hormones were robust in the sensitivity analysis, suggesting that omission of each trial did not have a significant effect on the results.
4 Discussion
There has been extensive research carried out regarding nut consumption and health outcomes, particularly brazil nuts, which are purportedly capable of reducing the CVD risk (Mozaffarian, 2016; Ros, 2015), hypercholesterolemia (Del Gobbo et al., 2015; Ros, 2015), diabetes (Asghari et al., 2017; Estruch et al., 2006), and thyroid function (Roman et al., 2014), and putatively mediated by selenium levels, and upregulated by Glutathione peroxidase (Thomson et al., 2008). However, whilst brazil nuts have been well reviewed, to the author’s knowledge, there exists no quantitative analysis elucidating the overall effect of brazil nuts on selenium levels and thyroid hormones; thus, we sought to conduct a systematic review and meta-analysis of the effect of brazil nuts on selenium levels, Glutathione peroxidase (GPx), and thyroid hormones. In accordance with the aforementioned aim, we found that brazil nut consumption yielded significant increases in plasma Selenium and GPx, but not thyroid hormones.
The essential micronutrient selenium is a tenet of selenoproteins in antioxidant and redox reactions, metabolism of thyroid hormones, immune system function, and reproductivity. Inadequate amounts of selenoproteins are strongly associated with oxidative stress and its incumbent diseases (Thomson et al., 2008). Cytosolic glutathione peroxidase-1 is an antioxidative enzyme that catalyzes hydrogen peroxide and organic peroxides via glutathione metabolism (Brigelius-Flohé, 1999). Furthermore, plasma selenium and GPx activity have been used as conduits to infer the bioavailability of selenium supplementation (Duffield et al., 1999; Levander et al., 1983; Thomson et al., 1985; Thomson et al., 1993). Plasma selenium is considered to represent short-term alterations in tissue selenium concentrations, whilst plasma or whole blood GPx measures are asserted to represent functional indexes of overall selenium status. Importantly, however, plasma GPx may only reflect short-term changes, as opposed to whole blood activity, which is believed to better reflect longer-term changes, and is attributed to the relatively long erythrocyte life span of 120 days. Indeed, we found that there is an inverse relation between dose of brazil nut and TSH levels, but this was not statistically significant (p = 0.26). Clearly, investigation of longer-term effects of brazil nut supplementation is warranted, particularly as we noted that the average duration of intervention was 11 weeks, ranging from 8 to 24 weeks. Interestingly, although selenium is regarded as essential in the effective functioning of the thyroid, we found no significant changes in any thyroid hormone. This finding is reflective of the equivocal reports in the literature regarding selenium and thyroid function, therein stymieing any overall consensus on TSH and T-hormones.
4.1 Putative mechanism
Selenium is known to possess several important antioxidant properties (Brigelius-Flohé et al., 2003; Nève, 1996; Rayman, 2000). GPx, a selenoprotein, acts to reduce hydrogen peroxide, lipid hydroperoxides, and thioredoxin reductases, which is believed to help and maintain the intracellular redox status (Rayman, 2000). Whilst selenoprotein P, an additional selenoprotein, is speculated to protect the endothelial cells against peroxynitrite and lipid peroxidation, respectively (Arteel et al., 1999; Burk and Hill, 2005). In humans, selenium supplementation has been shown to yield increases in enzymatic antioxidant activity (Monget et al., 1996; Tränkmann et al., 1999) and decreases lipid peroxidation (Brown et al., 2001; Salonen et al., 1991), whilst reductions in the production of inflammatory prostaglandins and leukotrienes has been mediated by the neutralization of peroxide intermediates (Rayman, 2000). In thromboxane synthesized prostaglandins, low, or inadequate, selenium may yield unwanted increases in platelet aggregability and vasoconstriction (Huang et al., 2002; Nève, 1996; Rayman, 2000). However, RCTs of selenium supplementation and platelet function, blood pressure levels, and lipid profile, have yielded equivocal findings (Brown et al., 2001; Salonen et al., 1991; Van et al., 1992).
Inflammation decreased by even moderate selenium supplementation because selenium has been shown beneficial effects in both immune function and oxidative stress (Broome et al., 2004; Esposito et al., 2004; Roy et al., 1994; Willet et al., 1995); Interestingly, fatty acid levels and selenium status are considered to represent vital modulators of the GPx expression, which may putatively impact the levels of oxidative stress and fatty acid metabolism within the endothelium (Sneddon et al., 2003). Such benefits are exemplified in the brazil nut, where its nutritional composition may confer numerous complementary health benefits; indeed, clinical trials have reported an increase in antioxidant defense capability after nuts consumption, because increase in erythrocyte catalase activity (Canales et al., 2007).
4.2 Strengths and limitations
The primary strength of this study was that, to the authors’ knowledge, this is the first meta-analysis to assess the impact of brazil nuts on selenium levels, Glutathione peroxidase, and thyroid hormones; and given the potential influence on clinical and complimentary practice, this may be considered a useful addition to the literature. Prior to this meta-analysis, the evidence base was relatively equivocal, and thus necessitated a meta-analytical assessment, which we have provided. We found that there is sufficient evidence for brazil nut consumption to elicit significant, beneficial effects on plasma selenium and GPx. An additional strength of the current meta-analysis is the heterogeneous nature of the included participants, which incorporated a range of demographics, ethnicities and ages. However, there are some limitations that need to be appreciated within this meta-analysis. Ad libitum diets may act to confound some of the results of the meta-analysis, however, this is a feature of nutritional research, and out of the control of this study. The small sample sizes of the studies was another limitation of this meta-analysis.
5 Conclusion
In conclusion, we found that there is sufficient evidence to support that brazil nut consumption, at an average dose of 9.42 mg/day for an average of 11 weeks can elicit significant improvements in plasma selenium and GPx. However, the literature base remains equivocal regarding the overall effect on thyroid hormones and necessitates a greater number of RCT’s and longitudinal investigations. Encouragingly, however, no deleterious side-effects of brazil nut consumption have been reported, meaning that its consumption is, likely, universally safe.
Funding
No fund.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J. Antimicrob. Chemother.. 2015;71(1):196-207.
- [Google Scholar]
- Nut consumption is associated with lower incidence of type 2 diabetes: the Tehran Lipid and Glucose Study. Diabetes Metab. 2017;43(1):18-24.
- [Google Scholar]
- Tissue-specific functions of individual glutathione peroxidases. Free Radical Biol. Med.. 1999;27(9–10):951-965.
- [Google Scholar]
- Selenium-dependent enzymes in endothelial cell function. Antioxid. Redox Signal.. 2003;5(2):205-215.
- [Google Scholar]
- An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr.. 2004;80(1):154-162.
- [Google Scholar]
- Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med.. 2001;345(22):1583-1592.
- [Google Scholar]
- Selenoprotein P: an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu. Rev. Nutr.. 2005;25:215-235.
- [Google Scholar]
- Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J. Am. Coll. Nutr.. 2007;26(3):225-232.
- [Google Scholar]
- Brazil nuts: nutritional composition, health benefits and safety aspects. Food Res. Int.. 2017;100:9-18.
- [Google Scholar]
- Nutritional status of selenium in Alzheimer's disease patients. Br. J. Nutr.. 2010;103(6):803-806.
- [Google Scholar]
- Selenium, selenoproteins and neurodegenerative diseases. Metallomics. 2015;7(8):1213-1228.
- [Google Scholar]
- Intake of partially defatted Brazil nut flour reduces serum cholesterol in hypercholesterolemic patients- a randomized controlled trial. Nutr. J.. 2015;14(1)
- [CrossRef] [Google Scholar]
- A randomized-controlled, double-blind study of the impact of selenium supplementation on thyroid autoimmunity and inflammation with focus on the GPx1 genotypes. J. Endocrinol. Invest.. 2015;38(10):1065-1074.
- [Google Scholar]
- Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr.. 2015;102(6):1347-1356.
- [Google Scholar]
- Consumption of Brazil nuts with high selenium levels increased inflammation biomarkers in obese women: a randomized controlled trial. Nutrition. 2019;63–64:162-168.
- [CrossRef] [Google Scholar]
- An estimation of selenium requirements for New Zealanders. Am. J. Clin. Nutr.. 1999;70(5):896-903.
- [Google Scholar]
- Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634.
- [Google Scholar]
- Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440-1446.
- [Google Scholar]
- Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann. Intern. Med.. 2006;145(1):1-11.
- [Google Scholar]
- The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928
- [Google Scholar]
- Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2011.
- Supplementation with Brazil nuts and green tea extract regulates targeted biomarkers related to colorectal cancer risk in humans. Br. J. Nutr.. 2016;116(11):1901-1911.
- [CrossRef] [Google Scholar]
- Role of selenium in cytoprotection against cholesterol oxide-induced vascular damage in rats. Atherosclerosis. 2002;162(1):137-144.
- [Google Scholar]
- Improvement of antioxidant status after Brazil nut intake in hypertensive and dyslipidemic subjects. Nutr. J.. 2015;14:54.
- [CrossRef] [Google Scholar]
- Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98(2):381-387.
- [Google Scholar]
- Bioavailability of selenium to Finnish men as assessed by platelet glutathione peroxidase activity and other blood parameters. Am. J. Clin. Nutr.. 1983;37(6):887-897.
- [Google Scholar]
- Selenium and cancer: a story that should not be forgotten-insights from genomics. In: Advances in Nutrition and Cancer. Springer; 2014. p. :145-166.
- [Google Scholar]
- Effect of 6 month supplementation with different combinations of an association of antioxidant nutrients on biochemical parameters and markers of the antioxidant defence system in the elderly. The Geriatrie/Min. Vit. Aox Network. Eur. J. Clin. Nutr.. 1996;50(7):443-449.
- [Google Scholar]
- Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187-225.
- [Google Scholar]
- Selenium as a risk factor for cardiovascular diseases. J. Cardiovasc. Risk. 1996;3(1):42-47.
- [Google Scholar]
- Brazil nut intake increases circulating miR-454-3p and miR-584-5p in obese women. Nutr. Res.. 2019;67:40-52.
- [CrossRef] [Google Scholar]
- Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: a randomized controlled pilot trial. Eur. J. Nutr.. 2016;55(1):107-116.
- [CrossRef] [Google Scholar]
- Supplementation with selenium and human immune cell functions. Biol. Trace Elem. Res.. 1994;41(1–2):103.
- [Google Scholar]
- Effects of antioxidant supplementation on platelet function: a randomized pair-matched, placebo-controlled, double-blind trial in men with low antioxidant status. Am. J. Clin. Nutr.. 1991;53(5):1222-1229.
- [Google Scholar]
- Regulation of selenoprotein GPx4 expression and activity in human endothelial cells by fatty acids, cytokines and antioxidants. Atherosclerosis. 2003;171(1):57-65.
- [Google Scholar]
- Assessment of requirements for selenium and adequacy of selenium status: a review. Eur. J. Clin. Nutr.. 2004;58(3):391.
- [Google Scholar]
- Selenium and iodine intakes and status in New Zealand and Australia. Br. J. Nutr.. 2004;91(5):661-672.
- [Google Scholar]
- Brazil nuts: an effective way to improve selenium status. Am. J. Clin. Nutr.. 2008;87(2):379-384.
- [CrossRef] [Google Scholar]
- Effects of supplementation with high-selenium wheat bread on selenium, glutathione peroxidase and related enzymes in blood components of New Zealand residents. Am. J. Clin. Nutr.. 1985;41(5):1015-1022.
- [Google Scholar]
- Long-term supplementation with selenate and selenomethionine: selenium and glutathione peroxidase (EC 1.11. 1.9) in blood components of New Zealand women. Br. J. Nutr.. 1993;69(2):577-588.
- [Google Scholar]
- Effect of administration of selenium and vitamin E on heart failure and ventricular arrhythmias in patients with acute myocardial infarct. Medizinische Klinik (Munich, Germany: 1983). 1999;94:78-80.
- [Google Scholar]
- Supplementation with selenium-rich bread does not influence platelet aggregation in healthy volunteers. Eur. J. Clin. Nutr.. 1992;46(6):445-450.
- [Google Scholar]
- Chemical composition of selected edible nut seeds. J. Agric. Food Chem.. 2006;54(13):4705-4714.
- [Google Scholar]
- Mediterranean diet pyramid: a cultural model forhealthy eating. Am. J. Clin. Nutr. 1995:61.
- [Google Scholar]
- Brazil nuts and associated health benefits: A review. Lwt-Food Sci. Technol.. 2009;42(10):1573-1580.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.01.019.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







