Translate this page into:
The angiotensin II type 1 receptor mediates the induction of oxidative stress, apoptosis, and autophagy in HUVECs induced by angiotensin II
⁎Corresponding author. mejalouli@imamu.edu.sa (Maroua Jalouli)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Angiotensin (Ang) II, which is the central effector of the renin-angiotensin system (RAS), is one of the principal mediators of vascular dysfunction in hypertension and cardiovascular diseases. Proper vascular function is mediated by oxidative stress, cell apoptosis, and autophagy. However, the underlying signaling pathways and the major RAS components involved in this process are still not fully understood. In the present study, the effect of Ang II on reactive oxygen species (ROS) production, apoptosis induction, and autophagy in human umbilical vein endothelial cells (HUVECs) was investigated. An Annexin V kit was used for the apoptosis analysis, and caspase-3/7 activities were measured with the Caspase-Glo 3/7 Assay Kit. ROS production was measured using a 2,7-dichlorodihydrofluorescein diacetate probe whereas detection of autophagy was performed using acridine orange staining. We found that Ang II increases oxidative stress via ROS production, cell apoptosis via caspase 3/7, and the mitochondrial membrane potential (MMP). Interestingly, losartan, being an antagonist of the angiotensin II type 1 receptor (AT1R), has demonstrated the ability to restore autophagy levels to that of the control group subsequent to its induction by Ang II. The latter can thus induce endothelial cell damage, through excessive oxidative stress and defective autophagy-related apoptosis, which can be inhibited by losartan. These findings reinforce the pivotal role played by the Ang II/AT1R axis in the pathogenesis of vascular damage and bolster our knowledge of the role played by ROS/autophagy-related apoptosis via AT1R in the pathogenesis of hypertension and vascular diseases.
Keywords
Endothelial cells
Angiotensin II
Oxidative stress
Autophagy
Apoptosis
1 Introduction
Dysregulation of the renin-angiotensin system (RAS) is associated with inflammation, oxidative stress, and vascular damage (Almutlaq et al., 2022). Furthermore, endothelial dysfunction triggers vascular injury and cardiovascular diseases. Local endothelial RAS components are involved in cellular homeostasis and in biological function, structure, and proliferation (Barhoumi et al., 2021). Hyperactivation of the RAS via the angiotensin (Ang) II/angiotensin II type 1 receptor (AT1R) axis is balanced by the Ang 1–7/MAS receptor (MASR) regulatory axis (Almutlaq et al., 2021). The active form of the latter is mainly modulated by Ang II, which is the main component of the RAS (O'Connor et al., 2022). Ang II receptors can be found in several cells, including endothelial cells. Thus, high plasma levels of Ang II stimulate endothelial cells and trigger vascular dysfunction through different pathways (Chrysanthopoulou et al., 2021). Given the significant rise in cardiovascular diseases globally and the correlation between RAS and the onset of cardiovascular diseases, potential treatment options have been proposed. One of these alternatives targets, angiotensin receptor blockers (ARBs) exert their pharmacological effects through the inhibition of Ang II, a hormone that has a pivotal pathophysiological role in renal and cardiovascular diseases. The competitive antagonism of Ang II receptors has been hypothesized to result in the selective suppression of Ang II, potentially leading to a decrease in negative effects and a potential enhancement in therapeutic effectiveness (Abraham et al., 2015). The Food and Drug Administration (FDA) in the United States has approved several ARBs for the treatment of hypertension. These ARBs, namely irbesartan, valsartan, losartan, and candesartan, are classified as non-peptide compounds. They are distinguished by the presence of biphenyl, tetrazole, benzimidazole, or nonbiphenyl nontetrazole moieties (Abraham et al., 2015). These ARBs exhibit a higher binding affinity towards ATR1 compared to ATR2, hence enabling them to effectively inhibit the actions of Ang II on ATR1.
Although it is known that Ang II induces endothelial dysfunction, the underlying pathways and cell responses to excessive Ang II levels are still not fully understood. Autophagy is involved in vascular biology and in cardiometabolic diseases (Yu et al., 2022). However, the signaling pathways responsible for these cellular effects are poorly understood. Autophagy induction in endothelial cells is a popular area of research due to the sensitivity of these cells to signal transduction and targeted therapies in cardiovascular diseases. Like any regulator of physiologic homeostasis such as reactive oxygen species (ROS), autophagy activation is not typically protective in that excessive autophagy worsens biological effects (An et al., 2019). Although Ang II induces ROS production in endothelial cells, the synergistic effect of oxidative stress induced by Ang II- and detrimental autophagy is unclear.
In the present study, we used an in vitro model of endothelial cells to test the effect of Ang II and related mechanisms. We found that Ang II increases cell autophagy, which could stimulate apoptosis via the caspase 3/7 pathway. Furthermore, autophagy activation can trigger ROS production as a hallmark of endothelial dysfunction. All Ang II effects are dependent on the AT1R axis. Our results highlight the role of autophagy in Ang II-induced endothelial dysfunction and emphasize the crucial function played by AT1R antagonists and the RAS as targets for treating endothelial dysfunction.
2 Materials and methods
2.1 Human umbilical vein endothelial cell (HUVEC) cultures
HUVECs were procured from ATCC (CRL-1730 USA) and cultured in DMEM/F12 medium supplemented with 10 % FBS and 1 % penicillin–streptomycin. The cells were seeded and incubated at 37 °C in a humidified atmosphere with 5 % CO2. The complete medium was replenished every 2 days by discarding the old medium and replacing it with fresh complete medium. The cells were treated for 24 h or 48 h with 100 nM Ang II or were pretreated for 1 h with 100 µM losartan (Sigma Cat# 61188-100MG) prior to the Ang II treatment.
2.2 Cytotoxicity assay
LDH release by HUVECs was measured after a 48-h treatment with 100 µM losartan, with 100 µM Ang II, or in combination to study their cytotoxicity (LDH assay kit, Abcam, Cambridge, UK).
2.3 Apoptosis assay
For apoptosis analysis, an Annexin V kit obtained from BD Biosciences (San Diego, CA, USA) was utilized. Following treatment with 100 nM Ang II or a 1-hour pre-treatment with 100 µM losartan, the cells were washed twice with cold PBS. Subsequently, they were resuspended in binding buffer and incubated with annexin V conjugated to FITC and propidium iodide (PI). After a 15-minute incubation period, the cells were assessed within 1 h using a BD FACS Calibur® flow cytometer (BD Biosciences, San Jose, CA, USA). The acquired data were analyzed using Cell Quest® Pro software (BD). A gating strategy was used to exclude doublet cells and to separate early and late apoptotic populations. To confirm the flow cytometry results, fluorescence microscopy was used to study apoptosis. Caspase 3/7 activity was measured in HUVECs to evaluate apoptosis signaling pathways.
The activities of caspase-3/7 in the HUVECs were evaluated with Caspase-Glo 3/7 Assay Kit (Promega, Madison, WI, USA). HUVECs were put into a 96-well plate at a density of 10,000 cells per well and subsequently exposed to losartan and Ang II for a period of 48 h. Then, the treated cells were exposed to 10 μL of the assay reagent at room temperature for 1 h, and luminescence was quantified to determine the levels of caspase-3/7 activity.
Detection of Apoptotic nuclear morphological Changes by fluorescence imaging. Hoechst 33,342 staining was utilized to monitor changes in the morphology of HUVECs undergoing apoptosis. Initially, cells were seeded onto coverslips placed within a six-well plate and allowed to incubate overnight. They were then exposed to various concentrations of losartan and Ang II for a 48-hour duration. After fixation with formaldehyde, Hoechst 33,342 dye was used to label the cells for a period of 10 min. Subsequently, the cells were washed with 1X PBS, and their nuclear morphological changes were examined using a fluorescence microscope (Olympus BX41, Tokyo, Japan) equipped with a digital camera and appropriate filters. The excitation wavelength employed was 346 nm, and the emission wavelength was 497 nm.
Since the Hoechst 33,342 staining results were estimated non-qualitatively, the reproducibility of results was maintained by performing the experiments at least three times, and all the slides were coded and observed by a single operator.
2.4 Measurement of the mitochondrial membrane potential (MMP) to evaluate mitochondrial function
HUVECs were treated with 100 nM Ang II alone or in combination with 100 µM losartan for 48 h. The cells were washed with 1X PBS (Phosphate Buffered Saline, pH 7.4) and were stained with rhodamine 123. Images were captured at 200X magnification using a compound Olympus BX41 microscope (Tokyo, Japan) equipped with a CCD camera and a fluorescence attachment.
2.5 Measurement of ROS production
To evaluate oxidative stress, ROS production was measured in cells using a 2,7-dichlorodihydrofluorescein diacetate (DCFH2-DA) probe (Thermo Fisher Scientific, Waltham, MA, USA) as described previously (Alamri et al., 2021). Briefly, the cell-permeant indicator DCFH2-DA is cleaved by intracellular esterases and is oxidized by ROS to generate the fluorescent DCF form. After treatments with 100 nM Ang II alone or in combination with 100 µM losartan for 48 h, the cells were washed in 1X PBS solution and were resuspended in 200 μL (final volume) before being incubated for 30 min with 10 μM DCFH2-DA at 37 °C. The cells were examined using a fluorescence microscope (Olympus BX41, Tokyo, Japan) equipped with charge-coupled device (CCD) camera and appropriate filter.
To quantitatively assess reactive oxygen species (ROS) levels, HUVECs were cultured in a 96-well plate with a black bottom and underwent the identical assay procedure explained earlier. The fluorescence intensity signal was captured using a microplate reader (BioTek®, Winooski, VA, USA) with an excitation wavelength of 485 nm and an emission wavelength of 528 nm. Subsequently, the measurements were compared to untreated cells, which were utilized as a reference and set at 100 %.
2.6 Detection of autophagy by acridine orange staining
HUVECs were cultured with 5 μg/mL acridine orange (AO) working solution for 15 min in the dark after being exposed to the indicated amounts of losartan and Ang II for 12 and 24 h. The stained cells were mounted on glass slides, rinsed with PBS, and examined with a compound Olympus BX41 microscope equipped with a CCD camera and a fluorescence attachment. For the relative quantification of acridine orange staining, we utilized the Zen 3.1 service (ZEN Light, Blue Edition, Germany) as the method. The quantification was performed using GraphPad Prism 9 software (GraphPad Software, San Diego, CA, USA).
2.7 Statistical analysis
Data are expressed as means ± SEM. Comparisons between groups were performed using SPSS (version 17.0, SPSS) and GraphPad Prism 9.3.0 (4 6 3) software with a one-way ANOVA followed by Tukey’s post hoc test (we considered p < 0.05 as statistically significant).
3 Results
3.1 Ang II cytotoxicity toward HUVECs
An LDH assay was used to evaluate the cytotoxicity of Ang II toward endothelial cells. While no significant effect was found in the losartan group, LDH release was significantly higher in the Ang II group compared to the control group. This effect was significantly reduced when adding losartan to Ang II (Fig. 1). However, the level of LDH in the (Los + Ang II) group was still significantly higher than that in the control group, which indicates the potential involvement of other pathways in addition to the AT1R pathway.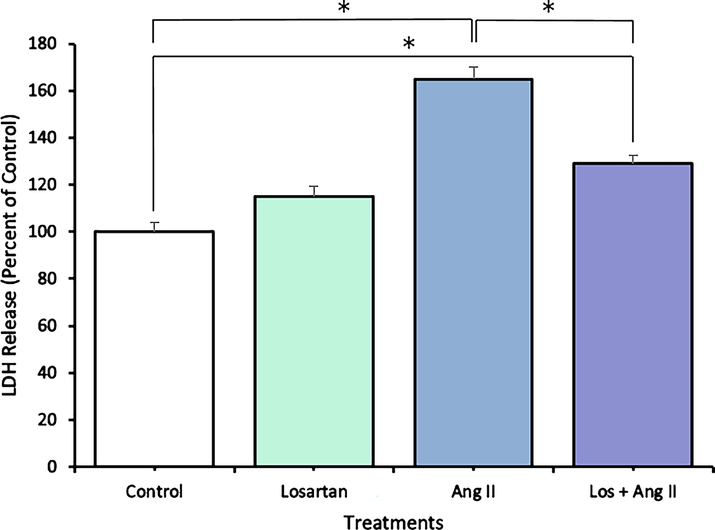
HUVEC cytotoxicity was measured by LDH release assays 48 h after treatments with losartan and Ang II alone or in combination. The data are expressed as means ± SEM from three independent experiments. *Significant (p < 0.05) compared with the untreated control.
3.2 Ang II triggers HUVEC apoptosis
HUVECs were used as an endothelial cell model. They were treated for 48 h to investigate the effect of Ang II on apoptosis (Figs. 2, 3, and 4). Ang II significantly increased the number of apoptotic and necrotic cells (Fig. 2A-E). To better explain apoptosis in response to the Ang II treatment, we performed flow cytometry to determine the percentages of early and late apoptotic cells. There was an increase in early and late apoptotic Ang II-treated cells compared to the control group (Fig. 3). Furthermore, Ang II-treated HUVECs stained with Hoechst 33,324 nuclear dye showed a significant increase in piknotic nuclei (Fig. 4).. Hoechst 33,324 is a cell-permeant fluorescent dye that attaches to structurally damaged DNA fragments, resulting in a more intense blue fluorescence emission. To elucidate the signaling pathway by which Ang II induces apoptosis, we measured caspase 3/7 levels, which were significantly higher in the Ang II group than in the control group (Fig. 5).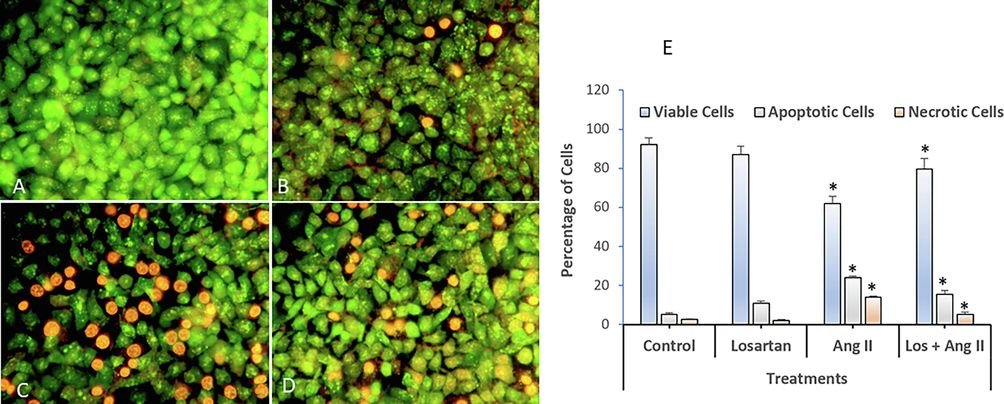
Dual staining and analysis by fluorescence microscopy of apoptotic morphological changes in HUVECs exposed to losartan and Ang II alone or in combination. (A) Control, (B) Losartan (100 µM), (C) Angiotensin II (100 nM), (D) Losartan + Ang II, and (E) Quantification of apoptotic and necrotic cells based on AO and ethidium bromide uptake in more than 300 cells (Magnification: 200X). The data are expressed as means ± SEM from three independent experiments. *Significant (p < 0.05) compared with the corresponding controls.
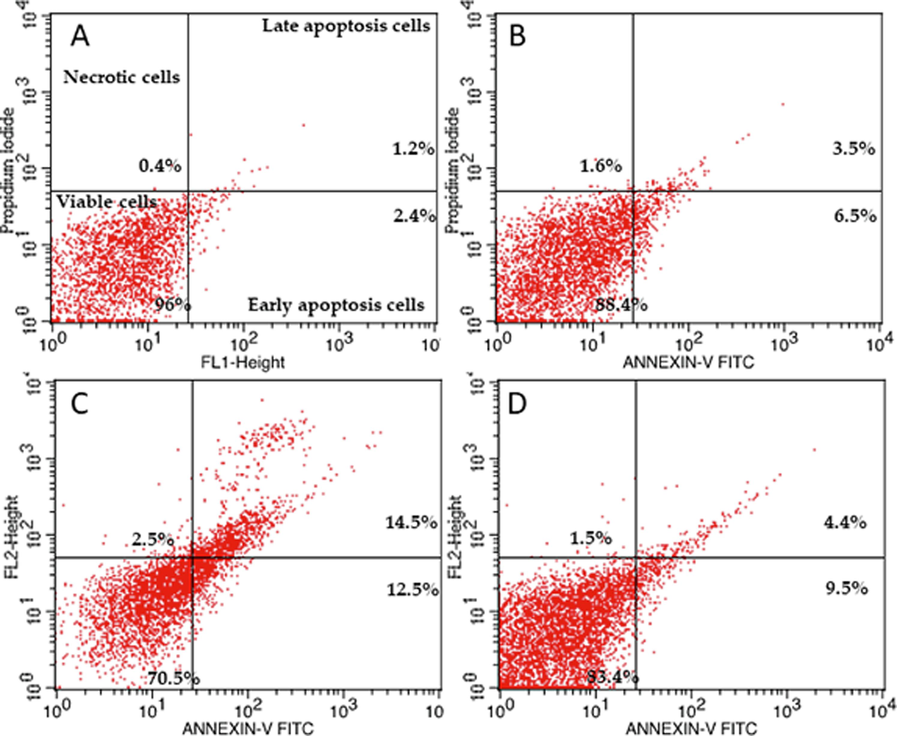
Flow cytometry profile of annexin V-FITC/PI staining of HUVECs showing the percentage of viable cells, early apoptotic cells, late apoptotic cells, and necrotic cells after a 48-h exposure. (A) Control, (B) Losartan (100 µM), (C) Ang II (100 nM), (D) Losartan + Ang II.
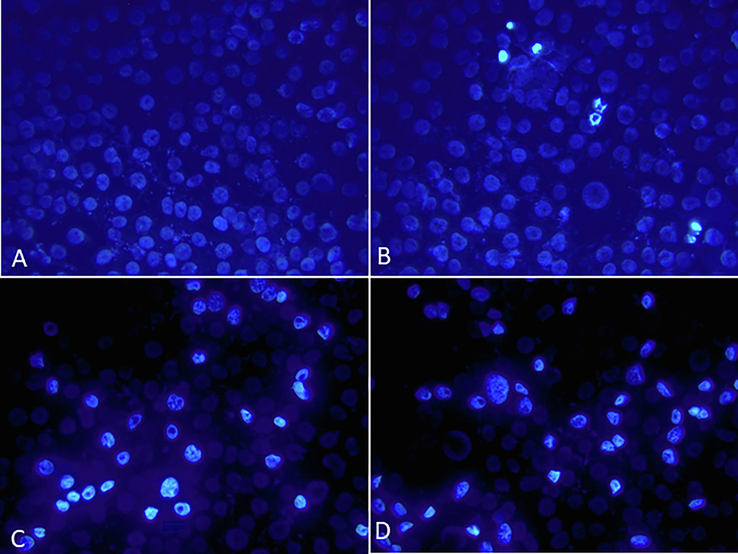
Representative images of losartan- and angiotensin II-treated HUVEC cells captured using a fluorescence microscope after staining with the nuclear dye Hoechst 33324. (A) Control, (B) Losartan (C) Ang II (D) Losartan + Ang II. Magnification 200X.
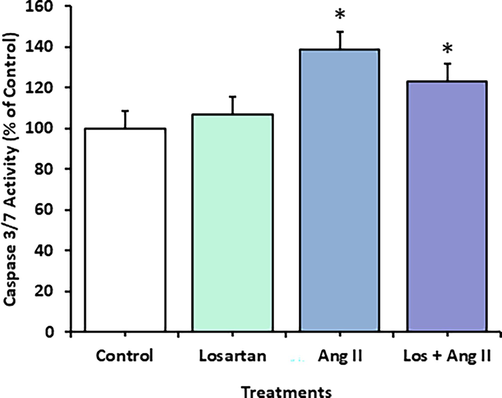
The activities of caspases 3 and 7 were measured in HUVECs using a caspase 3/7 assay fluorometric kit after a 48-h exposure to losartan and Ang II alone or in combination. The data are expressed as means ± SEM from three independent experiments (*Significant, p < 0.05).
3.3 Assessment of mitochondrial depolarization using the rhodamine 123 dye
Rhodamine 123 is a fluorescent cationic dye frequently employed to investigate mitochondrial function and membrane potential within cells. It was chosen for its capacity to specifically accumulate in the mitochondria of living cells. The process of mitochondrial energization leads to a reduction in rhodamine 123 fluorescence, and the rate at which the fluorescence decreases is directly linked to the mitochondrial membrane potential. To evaluate mitochondrial function, we analyzed MMP (Fig. 6A-D), which was clearly affected by the Ang II treatment (Fig. 6C). This effect was abrogated by blocking AT1R (Fig. 6B).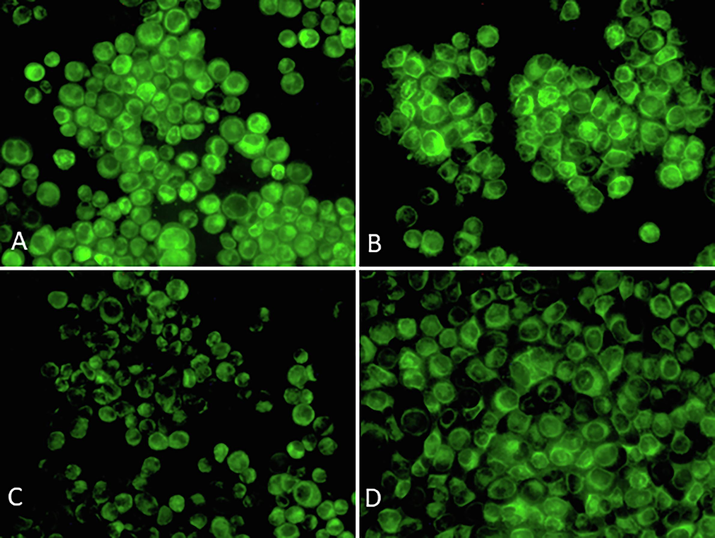
Analysis of MMP in HUVECs exposed to losartan and Ang II alone or in combination for 48 h and stained with rhodamine 123. (A) Control (B) Losartan (100 µM), (C) Ang II (100 nM), (D) Losartan + Ang II. Magnification 200X.
3.4 Ang II induces ROS production
ROS production is a hallmark of endothelial dysfunction. Cell function is modulated by ROS production, and abnormalities in this marker dysregulate metabolism and cell homeostasis. To investigate the effect of Ang II on the cellular redox balance in HUVECs, we analyzed DCF fluorescence (Fig. 7). Ang II significantly increased ROS production in by HUVECs (Fig. 7C) through the AT1R pathway while losartan blocked ROS production (Fig. 7B).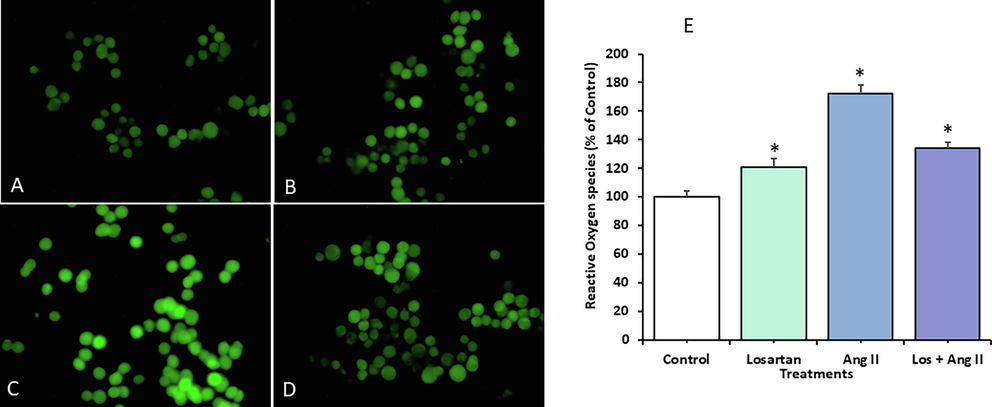
ROS levels in HUVECs exposed to losartan and Ang II alone or in combination for 48 h. The cells were observed by fluorescence microscopy after the cells were stained with the fluorescence marker carboxy-H2DCFDA. Representative images of (A) untreated control cells, (B) cells treated with 100 µM Losartan, (C) cells treated with100 nM Ang II, (D) cells treated with Losartan + Ang II (Magnification 200X). (E) Quantification of the fluorescence intensity (%) of exposed cells relative to the untreated control. The data are expressed as means ± SEM from three independent experiments (*Significant, p < 0.05).
3.5 Ang II stimulates autophagy in HUVECs
When observing HUVECs under fluorescence microscopy, it was observed that those treated with losartan, with or without Ang II, displayed normal acidic vesicular organelles (AVOs), which serve as an indicator of autophagy. This observation was in comparison to the control group (Fig. 8). Notably, HUVECs treated solely with Ang II exhibited a significant increase in AVOs, evident through the higher levels of green and orange fluorescence. This indicates that Ang II induced autophagy, while losartan restored autophagy levels to that of the control group.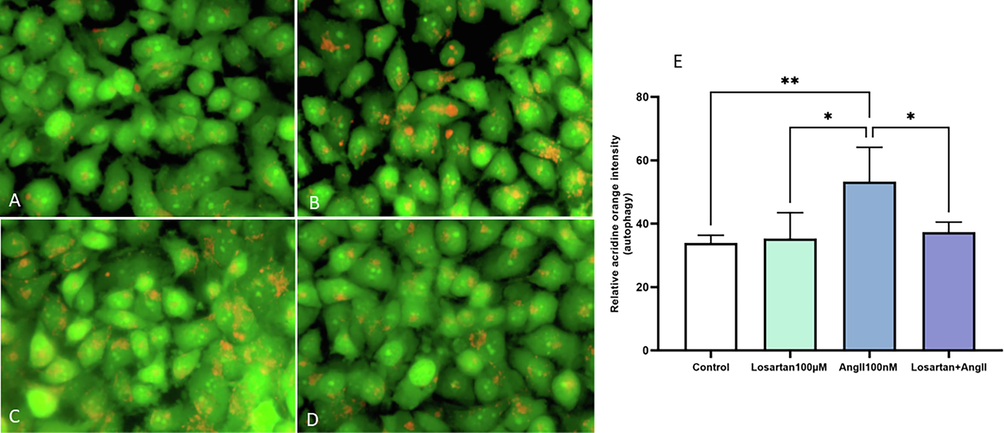
Detection of autophagy in HUVECs after a 48 of exposure to losartan and Ang II alone or in combination by AVO staining with AO. Representative fluorescence microscopy images of the (A) Control, (B) Losartan (100 µM), (C) Ang II (100 nM), and (D) Losartan + Ang II groups. Magnification 400X.
4 Discussion
Endothelial dysfunction is a hallmark of vascular damage associated with cardiovascular diseases (Nukala et al., 2023). The RAS, which is a major regulator of hypertension, is a fundamental system involved in all cardio-metabolic diseases, and RAS components are present in almost all tissues. Hyperactivation of the RAS in the vasculature induces pathophysiological vasoconstriction, which, in turn, induces vascular injury (Ting et al., 2023). We investigated the impact of Ang II on the cellular redox balance in HUVECs. Our findings revealed that when cells were exposed to Ang II, there was a significant increase in reactive oxygen species (ROS) production through the AT1R pathway because the introduction of losartan effectively inhibited ROS production. These results align with previous research that has demonstrated the involvement of various proinflammatory and proatherosclerotic factors, such as Ang II and TNFα, in the intrinsic generation of ROS by endothelial cells (Dimmeler and Zeiher, 2000). Additionally, they support the notion that Ang II-induced endothelial dysfunction is influenced by oxidative stress, inflammation, and the immune system (Caillon et al., 2017). The overproduction of reactive oxygen species (ROS) results in an imbalance between oxidation and antioxidation, which leads to oxidative stress (Ekhteiari Salmas et al., 2018; Ozdemir et al., 2021). This oxidative stress is implicated in the development of several cardiovascular illnesses, such as atherosclerosis, hypertension, and heart failure (Cai and Harrison, 2000). Moreover, oxidative stress alters many endothelial functions by, for example, modulating vasomotor tone (Versari et al., 2009). These effects are triggered by vascular smooth muscle cells and endothelial cells via local RAS activation, which is associated with changes in cell structure and function.
Interestingly, ROS are known to cause programmed cell death in a variety of cell types. Increased ROS production is an early sign of atherogenesis, indicating a connection between ROS and apoptosis (Dimmeler and Zeiher, 2000). When compared to the control, our results show that Ang II significantly increases the number of early and late apoptotic. This was likely due to a considerable increase in caspase 3/7 levels in the Ang II group. The physiological significance of these findings is supported by studies showing that ROS production and apoptosis can play pathophysiological roles in vascular homeostasis through their many effects on smooth muscle cells (Irani, 2000; Ma et al., 2023). In fact, pathological endothelial cell apoptosis is a major mechanism causing vascular damage. This process leads to a disarrangement of the structure of the endothelial layer and vascular injury characterized by pathological plasmatic protein infiltration due to vascular seepage, resulting in an increase in cell adhesion, procoagulant factor accumulation, and prothrombotic conditions (Rong et al., 2006; Zacharia et al., 2020).
During physiological tissue and cell homeostasis, autophagy can modulate protein and organelle turnover by removing dysfunctional proteins and organelles (Madden et al., 2014). Recent research has demonstrated that autophagy serves as a cellular mechanism for cell death and a response to different pathological conditions (Ravikumar et al., 2010). Notably, emerging evidence suggests a connection between reactive oxygen species (ROS) and autophagy, indicating that ROS production can both initiate autophagy and be controlled by autophagy itself (Li et al., 2015). The consequences of these interactions between ROS and autophagy can become evident in different clinical circumstances. To further investigate the role of Ang II in endothelial cell dysfunction and the mechanisms associated with cell apoptosis, oxidative stress, and autophagy, we examined processes, including ROS production, apoptosis induction via caspase3/7, and autophagy, in Ang II-treated HUVECs. Our results suggest that the induction of autophagy by Ang II is a pathological condition rather than a regular physiological process to clear abnormal proteins and inoperative organelles (Aghajan et al., 2012). Indeed, Ang II induces several physiological reactions inside the human body, encompassing the development of hypertension, renovascular hypertension, and renal disorders (Vargas et al., 2022). Previous investigations have demonstrated the pathogenic impact of Ang II, primarily through its interaction with the angiotensin receptor 1 (AT1R), a G protein-coupled receptor. The Ang II-AT1R interaction has been observed to elevate the intracellular calcium concentration in vascular smooth muscle and various other tissues, resulting in vasoconstriction, inflammation, and fibrosis (Kobori et al., 2007). This action can be inhibited by the angiotensin receptor blocker, losartan, as shown by our results, and in accordance with previous studies. Interestingly, we demonstrated that autophagy was highly induced after Ang II treatment in accordance with the increase of ROS, apoptosis, and Caspases 3/7. Therefore, it is plausible that the detrimental impact of Ang II is mediated by the elevation of ROS, which subsequently triggers the autophagic pathway and autophagy-related apoptosis. This process may contribute to the exacerbation of endothelial cell damage and the development of pathological conditions, rather than promoting cell survival. Indeed, Excessive autophagy can lead to an increase in apoptotic cell death, whereas an upregulation of autophagy in response to stress can mitigate apoptotic cell death (Maiuri et al., 2007; Liu et al., 2015). Numerous investigations have provided evidence that oxidative stress can induce apoptosis, autophagy, or both, leading to detrimental impacts on different tissues (An et al., 2019; Zhu et al., 2019). Nevertheless, the potent induction of autophagy in response to Ang II was effectively suppressed by losartan as the autophagy level was restored to the baseline of control when this AT1R blocker was employed. Further investigation into this dual mechanism should be explored in future research. The effects of Ang II are mainly mediated by AT1/2 receptors, which are expressed in almost all cells, including endothelial cells. To identify the signaling pathway by which Ang II induces autophagy and cell damage, we treated cells with the AT1R antagonist losartan prior to the Ang II treatment. As expected, the effect was completely reversed. However, further studies to test the effect of AT2R inhibition and confirm the specific signaling pathway by which Ang II/AT1R triggers autophagy, ROS production, and cell apoptosis, and induces endothelial cell dysfunction would be of interest.
Our findings need to be confirmed through in vivo experiments using an animal model infused with Ang II. Autophagy should be investigated in distinct organs implicated in Ang II-induced hypertension and vascular damage. This will help us pinpoint the precise role of Ang II-induced autophagy, unravel the associated signaling pathways, and identify potential novel treatment targets.
The study has certain limitations that need to be acknowledged. Firstly, there remains a lack of consensus regarding the suitability of the DCFH-DA assay for accurately detecting reactive oxygen species (ROS), primarily due to inherent limitations such as the establishment of reliable positive controls. The second limitation includes the examination of the signaling pathway associated with the pathogenic impact of Ang II in conjunction with the AT1R pathway. It is worth noting that previous studies have suggested that inhibiting the PI3K/AKT/mTOR signaling pathway could potentially result in excessive oxidative stress, impaired autophagy-related apoptosis, and subsequent cellular damage (Jalouli et al., 2022). Therefore, further examination of the PI3K/AKT/mTOR pathway is required to evaluate whether Ang II inhibits this pathway, thereby inducing autophagy and apoptosis. This may also provide insights into the underlying mechanisms by which losartan mitigates apoptosis, autophagy and ROS.
5 Conclusions
Endothelial dysfunction, a hallmark of vascular damage linked to cardiovascular diseases, involves the Renin-Angiotensin System (RAS), a major regulator of hypertension. RAS components are ubiquitous in tissues, and RAS hyperactivation in the vasculature induces pathophysiological vasoconstriction and subsequent vascular injury. Our study focused on Angiotensin II (Ang II) and its impact on cellular redox balance in HUVECs. We found that Ang II significantly increased ROS production through the AT1R pathway, which losartan treatment effectively mitigated. This aligns with previous research implicating various factors, including Ang II and TNFα, in ROS production by endothelial cells, linking oxidative stress to cardiovascular diseases. Excessive ROS contributes to endothelial dysfunction, affecting vasomotor tone and initiating apoptosis. Ang II elevated apoptosis and caspase 3/7 levels in HUVECs, highlighting its role in vascular damage. Furthermore, we explored the relationship between ROS and autophagy, revealing that Ang II-induced autophagy is pathological rather than a routine cellular process. Excessive autophagy can exacerbate apoptosis and ROS production. Further research is needed to pinpoint the specific Ang II/AT1R signaling pathway driving these processes. These findings underscore the importance of in vivo validation and exploring Ang II-induced autophagy in different organs to identify potential treatment targets.
Author contributions
Conceptualization, Maroua Jalouli; Formal analysis, Maroua Jalouli and Mohammed Al-zahrani; Investigation, Maroua Jalouli and Tlili Barhoumi; Supervision, Mohamed Chahine; Validation, Mohammed Al-zahrani and Mohamed Chahine; Visualization, Mohamed Chahine; Writing – original draft, Maroua Jalouli and Tlili Barhoumi.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RP23099).
8 Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
CRediT authorship contribution statement
Maroua Jalouli: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. Tlili Barhoumi: Investigation, Software. Mohammed Al-Zharani: Investigation. Mohamed Chahine: Visualization, Supervision.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-RP23099).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Obesity, autophagy and the pathogenesis of liver and pancreatic cancers. J. Gastroenterol. Hepatol.. 2012;27(Suppl 2):10-14.
- [Google Scholar]
- Stimulation of calcium influx and CK1alpha by NF-kappaB antagonist [6]-gingerol reprograms red blood cell longevity. J. Food Biochem.. 2021;45:e13545.
- [Google Scholar]
- The effect of local renin angiotensin system in the common types of cancer. Front. Endocrinol. (Lausanne).. 2021;12:736361
- [Google Scholar]
- Angiotensin II exaggerates SARS-CoV-2 specific T-cell response in convalescent individuals following COVID-19. Int. J. Mol. Sci.. 2022;23
- [Google Scholar]
- Interactions between oxidative stress, autophagy and apoptosis in A549 cells treated with aged black carbon. Toxicol. In Vitro. 2019;54:67-74.
- [Google Scholar]
- SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1-like-macrophages: potential role of angiotensin-converting enzyme inhibitor (perindopril) Front. Immunol.. 2021;12:728896
- [Google Scholar]
- Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res.. 2000;87:840-844.
- [Google Scholar]
- gammadelta T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation. 2017;135:2155-2162.
- [Google Scholar]
- Angiotensin II triggers release of neutrophil extracellular traps, linking thromboinflammation with essential hypertension. JCI Insight. 2021;6
- [Google Scholar]
- Reactive oxygen species and vascular cell apoptosis in response to angiotensin II and pro-atherosclerotic factors. Regul. Pept.. 2000;90:19-25.
- [Google Scholar]
- The effects of pollen, propolis, and caffeic acid phenethyl ester on tyrosine hydroxylase activity and total RNA levels in hypertensive rats caused by nitric oxide synthase inhibition: experimental, docking and molecular dynamic studies. J. Biomol. Struct. Dyn.. 2018;36:609-620.
- [Google Scholar]
- Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ. Res.. 2000;87:179-183.
- [Google Scholar]
- Allethrin promotes apoptosis and autophagy associated with the oxidative stress-related PI3K/AKT/mTOR signaling pathway in developing rat ovaries. Int. J. Mol. Sci.. 2022;23:6397.
- [Google Scholar]
- The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol. Rev.. 2007;59:251-287.
- [Google Scholar]
- ROS and autophagy: interactions and molecular regulatory mechanisms. Cell. Mol. Neurobiol.. 2015;35:615-621.
- [Google Scholar]
- ROS activates JNK-mediated autophagy to counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta Pharmacol. Sin.. 2015;36:1473-1479.
- [Google Scholar]
- Cyr61 mediates angiotensin II-induced podocyte apoptosis via the upregulation of TXNIP. J. Immunol. Res.. 2023;2023:8643548.
- [Google Scholar]
- Acute 7, 12-dimethylbenz [a] anthracene exposure causes differential concentration-dependent follicle depletion and gene expression in neonatal rat ovaries. Toxicol. Appl. Pharmacol.. 2014;276:179-187.
- [Google Scholar]
- Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol.. 2007;8:741-752.
- [Google Scholar]
- Modulation of lncRNA links endothelial glycocalyx to vascular dysfunction of tyrosine kinase inhibitor. Cardiovasc. Res.. 2023;119:1997-2013.
- [Google Scholar]
- The investigation of antioxidant and anti-inflammatory potentials of apitherapeutic agents on heart tissues in nitric oxide synthase inhibited rats via Nω-nitro-L-arginine methyl ester. Clin. Exp. Hypertens.. 2021;43:69-76.
- [Google Scholar]
- Regulation of mammalian autophagy in physiology and pathophysiology. Physiol. Rev.. 2010;90:1383-1435.
- [Google Scholar]
- ‘Pseudopalisading’necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol.. 2006;65:529-539.
- [Google Scholar]
- In vitro studies of the renin-angiotensin system in human adipose tissue/adipocytes and possible relationship to SARS-CoV-2: a scoping review. Adipocyte. 2023;12:2194034.
- [Google Scholar]
- Renin–angiotensin system: basic and clinical aspects—A general perspective. Endocrinología Diabetes y Nutrición (english Ed.). 2022;69:52-62.
- [Google Scholar]
- The ageing endothelium, cardiovascular risk and disease in man. Exp. Physiol.. 2009;94:317-321.
- [Google Scholar]
- Sox9 mediates autophagy-dependent vascular smooth muscle cell phenotypic modulation and transplant arteriosclerosis. iScience. 2022;25:105161
- [Google Scholar]
- Plasma signature of apoptotic microvesicles is associated with endothelial dysfunction and plaque rupture in acute coronary syndromes. J. Mol. Cell. Cardiol.. 2020;138:110-114.
- [Google Scholar]
- Abamectin induces apoptosis and autophagy by inhibiting reactive oxygen species-mediated PI3K/AKT signaling in MGC803 cells. J. Biochem. Mol. Toxicol.. 2019;33:e22336.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103180.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







