Translate this page into:
Synthesis of novel indole, 1,2,4-triazole derivatives as potential glucosidase inhibitors
⁎Corresponding authors. ramesh180769@gmail.com (Ramesh S Gani), avinash.kudva@gmail.com (Avinash Kundadka Kudva)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objective
In the present study a series of eleven bis-heterocyclic compounds with indole derivative carrying 1,2,4-triazole moiety were synthesized and assessed for their in vitro α-amylase and α-glucosidase inhibition activity.
Method
The synthesized compounds were characterized by using various spectroscopic techniques such as 1H NMR, IR and EI-MS. Initial in silico screening process was used to find potential ligands that were later evaluated for α-amylase and α-glucosidase inhibitory potential.
Results
The docking results revealed that the synthesized compounds were well accommodated in the binding pockets of α-glucosidase. Especially, 5e and 5j showed similar interaction pattern, as previously reported Casuarine-enzyme complex. In vitro analysis suggests that compounds 5a-5k showed varying degrees of α-amylase and α-glucosidase inhibitory activity. Amongst them, 5e and 5j demonstrated good enzyme inhibition while remaining compounds showed low to moderate inhibitory potential.
Conclusions
Addition of 2,5 dimethoxy substituent (2,5-dimethoxybenzaldehyde) (5e) or hydroxy, methoxy substituents (6-methoxy-2-naphthol aldehyde) (5j) at ortho and meta position exhibited good α-amylase and α-glucosidase inhibition. Hence this study provides several insights on improving the pharmacological profile of triazole containing compounds that can be adopted to design and develop novel glucosidase inhibitors.
Keywords
α-Amylase
α-Glucosidase
Molecular docking
Indole
Triazole
1 Introduction
Hyperglycemia is one of the characteristics of diabetes caused due to increased blood glucose and impaired insulin levels in the body. Upon consumption of food, α-amylase and α-glucosidase found in small intestine brush border, breaks down the complex carbohydrates into lower sugars, mainly disaccharides, and monosaccharides. As there is elevated enzymatic activity during digestion, a rapid increase in blood glucose levels is imminent. Therefore, the hydrolyzing enzymes, namely, α-amylase and α-glucosidase, have been potential therapeutic targets.
Reduction of postprandial hyperglycemia is one of the critical medicinal involvement to cure type-2 diabetes mellitus (Hinnen, 2015; Teng and Chen, 2017). Postprandial hyperglycemia can be controlled by preventing these carbohydrate hydrolyzing enzymes. Among these, α-amylase breaks down the insoluble starch by hydrolyzing the α-bonds, while, α-glucosidase digests starch into simple glucose subunits. Thus these enzymes increase postprandial blood glucose levels. Hence these enzymes are a potential target for the development of anti-diabetic drugs (Scott and Spencer, 2000). The literature review indicates that many natural products possess α-glucosidase inhibitory action (Sun et al., 2016; Unnikrishnan and Jayasri, 2018). Also, various sugar derivatives such as ethanolamine phenyl 6-deoxy-6(morpholine-4-yl)-b-D-glucopyranoside, C-glucopyranosyl arene, and heteroarenes derivatives have been reported as α-glucosidase inhibitors (Balbaa et al., 1999; Bokor et al., 2017; Zhang et al., 2016). At present acarbose, voglibose and miglitol, are frequently used α-glucosidase inhibitors that efficiently prevent or prolong digestion of carbohydrates and reduce the rate of glucose absorption, thereby regulate postprandial hyperglycemia (Joshi et al., 2015; Matsuo et al., 1992; Scott and Spencer, 2000).
The indole moiety containing benzene ring fused with 4 and 5 positions of pyrrole ring are widely spread in nature. Indole and its derivatives are well known to possess a broad spectrum of biological properties such as anticancer, antiviral, antioxidant and antibacterial activities (Agarwal et al., 2005; Macdonough et al., 2013; Yamamoto and Kurazono, 2007; Zhang et al., 2016). A comprehensive literature survey revealed that indole-based compounds and their derivatives are widely used for treating diabetes and diabetic-related complications (Sravanthi and Manju, 2016). A variety of indole derivatives such as Indolyl xanthene, Indole carbohydrazide,N-bicyclo-5-chloro-1H-indole-2-carboxamide, and 5-chloro-1H-Indole carbohydrazide has been recently shown to have inhibitory activities against hydrolyzing enzymes like α-amylase and α-glucosidase (Noreen et al., 2017; Onda et al., 2008; Taha et al., 2018). Earlier reports have shown that Indole-pyrimidine based heterocyclic compounds had excellent inhibitory activity against carbohydrate degrading enzymes such as α-glucosidase and amylase (Avula et al., 2018; Gong et al., 2017; Imran et al., 2017). For instance, 2,3-indolobetulinic acid showed 4.5 times more activity than the parent triterpenoid with IC50 of 0.5 μM (Khusnutdinova et al., 2019). Nazir et al. had earlier synthesized indole based hybrid oxadiazole derivatives, all synthesized compounds showed good to moderate inhibition potential compared to acarbose (Nazir et al., 2018). In another study, indole fused benzylidene derivatives were demonstrated to be α-glucosidase inhibitors and hence proposed for use in management of hyperglycemia in patients with type 2 diabetes mellitus (Kaur et al., 2018).
More recently, triazoles that are N-substituted heterocyclic compounds have been shown to possess anti-diabetic properties. Recently 4-amino-1,2,4-triazole derivatives containing different types of aldehydes were shown to have good in vitro α-glucosidase and α-amylase inhibitory activities (Yeye et al., 2020). Hybrid heterocycles comprising benzothiazole-triazole derivatives were shown to possess moderate α-glucosidase inhibitory activity compared to standard acarbose (Gong et al., 2017). A 2–6-di(substituted phenyl)thiazole[3,2-b]-1,2,4-triazole was shown to have excellent inhibitory activity against α-amylase and α-glucosidase enzymes (Channar et al., 2017). Furthermore, several indolyl xanthene, indole carbohydrazide, indole hydrazone, and triazole derivatives were shown to possess inhibitory activities as compared to standard acarbose (Avula et al., 2018; Chavan et al., 2017; Imran et al., 2017; Saeedi et al., 2019; Taha et al., 2018).
Hence biological importance of fused-heterocyclic compounds containing indole and 1, 2, 4-triazole with their pharmacological potential has thereby made them extremely attractive research targets. Based on the above essential information, we have designed and synthesized a total of eleven different 1,2,4-triazole derivatives containing indole moiety and further evaluated their α-amylase, α-glucosidase inhibitory potential by using in vitro and in silico methods.
2 Materials and methods
2.1 Experimental details
All the analytical grade chemicals used in this experimental work were procured from Merck India and used without further purification. Melting points were recorded by the open capillary method and are uncorrected. The IR spectra were recorded on a Shimadzu FT-IR 157 spectrophotometer. 1H NMR spectra were recorded using CdCl3/DMSO‑d6 as solvent and TMS as an internal standard on a Bruker 400 MHz NMR spectrometer. The mass spectra were recorded on a low-resolution SHIMADZU mass spectrometer operating at 70 eV. The purity of the compounds was checked on completion of the reaction by thin-layer chromatography (TLC) on pre-coated silica gel plates.
2.1.1 Synthesis of 1-cyclopropyl-3-ethoxycarbonyl-2-methylindole-5-(1-methylethoxy acetic acid hydrazide (Compound 4)
The starting material 1-cylopropyl-3-ethoxycarbonyl-5-hydroxy-2-methyl Indole and 1-cyclopropyl-2-methyl-3-ethoxycarbonyl-5-(1-ethoxycarbonyl-1-methoxy)-indole were prepared as reported earlier (Gani et al., 2002). 1-cyclopropyl-3-ethoxycarbonyl-2-methylindole-5-(1-methylethoxy acetic acid hydrazide was prepared by dissolving 1-cyclopropyl-2-methyl-3-ethoxycarbonyl-5-(1-ethoxycarbonyl-1-methoxy)-indole (20 g, 0.0579 mol) and 99% hydrazine hydrate (15 mL) in 200 mL ethanol, in addition to this 1–5 drops pyridine was added. The whole reaction mass initially stirred at room temperature for 1 h. Then subjected to reflux on a water bath for 4–5 h. The completion of the reaction was analyzed by TLC using a mobile phase (Hexane: ethyl acetate; ratio 4:1). After completing the reaction, ethanol was distilled out to half its volume. Then cool the reaction mass at 10 °C, filtered, washed with ethanol, and dried under vacuum at 50–55 °C for about 6 h. The final yield obtained was 72%, with a melting point at 154–158 °C.
2.1.2 Ethyl1-cyclopropyl-5-((5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)methoxy)-2-methyl-1H-indole-3-carboxylate (Compound 5a)
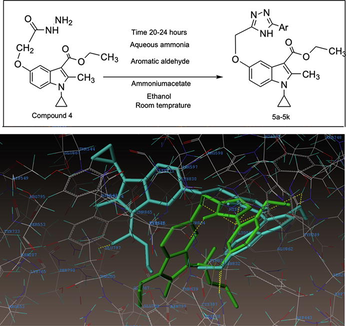
In a 100 mL round bottom flask placed 1 g (0.003 mol) of Compound 4, 0.42 g (0.003 mol) of Anisaldehyde (aromatic aldehyde), 0.47 g (0.006 mol) of ammonium acetate and added 15 mL ethanol, shake the mixture well and stirred the reaction mass at room temperature for 22–24 h. The progress of the reaction monitored by TLC by using the mobile phase (Hexane: Ethyl acetate; ratio 4:1). After 24 h, the reaction was complete, and then the reaction mass was neutralized with aqueous ammonia. A white color compound was precipitated out. The solid was filtered and washed with water, dried at 55–60 °C for 10 h. Yield 78%; Melting point: 191–181 °C. The same process was used for remaining aromatic aldehydes (Table 1; Compounds 5b-5k).
Code
Structure
Molecular weight
Molecular formula
5a
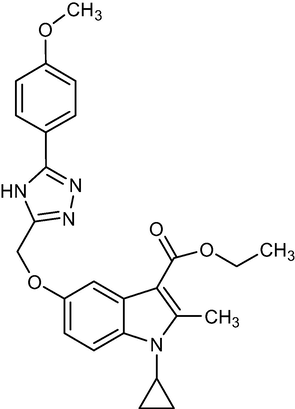
446.56
C25H26N4O4
5b
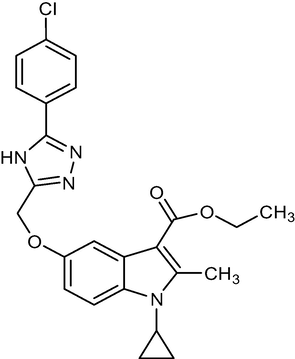
450.92
C24H23ClN4O3
5c
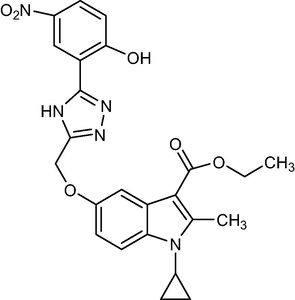
477.47
C24H23N5O6
5d
461.47
C24H23N5O5
5e
476.52
C26H28N4O5
5f
485.36
C24H22Cl2N4O3
5g
512.56
C29H28N4O5
5h
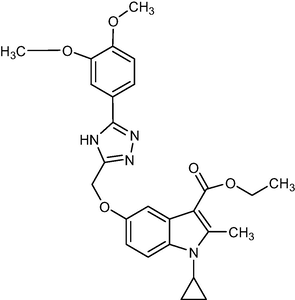
476.52
C26H28N4O5
5i
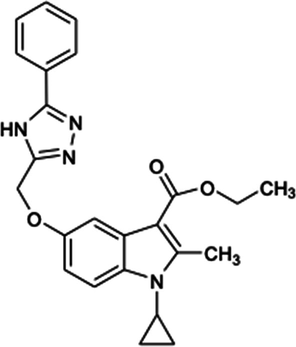
416.47
C24H24N4O3
5j
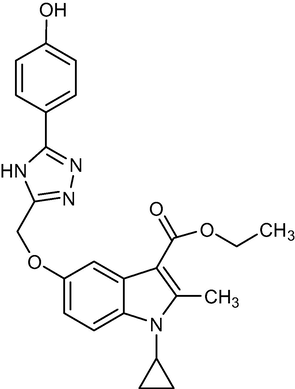
432.47
C24H24N4O4
5k
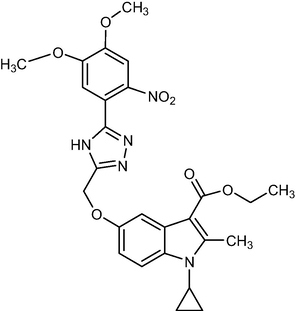
521.52
C26H27N5O7
Purity confirmation: After isolation, the product was dried at 55–60 °C for 10 h. Single product spot was observed on TLC plate, and it was confirmed by a clean 1H NMR spectrum; hence, it was concluded that the product was pure. The derived compounds were sequentially labelled as 5a-5k (the experimental procedures are reported in supplementary file).
2.1.3 Ethyl-1-cyclopropyl-5-((5-(4-methoxyphenyl)-4H-1,2,4-triazol-3-yl)methoxy)-2-methyl-1H-indole-3-carboxylate (5a)
Yield:78%.m.p.179–181 °C;IR;3190 cm−1(N–H-Str),3065 cm−1(AromC-H-Str),1685 cm−1(C = O),1HNMR(400 MHz,DMSO‑d6):δ3.80(s,3H,O-CH3),5.12(s,2H,C-5,O-CH2),1.35(t,3H,C-3,esterCH3),4.28(q,2H,C-3,–OCH2),2.75(s,3H,C-2,CH3),11.48(s,1H,triazoleNH),4.6(m,1H,N-CH),0.9–1.2(m,4H,Cycloprophylmethylene),6.8(d,J = 7.6 Hz,1H),6.96(d,J = 9.2 Hz,1H),7.01(d,J = 4.4 Hz,1H)7.42(s,1H),7.49(d.J = 9.2),7.53(d,J = 4.4 Hz),7.6(d,J = 7.6,1H)EIMSm/z (% Rel. Abund.) 489(M + H + 41],(10),256.75(10),217(10),2.
2.1.4 Ethyl-5-((5-(4-chlorophenyl)-4H-1,2,4-triazol-3-yl)methoxy)-1-cyclopropyl-2-methyl-1H-indole-3-carboxylate (5b)
Yield:65%.m.p.167–169 °C;IR;3311 cm−1(N–H-Str),3099 cm−1(AromC-H-Str),1695 cm−1(C = O), 1HNMR(400 MHz, DMSO‑d6):δ5.14(s,2H,C-5,O-CH2),1.31(t,3H,C-,esterCH3),4.18(q,2H,C-3,–OCH2),2.75(s,3H,C-2,CH3),11.50(s,1H,triazoleNH),4.65(m,1H,N-CH),0.9–1.22(m,4H,Cycloprophylmethylene),6.86(d,J = 2.8 Hz,1H),6.93(d,J = 2.4 Hz),6.90(d,J = 6.8 Hz,1H),6.97(d,J = 2.8 Hz,1H),7.01(s,1H),7.01(d,J = 2.4 Hz,1H),6.97(d,J = 7.6 Hz,1H).:EI MS m/z (% Rel. Abund.) 452(M + 1],(20),382(10),338(10)224(10)178(1 0 0).
2.1.5 Ethyl-1-cyclopropyl-5-((5-(2-hydroxy-5-nitrophenyl)-4H-1,2,4-triazol-3-yl)methoxy)-2-methyl-1H-indole-3-carboxylate (5c)
Yield:87%.m.p.117–120 °C;IR;3394 cm−1(O–H-Str),3344 cm−1(N–H-Str),3044 cm−1(AromC-H-Str),1678 cm−1(C = O),1HNMR(400 MHz, DMSO‑d6):δ5.14(s,2H,C-5,O-CH2),1.34(t,3H,C-3, esterCH3),4.28(q,2H,C-3,–OCH2),2.76(s,3H,C-2,CH3),11.50(s,1H,triazole NH),4.67(m,1H,N-CH),0.9–1.22(m,4H,Cycloprophylmethylene),6.86–8.62(all-aromatic protons), 5.09(s,1H,OH).(:EI MS m/z (% Rel. Abund.) 479(M + 1].
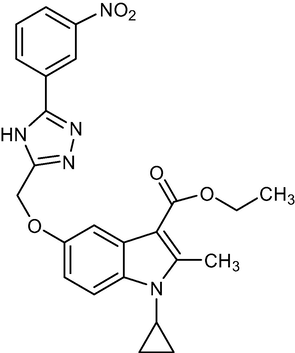
2.1.6 Ethyl-1-cyclopropyl-2-methyl-5-((5-(3-nitrophenyl)-4H-1,2,4-triazol-3-yl)methoxy)-1H-indole-3-carboxylate (5d)
Yield:65%.m.p.160–164 °C;IR;3373 cm−1(N–H-Str),3044 cm−1(AromC-H-Str),1676 cm−1(C = O),1HNMR(400 MHz, DMSO‑d6):δ5.19(s,2H,C-5,O-CH2),1.34(t,3H,C-3,esterCH3),4.26(q,2H,C-3,–OCH2),2.75(s,3H,C-2,CH3),11.40(s,1H,triazole NH), 4.71(m,1H,N-CH),0.9–1.25(m,4H,Cycloprophylmethylene),6.86–8.48(all-aromatic protons),:EI MS m/z (% Rel. Abund.) 509(M + H + 46],(20),463(70),256(10)0.224(10),206(1 0 0).
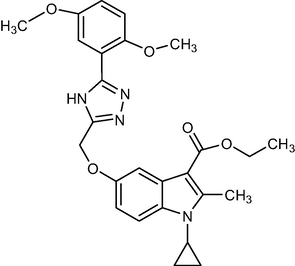
2.1.7 Ethyl1-cyclopropyl-5-((5-(2,5-dimethoxyphenyl)-4H-1, 2, 4-triazol-3-yl) methoxy)-2-methyl-1H-indole-3-carboxylate (5e)
Yield:88%.m.p.180–184 °C;IR;3380 cm−1(N–H-Str),3010 cm−1(AromC-H-Str),1689 cm−1(C = O),1HNMR(400 MHz,DMSO‑d6):δ3.73(s,3H,O-CH3),3.74(s,3H,O-CH3)1.35(t,3H,C-3,esterCH3),4.27(q,2H,C-3,–OCH2),2.75(s,3H,C-2,CH3),11.59(s,1H,triazole-H), 4.65(m,1H,N-CH),0.9–1.27(m,4H,Cycloprophylmethylene),6.88–7.52(all-aromaticprotons),EI-MS,m/z (% Rel. Abund.) 480(M + 1 + Li) adduct.
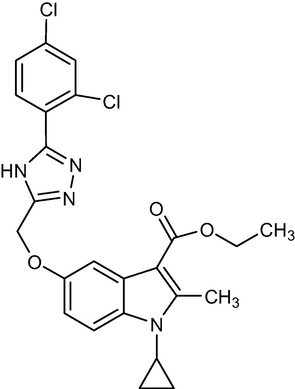
2.1.8 Ethyl-1-cyclopropyl-5-((5-(2,4-dichlorophenyl)-4H-1,2,4-triazol-3-yl)methoxy)-2-methyl-1H-indole-3-carboxylate (5f)
Yield:59%.m.p.187–189 °C;IR;3170 cm−1(N–H-Str),3010 cm−1(AromC-H-Str),1693 cm−1(C = O),1HNMR(400 MHz,DMSO‑d6):1.34(t,3H,C-3,esterCH3),4.26(q,2H,C-3,–OCH2),2.75(s,3H,C-2,CH3),11.85(s,1H,triazoleNH),4.68(m,1H,N-CH),0.9–1.27(m,4H,Cycloprophylmethylene),6.88–8.02(all-aromaticprotons),:EIMSm/z (% Rel. Abund.) 485(M + 1],(25),326(10),256(10),228(10),178(1 0 0).
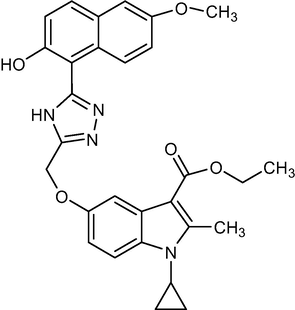
2.1.9 Ethyl-1-cyclopropyl-5-((5-(2-hydroxy-6-methoxynaphthalen-1-yl)-4H-1,2,4-triazol-3-yl)methoxy)-2-methyl-1H-indole-3-carboxylate (5 g)
Yield:73%.m.p.177–178 °C;IR;3344 cm−1(O–H-Str),3190 cm−1(N–H-Str),3059 cm−1(AromC-H-Str),1678 cm−1(C = O),1HNMR(400 MHz,DMSO‑d6):1.33(t,3H,C-3,esterCH3),4.27(q,2H,C-3,–OCH2),2.76(s,3H,C-2,CH3),11.8(s,1H,triazoleNH),4.68(m,1H,N-CH),0.9–1.27(m,4H,Cycloprophylmethylene),6.91–8.14(all-aromaticprotons),3.89(s,3H,O-CH3),3.79(s,1H,OH).
2.1.10 Ethyl-1-cyclopropyl-5-((5-(3,4-dimethoxyphenyl)-4H-1,2,4-triazol-3-yl)methoxy)-2-methyl-1H-indole-3-carboxylate (5 h)
Yield:81%.m.p.140–143 °C;IR;3188 cm−1(N–H-Str),3040 cm−1(AromC-H-Str),1670 cm−1(C = O),1HNMR(400 MHz,DMSO‑d6):1.33(t,3H,C-3,esterCH3),4.27(q,2H,C-3,–OCH2),2.76(s,3H,C-2,CH3),11.8(s,1H,triazoleNH),4.65(m,1H,N-CH),0.9–1.27(m,4H,Cycloprophylmethylene),6.86–7.53(all-aromaticprotons),3.79(s,3H,O-CH3),3.80(s,3H,O-CH3).:EIMSm/z (% Rel. Abund.) 478(M + 1) (10)0.256(10), 178(1 0 0).
2.1.11 Ethyl-1-cyclopropyl-2-methyl-5-((5-phenyl-4H-1,2,4-triazol-3-yl)methoxy)-1H-indole-3-carboxylate (5i)
Yield:51%.m.p.152–154 °C;IR;3111 cm−1(N–H-Str),3070 cm−1(AromC-H-Str),1680 cm−1(C = O),1HNMR(400 MHz,DMSO‑d6):1.34(t,3H,C-3,esterCH3),4.28(q,2H,C-3,–OCH2),2.75(s,3H,C-2,CH3),11.63(s,1H,triazoleNH),4.65(m,1H,N-CH),0.9–1.27(m,4H,Cycloprophylmethylene),6.87–7.70(all-aromaticprotons),EIMSm/z (% Rel. Abund.) 418(M + 1) (10)0.418(10), 256(10), 206(1 0 0).
2.1.12 Ethyl-1-cyclopropyl-5-((5-(4-hydroxyphenyl)-4H-1, 2, 4-triazol-3-yl) methoxy)-2-methyl-1H-indole-3-carboxylate (5j)
Yield:81%.m.p.156–157 °C;IR;3340 cm−1(O–H-Str3207cm−1(N–H-Str),3072 cm−1(AromC-H-Str),1686 cm−1(C = O),1HNMR(400 MHz,DMSO‑d6):1.34(t,3H,C-3,esterCH3),4.26(q,2H,C-3,–OCH2),2.76(s,3H,C-2,CH3),11.40(s,1H,triazoleNH),4.63(m,1H,N-CH),0.9–1.27(m,4H,Cycloprophylmethylene),6.87–7.54(all-aromatic protons),4.26(s.1H,OH)EI-MS m/z (% Rel. Abund.) 475(M + H + 41) (10)0.418(10), 256(10), 206(1 0 0).
2.1.13 Ethyl-1-cyclopropyl-5-((5-(4,5-dimethoxy-2-nitrophenyl)-4H-1,2,4-triazol-3-yl)methoxy)-2-methyl-1H-indole-3-carboxylate (5 k)
Yield:58.%.m.p.190–196 °C;IR;3334 cm-1(O–H-Str3213cm-1(N–H-Str),3072 cm-1(AromC-H-
Str),1686 cm-1(C = O),1HNMR(400 MHz,DMSO‑d6):1.36(t,3H,C-3,esterCH3),4.29(q,2H,C-3,–OCH2),2.76(s,3H,C-2,CH3),5.19(m,1H,N-CH),0.9–1.27(m,4H,Cycloprophylmethylene),6.87–7.54(all-aromaticprotons),4.26(s.1H,OH),3.93(s,6H,OCH3) EI-MS m/z (% Rel. Abund.) 523(M + 1) (10)0.461(10), 206(1 0 0).
2.2 Molecular docking studies with α-glucosidase
The docking simulations were performed using N-terminal human maltase-glucoamylase protein (α-Glucosidase) from the human intestine (PDB ID: 3CTT) obtained from RCSB Protein Data Bank (http://www.rcsb.org/pdb) (Cardona et al., 2009). The docking was performed according to the method described earlier (Chen et al., 2020; Wu et al., 2017). The protein was prepared by adding polar hydrogen atom with Gasteiger-Huckel charges and the removal of water molecules. The enzyme energy minimized using Gasteiger–Huckel charges and Tripos force field in the Sybyl software suite (Clark et al., 1989; Homer et al., 2008). The 3D structures of the ligands 5a-5k were generated by the SKETCH module implemented in the SYBYL-X 2.0 program (Tripos Inc., St. Louis, USA), and its energy-minimized conformation was obtained using the Powel method in SYBYL software. The resultant ligands were subjected to docking run at the ligand-binding site of the α-glucosidase by Surflex dock in SYBYL-X 2.0 program. Finally, Surflex dock module was used to predict and examine the binding modes of the synthesized compounds at the active site of the α-glucosidase enzyme. The general parameter such as starting conformations, max conformations, angstroms to expand search grid and the number of rotatable bonds were set as 0, 20, 6, and 100, respectively. A soft grid treatment and active spin alignment method were performed. The number of spins and density of search per alignment was set at 12 and 3, respectively. From the analysis ligand-receptor complex with the maximum total score was considered as the most stable conformation. The H-bonding interactions between amino acid residues and their bond distances with synthesized compounds were noted and compared to enzyme inhibitor, Casuarine, as a reference molecule (see supplementary file).
2.3 α-Amylase inhibition assay
The α-amylase inhibitory assay was determined using a method adapted from Kwon et al. with minor modifications (Kwon et al., 2008). The synthesized compounds 5a-5k were dissolved in 1% dimethyl sulfoxide (DMSO) aqueous solution, while standard acarbose was prepared in distilled water. A reaction mixture was prepared to contain 100 μL of standard or compounds (20 to 100 µg/mL), 200 μL of sodium phosphate buffer (20 mM, pH 6.9) and 100 μL of α-amylase (1 U/mL). The mixture was preincubated at 25 °C for 10 min, then 200 μL of 1% starch in the above buffer was added and incubated for another 10 min. The reactions were stopped by adding 1 mL of 3,5-Dinitrosalicylic acid and placing on a boiling water bath for 5 min. The boiled reaction mixture was cooled to room temperature, diluted to 1:5 ratio with water, and absorbance was measured in a UV–visible spectrophotometer at 540 nm. The experiment was done in triplicates, and the percentage of inhibition of enzyme activity was calculated as:where AControl is absorbance of the control and ATreatment is absorbance upon treatment with various compounds. The percentage of α-amylase inhibitory activity is given in the Table 2. All results expressed are mean of three individual replicates (n = 3 ± S.D).
Compound
Concentration/% inhibition activity
20 µg/mL
40 µg/mL
60 µg/mL
80 µg/mL
100 µg/mL
4
21.01 ± 0.34
29.10 ± 0.74
45.09 ± 0.72
54.31 ± 0.91
61.03 ± 0.34
5a
18.41 ± 0.48
28.05 ± 0.95
34.02 ± 0.68
42.29 ± 0.74
51.07 ± 0.68
5d
20.71 ± 0.49
28.01 ± 0.72
38.63 ± 0.56
47.50 ± 0.83
59.03 ± 0.89
5e
30.05 ± 0.51
39.04 ± 0.78
48.05 ± 0.61
54.30 ± 0.45
63.14 ± 0.75
5h
17.30 ± 0.38
24.01 ± 0.65
32.10 ± 0.54
40.91 ± 0.43
51.07 ± 0.79
5i
20.19 ± 0.76
31.10 ± 0.44
42.81 ± 0.66
51.02 ± 0.72
60.12 ± 0.61
5j
30.08 ± 0.59
40.11 ± 0.76
54.05 ± 0.56
62.09 ± 0.85
71.29 ± 0.75
5k
20.50 ± 0.84
28.70 ± 0.44
31.40 ± 0.78
40.61 ± 0.59
50.13 ± 0.64
Acarbose
40.10 ± 0.75
51.15 ± 0.96
70.10 ± 0.83
82.70 ± 0.72
94.02 ± 0.54
2.4 α-Glucosidase inhibition assay
The α-Glucosidase inhibitory activity was determined as described earlier by Kim et al. with slight modifications (Kim et al., 2004). A reaction mixture containing 100 μL of synthesized compound 5a-5k or acarbose (20 to 100 µg/mL) was mixed with 50 μL of α-glucosidase (1 U/mL) prepared in 0.1 M phosphate buffer (pH 6.9). The mixture was preincubated at 37 °C for 20 min, then 10 μL of 10 mM p-nitrophenyl-α-D-glucopyranoside (pNPG) in the above buffer was added and incubated for another 30 min. The reactions were stopped by adding 650 μL of 1 M sodium carbonate, and the absorbance was measured in a UV–visible spectrophotometer at 405 nm. DMSO (1%) was used as a solvent control. The experiment was done in triplicates, and the percent inhibition of enzyme activity was calculated as:where AControl is absorbance of the control and ATreatment is absorbance of treated sample. The percentage of α-glucosidase inhibitory activity is given in the Table 3. All results expressed are mean of three individual replicates (n = 3 ± S.D).
Compound
Concentration/% inhibition activity
20 µg/mL
40 µg/mL
60 µg/mL
80 µg/mL
100 µg/mL
4
20.13 ± 0.64
29.01 ± 0.54
35.99 ± 0.72
48.19 ± 0.41
61.51 ± 0.54
5a
22.06 ± 0.63
30.01 ± 0.55
40.04 ± 0.82
52.81 ± 0.54
61.05 ± 0.74
5d
20.31 ± 0.59
29.06 ± 0.76
39.21 ± 0.74
48.99 ± 0.63
57.10 ± 0.99
5e
30.07 ± 0.41
40.19 ± 0.81
50.23 ± 0.71
60.02 ± 0.55
70.24 ± 0.86
5h
18.19 ± 0.68
26.02 ± 0.72
35.01 ± 0.54
46.13 ± 0.53
55.09 ± 0.33
5i
23.11 ± 0.49
31.08 ± 0.56
43.15 ± 0.78
51.05 ± 0.67
60.04 ± 0.66
5j
30.40 ± 0.69
42.06 ± 0.76
51.09 ± 0.51
62.20 ± 0.75
71.02 ± 0.69
5k
21.45 ± 0.64
29.05 ± 0.49
38.02 ± 0.52
44.31 ± 0.71
56.01 ± 0.64
Acarbose
40.10 ± 0.75
51.15 ± 0.96
70.10 ± 0.83
82.70 ± 0.72
94.02 ± 0.54
2.5 Statistical analysis
The enzyme assays were performed in triplicates (n = 3), and the results were expressed as mean ± Standard deviation. Un-paired two-tailed t-test performed to compare the mean of each treated group with the mean of the control group using GraphPad prism software (Graph Pad software Inc., San Diego, USA). p values ≤ 0.05 was considered as statistically significant.
3 Results and discussion
3.1 Chemistry
The direction of synthesis of the prepared compounds (5a-5k) was as outlined in the synthesis Scheme 1. These compounds were subjected to various spectroscopic techniques, namely, 1H NMR and EI-MS to confirm the structures (data shown in supplementary files). The elemental analyses were found to correspond with the calculated values for all the compounds in Scheme 1. The starting material indole hydrazide (compound-4) was prepared by Nenitzescu reaction and used further to synthesize compounds 5a-5k (Eiden and Kucklander, 1971; Gani et al., 2002).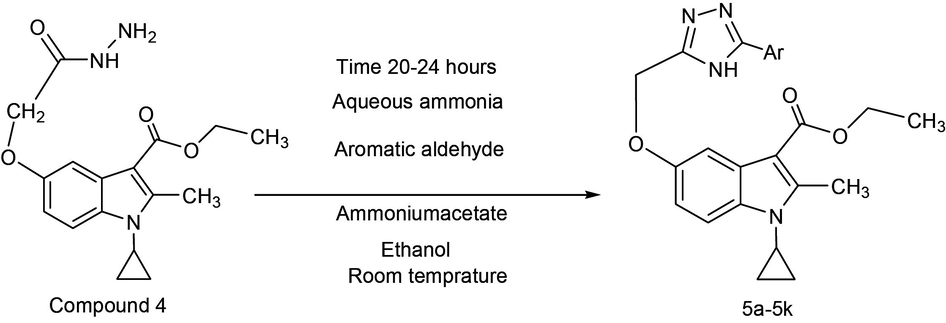
Synthesis of Indole fused triazole derivatives (5a-5k).
Indole fused with triazole derivatives (5a-5k) were prepared by a condensation reaction between 1-cyclopropyl-3-ethoxycarbonyl-2-methylindole-5-(1-methylethoxy acetic acid hydrazide) with aqueous ammonia, ammonium acetate, ethanol, and different aromatic aldehyde. The reaction mass was stirred for 20–24 h at room temperature. As a result of this novel bis-heterocyclic compounds containing triazole and indole were obtained in moderate to good yield, as shown in Table S1 (supplementary file). The infrared spectral pattern of all compounds 5a-5k is presented in the experimental section in which for all compounds C-3 carbonyl functional group peaks exhibit in the region between 1670 and 1695 cm−1. The band due to √NH of 1,2,4 triazole ring were observed in the range of 3180–3394 cm−1. The presence of the number of protons in the synthesized compounds was recognized by 1HNMR spectroscopic technique. The 1HNMR spectra data were recorded in DMSO‑d6. All the results have been presented in the experimental section. For all the compounds, a singlet peak at range δ 2.4–2.75 ppm indicates the presence of three protons, which is corresponding to the methyl group present at C-2 position of the indole ring. The spectra showed multiplate at the range between δ 7–8 ppm corresponding to all aromatic protons. δ 11–12 ppm exhibited as a singlet, which is corresponding to NH proton present in the 1,2,4 triazole ring. A singlet at the range between δ 5–5.2 ppm indicates about the presence of methylene protons at C-5 position of the indole ring. A quartet at a range between δ 4.2–4.5 ppm corresponds to the presence of methylene protons at C-3 position of the indole ring. A triplet δ 1.1–1.4 ppm is corresponding to the presence of methyl group at C-3 position of indole moiety. A multiplate δ 4–4.8 is corresponding to the presence of one proton of (N-CH) cyclopropyl ring. A multiplate in the range between at δ 0.9–1.2 ppm corresponding to four protons due to presence of two methylene protons appears in the cyclopropyl ring. Based on all these available information, the structures were determined. The molecular weight of synthesized compounds were recorded on a low-resolution mass spectrometer operating at 70 eV. The molecular weight of all compounds was precisely matching with the calculated molecular mass.
3.2 Molecular docking studies
A virtual screening mechanism using the Consensus-scoring method was applied in order to obtain potential ligands that bind the active site of α-glucosidase. In the beginning, synthesized molecules were built using SKETCH module implemented in the SYBYL program. The X-ray structure of N-terminal human maltase-glucoamylase (α-glucosidase), PDB ID: 3CTT, was obtained from protein data bank and used for docking calculations. The docking was performed with Surflex-Dock program interfaced with Sybyl-X 2.0. The compiled docked view for all the compounds (5a-5k) is shown in Fig. S1 (supplementary file). Based on the analysis, it was found that all the ligands were fitting well into the active site and had the most favorable docking scores, as shown in Table S2 (supplementary file). Among these 5e and 5j had the best Consensus scores. Hence, further docking analysis of binding region and interactions with amino acid residues in the active site were analyzed for these two ligands.
As depicted in Fig. 1, compound 5e makes four hydrogen-bonding interactions at the active site of the enzyme, hydrogen of –NH of triazole ring makes hydrogen bonding interaction with the oxygen of ASP542 (NH–---O-ASP542, 2.53 Å), an oxygen atom of methoxy group makes hydrogen bonding interaction with the hydrogen of ARG526 (O----H-ARG526, 1.88 Å), the hydrogen atom of methyl group present at the second position of indole ring makes hydrogen bonding interaction with the oxygen atom of LEU577 (H----O-LEU577, 2.40 Å). The methoxy group's oxygen atom at the fifth position in the phenyl ring makes hydrogen bonding interaction with the hydrogen of ASP327 (O----H-ASP327, 2.57 Å).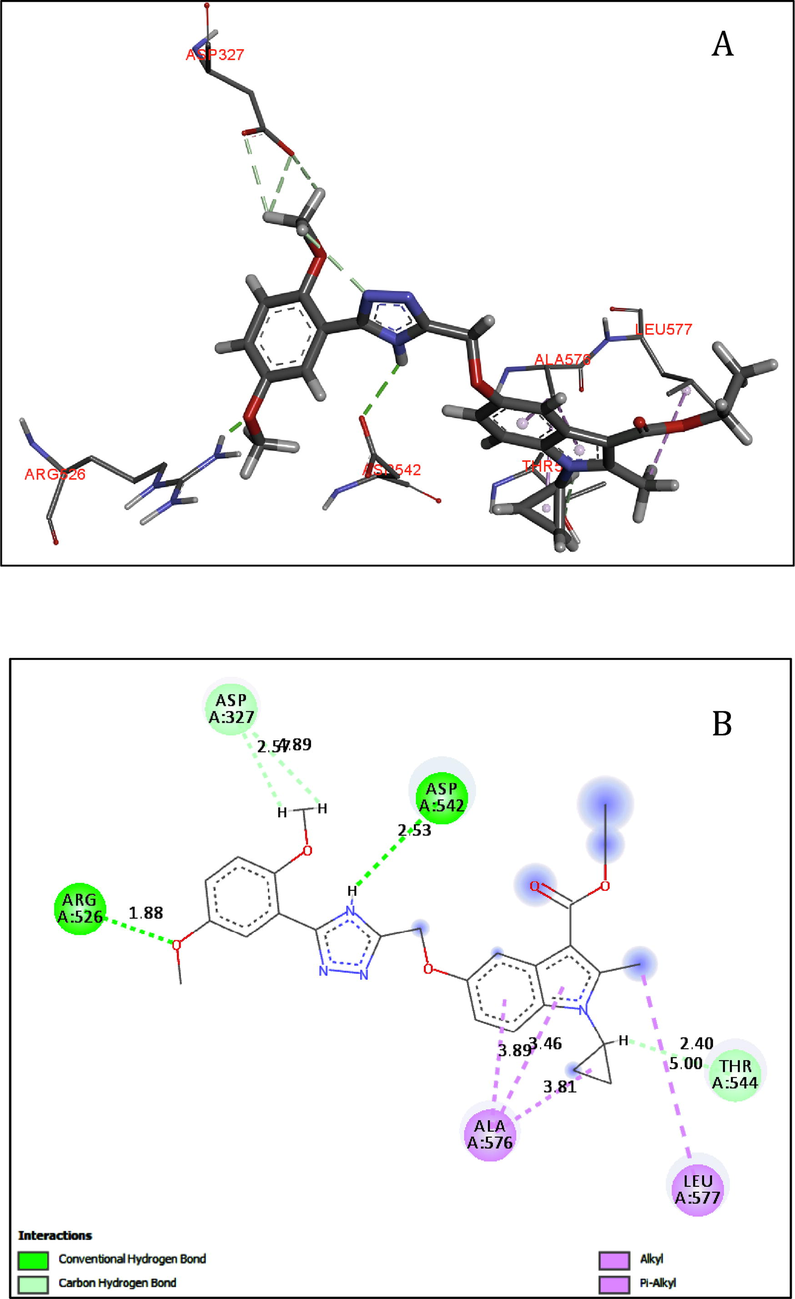
(A-B). Docked view of compound 5e at the active site of the enzyme Maltase-glucoamylase (α-glucosidase).
As shown in Fig. 2, compound 5j makes two hydrogen-bonding interactions at the active site of the enzyme, oxygen atom of the carbonyl group of carboxylate group present at the third position of indole ring makes hydrogen bonding interaction with the hydrogen of THR205 (O----H-GLN603, 2.05 Å) and the hydrogen atom of methyl group present at carboxylate makes hydrogen bonding interaction with the oxygen of GLU300 (H----H-GLU300, 4.98 Å).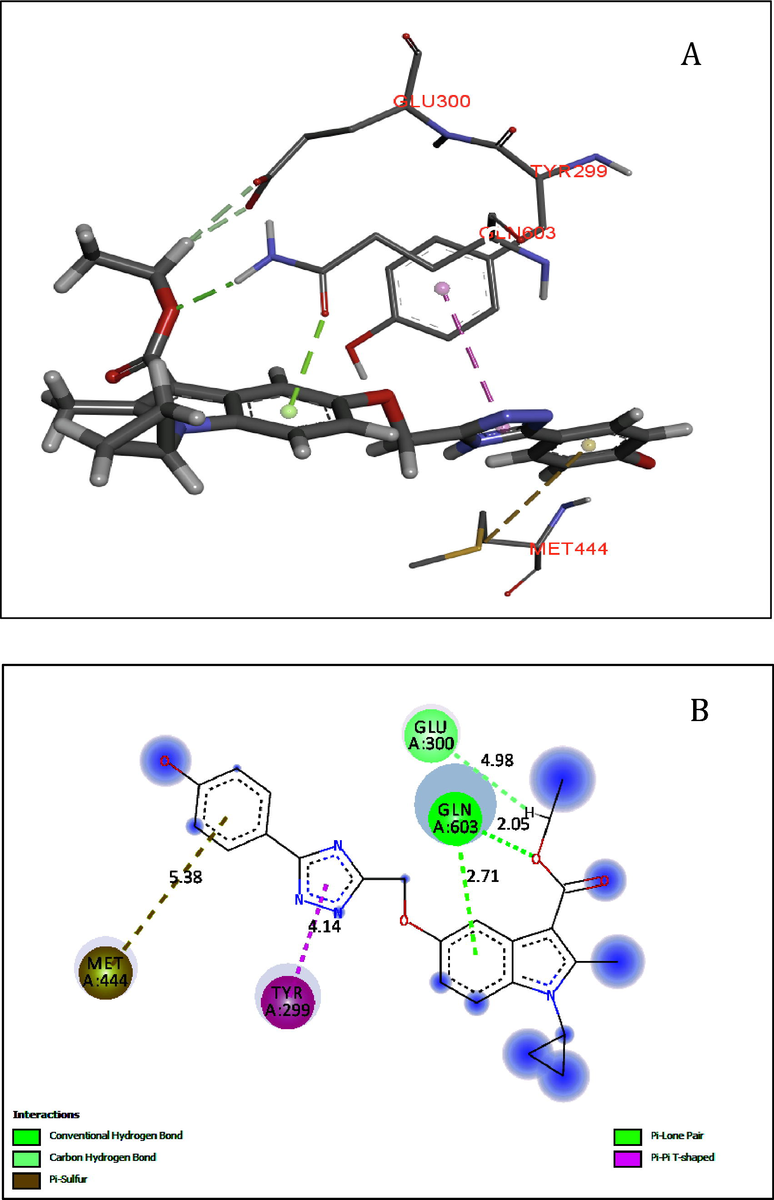
(A-B). Docked view of compound 5j at the active site of the enzyme Maltase-glucoamylase (α-glucosidase).
The binding simulation of ligand, Casuarine, a well-known inhibitor with α-glucosidase protein active site, was used as reference ligand. As reported earlier and also analyzed by us, casuarine showed eight bonding interactions. The docked view of the same is shown in Fig. S2 (supplementary file) (Cardona et al., 2009). The docking results revealed that the synthesized compounds are well accommodated in the same binding pocket of α-glucosidase. Moreover, the synthesized compounds bind similarly to the protein as that of the Casuarine-enzyme complex.
3.3 In vitro α-amylase inhibitory potential
We have synthesized eleven indole derivatives containing 1, 2, 4-triazole moiety, and screened for α-amylase inhibitory potential. Tested compounds showed a variable degree of α-amylase inhibition ranging from 17.30 ± 0.68 to 71.29 ± 0.75 at a concentration range from 20 to 100 μg/mL. While at similar concentrations, standard acarbose showed 40.10 ± 0.75 to 94.02 ± 0.54 respectively. Among the compounds, 5e showed good α-amylase inhibition potential (30.05 ± 0.51, 39.04 ± 0.78, 48.05 ± 0.61, 54.30 ± 0.45, 63.63 ± 0.75 at concentration gradient 20 to 100 μg/mL respectively). Likewise compound 5j showed similar α-amylase inhibitory potential (30.08 ± 0.59, 40.11 ± 0.76, 54.05 ± 0.56, 62.09 ± 0.85, 71.29 ± 0.75 at concentration 20 to 100 μg/mL respectively). Remaining compounds including the parent molecule (compound-4) showed moderate to low inhibitory potential when compared to standard acarbose (Table 2). The DMSO (1%) did not affect the enzyme activity (data not shown). α-Amylase facilitates the digestion of complex carbohydrates by breaking 1,4-glycosidic linkages of polysaccharides to disaccharides. Hence inhibition of α-amylase is an important step in controlling hyperglycemia in humans. In this study, compounds 5e, and 5j retained 69–75% inhibitory activity compared to standard acarbose.
3.4 In vitro α-glucosidase inhibitory potential
α-Glucosidase is a carbohydrate hydrolyzing enzyme responsible for the conversion of disaccharides to monosaccharides. This enzyme's inhibition has been correlated with improved postprandial hyperglycemia and decreased insulin requirements in diabetic patients (Hanefeld et al., 2004). In our study, tested compounds showed a variable degree of α-glucosidases inhibition, ranging from 18.19 ± 0.68 to 71.02 ± 0.69 at a concentration gradient 20 to 100 μg/mL respectively. Compound 5e showed good α-glucosidase inhibition potential (30.07 ± 0.41 to 70.24 ± 0.86). Similarly, compound 5j had inhibitory potential between 30.04 ± 0.69 to 71.02 ± 0.69 respectively. Remaining compounds showed lower α-glucosidase inhibitory potential (Table 3). The DMSO (1%) did not affect the enzyme activity (data not shown). Thus it can be concluded that compounds 5e and 5j confer inhibitory effects on α-glucosidase; however, at moderate levels (∼75%) compared to standard acarbose.
All structural features such as, 5-substituted indole moiety, aryl substituted 1, 2 4 triazole play an important role in the α-glucosidase and α-amylase inhibition. Addition of 2,5 dimethoxy substituent (2,5-dimethoxybenzaldehyde) at ortho and meta position in compound 5e and hydroxy, methoxy substituents (6-methoxy-2-naphthol aldehyde) at ortho and meta position in compound 5j have exhibited good inhibitory activities against α-glucosidase enzymes. The remaining compounds had low to moderate α-glucosidase inhibitory potential. Earlier studies had shown similar observation, where Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, derived from the marine algae, had good α-glucosidase inhibitory activity (Liu et al., 2011). Also, it is shown that methoxy-substituted benzohydrazide derivatives had good antioxidant and α-glucosidase inhibitory activity attributed to the methoxy group at the meta and ortho position on the benzene ring (Prachumrat et al., 2018). These reports hence substantiate our observation on 5e and 5j being better ligands among the synthesized compounds.
4 Conclusion
In this study, we prepared eleven bis-heterocyclic compounds with indole as a core moiety carrying 1, 2, 4 triazole ring. All newly synthesized compounds were characterized by various spectroscopic techniques, namely, 1H NMR, IR and EI-MS to confirm the structures. These were further evaluated against α-amylase and α-glucosidase enzymes for their inhibitory potential. Among these compounds, 5e and 5j showed comparable α-amylase and α-glucosidase inhibitory potential as that of standard acarbose. Furthermore, molecular docking studies revealed that synthesized compounds were well accommodated in the binding pockets of the α-glucosidase enzyme. The synthesized compounds similarly bind to the protein as that of the previously reported casuarine-enzyme complex. From the above studies, it was observed that compounds containing 2,5-dimethoxybenzaldehyde or 6-methoxy-2-naphthol aldehyde derivatives appear to favor enzyme inhibition. These findings will help us continue our efforts towards improving the pharmacological profile of these triazole containing compounds. And also provides several clues that can be adopted to design and develop novel α-glucosidase inhibitors.
Acknowledgments
The authors wish to thank the Management, Strides Research and Specialty Chemicals Limited, 120 A & B, Industrial Area, Baikampady, Mangalore 574199, India for needful support to carry out this research work. Dr. Shrinivas D. Joshi is thankful to Vision Group on Science and Technology (Govt. of Karnataka), Bangalore, India [VGST letter Ref No. VGST/GRD-567/2016-17/2017-18/183 dated 31-05-2018] for financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of substituted indole derivatives as a new class of antimalarial agents. Bioorg. Med. Chem. Lett.. 2005;15(12):3133-3136.
- [Google Scholar]
- Synthesis of 1H–1,2,3-triazole derivatives as new alpha-glucosidase inhibitors and their molecular docking studies. Bioorg. Chem.. 2018;81:98-106.
- [Google Scholar]
- Inhibition of some hepatic lysosomal glycosidases by ethanolamines and phenyl 6-deoxy-6-(morpholin-4-yl)-beta-D-glucopyranoside. Carbohydr. Res.. 1999;317(1–4):100-109.
- [Google Scholar]
- C-glycopyranosyl arenes and hetarenes: synthetic methods and bioactivity focused on antidiabetic potential. Chem. Rev.. 2017;117(3):1687-1764.
- [Google Scholar]
- Total syntheses of casuarine and its 6-O-alpha-glucoside: complementary inhibition towards glycoside hydrolases of the GH31 and GH37 families. Chem.-Eur. J.. 2009;15(7):1627-1636.
- [Google Scholar]
- Design and synthesis of 2,6-di(substituted phenyl)thiazolo[3,2-b]-1,2,4-triazoles as alpha-glucosidase and alpha-amylase inhibitors, co-relative Pharmacokinetics and 3D QSAR and risk analysis. Biomed. Pharmacother.. 2017;94:499-513.
- [Google Scholar]
- Iminosugars spiro-linked with morpholine-fused 1,2,3-triazole: synthesis, conformational analysis, glycosidase inhibitory activity, antifungal assay, and docking studies. ACS Omega. 2017;2(10):7203-7218.
- [Google Scholar]
- Design, synthesis and alpha-glucosidase inhibition study of novel embelin derivatives. J. Enzyme Inhib. Med. Chem.. 2020;35(1):565-573.
- [Google Scholar]
- Validation of the general purpose tripos 5.2 force field. J. Comput. Chem.. 1989;10(8):982-1012.
- [Google Scholar]
- Synthesis of 5-and 6-hydroxy-indole derivatives by the method of Nenitzescu. 1. Synthesis of antiphlogistics in indole derivatives. Arch. Pharm. Ber Dtsch Pharm. Ges.. 1971;304(1):57-64.
- [Google Scholar]
- Chemoselective reaction of indole dicarboxylate towards hydrazine hydrate: synthesis and antimicrobial activity of some new oxadiazolyl methoxyindole derivatives. Indian J. Heterocy. Ch.. 2002;12(1):25-28.
- [Google Scholar]
- Synthesis, in vitro alpha-glucosidase inhibitory activity and molecular docking studies of novel benzothiazole-triazole derivatives. Molecules. 2017;22(9)
- [Google Scholar]
- Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur. Heart J.. 2004;25(1):10-16.
- [Google Scholar]
- Therapeutic options for the management of postprandial glucose in patients with type 2 diabetes on basal insulin. Clin. Diabetes. 2015;33(4):175-180.
- [Google Scholar]
- SYBYL Line Notation (SLN): a single notation to represent chemical structures, queries, reactions, and virtual libraries. J. Chem. Inf. Model.. 2008;48(12):2294-2307.
- [Google Scholar]
- Synthesis and biological evaluation of indole derivatives as alpha-amylase inhibitor. Bioorg. Chem.. 2017;73:121-127.
- [Google Scholar]
- Therapeutic potential of alpha-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin. Pharmacother.. 2015;16(13):1959-1981.
- [Google Scholar]
- Novel indolyl linked para-substituted benzylidene-based phenyl containing thiazolidienediones and their analogs as alpha-glucosidase inhibitors: synthesis, in vitro, and molecular docking studies. Med. Chem. Res.. 2018;27(3):903-914.
- [Google Scholar]
- Structural modifications of 2,3-indolobetulinic acid: design and synthesis of highly potent alpha-glucosidase inhibitors. Bioorg. Chem.. 2019;88:102957
- [Google Scholar]
- A novel alpha-glucosidase inhibitor from pine bark. Carbohydr. Res.. 2004;339(3):715-717.
- [Google Scholar]
- In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol.. 2008;99(8):2981-2988.
- [Google Scholar]
- Synthesis and alpha-glucosidase inhibitory mechanisms of bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a potential marine bromophenol alpha-glucosidase inhibitor. Mar. Drugs. 2011;9(9):1554-1565.
- [Google Scholar]
- Synthesis and biological evaluation of indole-based, anti-cancer agents inspired by the vascular disrupting agent 2-(3′-hydroxy-4′-methoxyphenyl)-3-(3″,4″,5″-trimethoxybenzoyl)-6-methoxyindole (OXi8006) Bioorg. Med. Chem.. 2013;21(21):6831-6843.
- [Google Scholar]
- Matsuo, T., Odaka, H., Ikeda, H., 1992. Effect of an intestinal disaccharidase inhibitor (AO-128) on obesity and diabetes. Am J Clin Nutr 55(1 Suppl), 314S-317S.
- New indole based hybrid oxadiazole scaffolds with N-substituted acetamides: as potent anti-diabetic agents. Bioorg. Chem.. 2018;81:253-263.
- [Google Scholar]
- Synthesis of alpha amylase inhibitors based on privileged indole scaffold. Bioorg. Chem.. 2017;72:248-255.
- [Google Scholar]
- Design, synthesis, and pharmacological evaluation of N-bicyclo-5-chloro-1H-indole-2-carboxamide derivatives as potent glycogen phosphorylase inhibitors. Bioorg. Med. Chem.. 2008;16(23):10001-10012.
- [Google Scholar]
- Synthesis, crystal structure, antioxidant, and alpha-glucosidase inhibitory activities of methoxy-substituted benzohydrazide derivatives. Crystallogr. Rep.. 2018;63(3):405-411.
- [Google Scholar]
- Design, synthesis, in vitro, and in silico studies of novel diarylimidazole-1,2,3-triazole hybrids as potent alpha-glucosidase inhibitors. Bioorg. Med. Chem.. 2019;27(23):115148
- [Google Scholar]
- Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs. 2000;59(3):521-549.
- [Google Scholar]
- Indoles — a promising scaffold for drug development. Eur. J. Pharm. Sci.. 2016;91:1-10.
- [Google Scholar]
- Bioactivity-guided isolation and purification of alpha-glucosidase inhibitor, 6-O-D-glycosides, from Tinta Cao grape pomace. J. Funct. Foods. 2016;23:573-579.
- [Google Scholar]
- Synthesis, alpha-amylase inhibitory potential and molecular docking study of indole derivatives. Bioorg. Chem.. 2018;80:36-42.
- [Google Scholar]
- alpha-Glucosidase and alpha-amylase inhibitors from seed oil: a review of liposoluble substance to treat diabetes. Crit. Rev. Food Sci. Nutr.. 2017;57(16):3438-3448.
- [Google Scholar]
- Marine algae as a prospective source for antidiabetic compounds – a brief review. CDR. 2018;14(3):237-245.
- [Google Scholar]
- Synthesis and biological evaluation of novel ursolic acid analogues as potential alpha-glucosidase inhibitors. Sci. Rep.. 2017;7:45578.
- [Google Scholar]
- A new class of anti-MRSA and anti-VRE agents: preparation and antibacterial activities of indole-containing compounds. Bioorg. Med. Chem. Lett.. 2007;17(6):1626-1628.
- [Google Scholar]
- Syntheses, in vitro alpha-amylase and alpha-glucosidase dual inhibitory activities of 4-amino-1,2,4-triazole derivatives their molecular docking and kinetic studies. Bioorgan. Med. Chem.. 2020;28(11)
- [Google Scholar]
- Synthesis and hypoglycemic activity of 9- O -(lipophilic group substituted) berberine derivatives. Bioorg. Med. Chem. Lett.. 2016;26(19):4799-4803.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2020.09.026.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







