Translate this page into:
Synthesis, antiglycation and antioxidant potentials of benzimidazole derivatives
⁎Corresponding author. mtaha@iau.edu.sa (Muhammad Taha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Benzimidazole derivatives 1–20 were synthesized and evaluated for antiglycation and antioxidant potentials. Among the series some analogs showed antiglycating potential ranging in between 182.30 ± 1.20 and 473.51 ± 2.17 when compared with standard rutin (IC50 value 295.09 ± 1.04 µM) and for antioxidant potential ranging between 22.42 ± 0.26 and 82.30 ± 1.33 when compare with standard Propyl gallate (IC50 value 29.20 ± 1.25). Compound 2, 6, 10 and 19 showed potent antioxidant and antiglycation inhibitory potentials. Compounds 7, 11, 13, 15 and 20 showed moderate antiglycating potential with IC50 values 473.51 ± 2.17, 325.20 ± 1.70, 440.0 ± 3.60, 370.60 ± 2.80 and 415.20 ± 3.20 μM, and these compounds also showed excellent antioxidant potential with IC50 values 73.51 ± 1.17, 45.63 ± 0.92, 82.30 ± 1.33, 75.41 ± 1.51, 40.60 ± 0.80 and 64.92 ± 1.41 μM respectively. The remaining compounds 1, 3, 4, 5, 8, 9, 12, 14, 16, 17and 18 were found inactive.
Keywords
Synthesis
Benzimidazole
Antiglycating
Antioxidant
SAR
1 Introduction
Discovery of antiglycating agents is a vital tactic to cure diabetic problems, since at present, number of active antiglycating agents is neglected and there is an urgent demand of new compounds to be identified (Ahmed, 2005). As the hostile occurrence of type-2 diabetes is rising, its damaging effects are frequently abetting the production of advanced glycation end products (Brownlee, 1994). These advanced glycation end products (AGEPs) are significant pathogenic intermediaries of approximately all diabetic problems (Peppa et al., 2008). Glycation is a chaotic process which can take place endogenously (Kellow and Savige, 2013) and modifies biomolecules (Misciagna and Michele, 2007). The process is a non-enzymatic reaction of proteins with sugars (Ho et al., 2010). These modifications lead to impaired protein functions (Goodarzi et al., 2010) and perhaps contributes to microvascular diseases that slows down wound healings of diabetic patients, associated complications and aging-related sicknesses for instance cataracts, retinopathy, renal dysfunction and arteriosclerosis (Rodriguez and Jarvis, 2012; Anguizola et al., 2013; Akash, et al., 2013). Chemical entities that inhibit or slows down the process of glycation play a pivotal role in the treatment of diabetic complications. Exploration for new antiglycating agents is, therefore, still of great scientific interest (Ahmed, 2005). In recent years, certain food stuffs have been identified and attracted attention as having antiglycating and antioxidant properties (Deetae et al., 2012).
The most of diseases are linked with amplified oxidative stress due to either the changes in generation of free radicals or the changes antioxidant balanced activity (Tessier et al., 2009). The major methods of antioxidant resistance comprise enzymes CATs, SODs, and GH-PXs.
The a number of vitamins and micronutrients are energetic in reducing free-radical species or necessary as cofactors for inhibitions enzymes (Pinzani et al., 2010). Equally defensive and chain contravention antioxidants have a control over the oxidative stress that goes together with disease and aging (Jacomelli et al., 2010). Lately, The concentration in natural product antioxidants such as phenolic compounds and vitamin E (α-tocopherol). Mainly phenolic compounds are usually separated into flavonoids, stilbenes, tannins, phenolic acids and lignans (Dai and Mumper, 2010).
Compounds having benzimidazoles core are general and significant substructures occurring in nature and other bioactive compounds. They do something as glucagon receptor anagonists (Chang et al., 2001), β-Raf kinase (Takle et al., 2006), α-glucosidase (Taha et al. (2016a)), β-glucuronidase (Taha et al., 2015), antiglycation (Taha et al. (2016b)), CB1 cannabinoid receptor antagonists (Eyers et al., 1998), carbonic anhydrase (Khan et al., 2013a; Khan et al. (2013b)). Several imidazoles work as antibacterial (Antolini et al., 1999), antibiotics (Brogden et al., 1978) fungicides (Santo et al., 2005), anti-inflammatory agents (Brimblecombe et al., 1975), antidiabetic and antihypertensive (Pathan et al., 2006; Bellina et al., 2007).
2 Results and discussion
2.1 Chemical synthesis
Benzimidazole derivatives 1–20 were synthesized by treating of 3,5-dichlorobenzene-1,2-diamine with of arylaldehyde. Finally the precipitates were separated by filter and the crude product was recrystalized in ethyl acetate to afford pure crystal and characterized by different spectroscopic methods (See Scheme 1).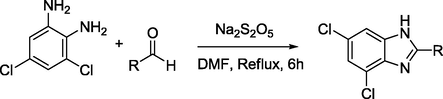
Synthesis of Benzimidazole derivatives (1–20).
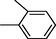
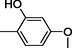
Benzimidazole derivatives 1–20 (Table 1) were subjected to various spectroscopic analysis and their data partially matched with the similar type of derivatives previously published (Alkahtani et al., 2012; Tunçbilek et al., 2009).
No.
Structure
No.
Structure
1
11
2
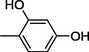
12
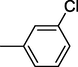
3
13
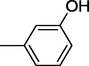
4
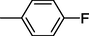
14
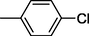
5
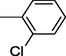
15
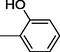
6
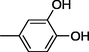
16
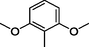
7
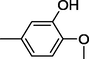
17
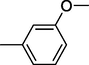
8
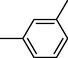
18
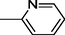
9
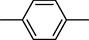
19
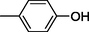
10
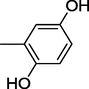
20
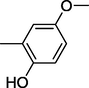
2.2 Biological activity
2.2.1 Antiglycation activity
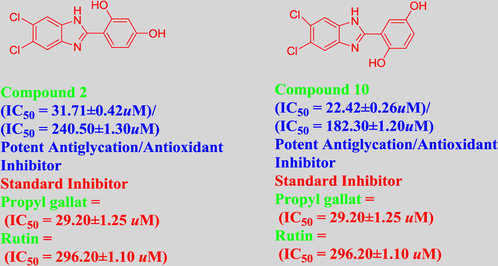
All synthesized benzimidazole derivatives (1–20) were evaluated for their antiglycation potential. Among the series, compounds 2 (IC50 = 240.50 ± 1.30 µM), 10 (IC50 = 182.30 ± 1.20 µM), and 19 (IC50 = 288.60 ± 1.30 µM) showed most potent antiglycation activity if compared with the standard rutin (IC50 = 295.09 ± 1.04 µM) (Table 2). Structure activity relationships were mainly based on substitution pattern on aldehyde phenyl ring. Compound 10, a 2,5-dihydroxy analog was found to be the most active among the series. If we compare compound 10 with compound 2, a 2,4-dihydroxy analog, and compound 6, a 3,4-dihydroxy, compound 10 is superior. The difference in their potential is mainly due to the position difference of substituents. The greater potential shown by these compounds might be due to the acetal formation with fructose carbonyl group. Similarly compound 19, a para hydroxyl analog was found to the third most active compound among the series. The decline in the potential of this compound might be due to less number of hydroxyl group on phenyl ring if we compare it with compound 10 and 2. By comparing compound 19 with other monohydroxy analogs like 13, a meta hydroxyl analog and 15, a ortho hydroxyl analog, compound 19 is superior. The difference in their potential is mainly due the position difference of hydroxyl group on phenyl ring. By bringing about other substituents like chloro, flouro or methyl group on phenyl ring makes it completely inactive. So, it was concluded that only those analogs showed antiglycation potential among these synthesized scaffolds which have hydroxyl group on phenyl ring. The number and position of hydroxyl further effect their potential. SEMa is the standard error of the mean, NAb Not active, Rutin, c standard inhibitor for glycation activity.
No.
Antioxidant (IC50 ± SEM µM)
Antiglycation (IC50 + SEM µM)
No.
Antioxidant (IC50 ± SEM µM)
Antiglycation (IC50 ± SEM µM)
1
N.A.
N.A.
11
45.63 ± 0.92
325.20 ± 1.70
2
31.71 ± 0.42
240.50 ± 1.30
12
N.A.
N.A.
3
N.A.
N.A.
13
82.30 ± 1.33
440.0 ± 3.60
4
N.A.
N.A.
14
N.A.
N.A.
5
N.A.
N.A.
15
75.41 ± 1.51
370.60 ± 2.80
6
29.14 ± 0.47
310.20 ± 1.60
16
N.A.
N.A.
7
73.51 ± 1.17
473.51 ± 2.17
17
N.A.
N.A.
8
N.A.
N.A.
18
N.A.
N.A.
9
N.A.
N.A.
19
40.60 ± 0.80
288.60 ± 1.30
10
22.42 ± 0.26
182.30 ± 1.20
20
64.92 ± 1.41
415.20 ± 3.20
(Propyl gallate) 29.20 ± 1.25
(Rutin) 296.20 ± 1.10
(Propyl gallate) 29.20 ± 1.25
(Rutin) 296.20 ± 1.10
2.2.2 Antioxidant activity
Benzimidazole derivatives 1–20 were also evaluated for their antioxidant potential. Among the series, compounds 6 (IC50 = 29.14 ± 0.47 µM) and 10 (IC50 = 22.42 ± 0.26 µM) showed potent antioxidant activity as compared to standard propyl gallate (IC50 = 29.20 ± 1.25 µM). The greater potential of these compounds i.e. 6, a 3,4-dihydroxyanalogand 10, a 2,5-dihydroxyanalogmight be due to the stabilization of phenoxy radical formed during this assay. The other dihydroxy analog like Compound 2 (IC50 = 31.71 ± 0.42 µM) also showed good potential. The small difference in the activities of these analogs might be due to the position difference of substituents. Other analogs like 7 (IC50 = 73.51 ± 1.17 µM), 11 (IC50 = 45.63 ± 0.92 µM), 13 (IC50 = 82.30 ± 1.33 µM), 15 (IC50 = 75.41 ± 1.51 µM), 19 (IC50 = 40.60 ± 0.80 µM) and 20 (IC50 = 64.92 ± 1.41 µM) having hydroxyl group also displayed better antioxidant activity. The difference in the activities of these compounds were mainly affected by the number and position of substituents. The remaining compounds were found inactive. The antioxidant potential of all compounds evaluated as reported Anouar et al. (2013), Khan et al. (2011, 2012), Taha et al. (2014).
3 Conclusion
In this study, benzimidazole derivatives 2 and 10 have been identified as potent inhibitors of both glycation of proteins and oxidative damages. It was found that most of the active compounds possess hydroxyl substitutions.
It was also observed that substituents such as methyl, methoxy and halides do not play any significant role in inhibiting glycation and oxidative potentials. But the halides, methoxy and methyl moiety has not role to inhibit the glycation and oxidation process. These results suggest benzimidazole class of compounds is an effective class of lead molecules that may act as dual inhibitors of both glycation of proteins and the oxidative damages caused by free radicals.
These finding suggest that benzimidazole is an effective class of compounds or a lead molecule that may act as dual inhibitors of both glycation of proteins and the oxidative damages caused by free radicals
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Advanced glycation end products-role in pathology of diabetic complications. Diabetes. Res. Cli. Prac.. 2005;67:3-21.
- [Google Scholar]
- Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J. Cell Biochem.. 2013;114(3):525-531.
- [Google Scholar]
- Synthesis and biological evaluation of benzo[d]imidazole derivatives as potential anti-cancer agents. Bioorg. Med. Chem. Lett.. 2012;22(3):1317-1321.
- [Google Scholar]
- Antioxidant properties of phenolic Schiff bases: structure-activity relationship and mechanism of action. J. Comput. Aided. Mol. Des.. 2013;27:951-964.
- [Google Scholar]
- Analogues of 4,5-bis (3,5-dichlorophenyl)-2-trifluoromethyl-1H-imidazole as potential antibacterial agents. Bioorg. Med. Chem. Lett.. 1999;9:1023-1028.
- [Google Scholar]
- Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles. Tetrahedron. 2007;63:4571-4624.
- [Google Scholar]
- Metronidazole in anaerobic infections: a review of its activity, pharmacokinetics and therapeutic use. Drugs. 1978;16:387-417.
- [Google Scholar]
- Substituted imidazoles as glucagon receptor antagonists. Bioorg. Med. Chem. Lett.. 2001;11:2549-2553.
- [Google Scholar]
- Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15 7313e7352
- [Google Scholar]
- Antioxidant and anti-glycation properties of Thai herbal teas in comparison with conventional teas. Food Chem.. 2012;133:953-959.
- [Google Scholar]
- Conversion of SB 203580-insensitive MAP kinase family members to drug-sensitive forms by a single amino-acid substitution. Chem. Biol.. 1998;5:321-328.
- [Google Scholar]
- Study of nonenzymaticglycation of transferrin and its effect on iron-binding antioxidant capacity. Iran. J. Basic Med. Sci.. 2010;13:194-199.
- [Google Scholar]
- Comparison of anti-glycation capacities of several herbal infusions with that of green tea. Food Chem.. 2010;122:768-774.
- [Google Scholar]
- Dietary extra-virgin olive oil rich in phenolic antioxidants and the aging process: Long-term effects in the rat. J. Nutri. Biochem.. 2010;21:290-296.
- [Google Scholar]
- Dietary advanced glycationendproduct restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic review. Eur. J. Clin. Nutr.. 2013;67:239-248.
- [Google Scholar]
- Synthesis of benzophenonehydrazone schiff bases and their in vitro antiglycating activities. Med. Chem.. 2013;9:588-595.
- [Google Scholar]
- Acylhydrazide schiff bases: synthesis and antiglycation activity. J. Pak. Chem Soc.. 2013;35:929-937.
- [Google Scholar]
- Synthesis of 2 4 6-Trichlorophenyl hydrazones and their inhibatory potenial against glycation. Med. Chem.. 2011;7:572-580.
- [Google Scholar]
- 2,4,6-Trichlorophenylhydrazine schiff bases as DPPH radical and super oxide anion scavengers. Med. Chem.. 2012;8:452-461.
- [Google Scholar]
- Non-enzymatic glycated proteins in the blood and cardiovascular disease. Curr. Pharm. Des.. 2007;13:3688-3695.
- [Google Scholar]
- Microwave-assisted facile synthesis of 2-substituted 2-imidazolines. Arkivoc. 2006;15:205-210.
- [Google Scholar]
- Glucose advanced glycation end products, and diabetes complications: what is new and what is works. Clin. Diabetes. 2008;21:186-187.
- [Google Scholar]
- Red or white wine assumption and serum antioxidant capacity. Arch. Gerontol. Geriatrics. 2010;51:72-74.
- [Google Scholar]
- Atomic force microscopy: an enabling nanotechnology for diabetes research. In: Le L.A., Hunter R.J., Preedy V.R., eds. Nanotechnology & Nanomedicine in Diabetes. Enfield, New Hampshire: Science Publishers; 2012. p. :34-58.
- [Google Scholar]
- Antifungal agents.Nsubstituted derivatives of 1-[(aryl)(4-aryl-1H-pyrrol-3-yl)methyl]-1H-imidazole: synthesis, anti-Candida activity, and QSAR studies. J. Med. Chem.. 2005;48:5140-5153.
- [Google Scholar]
- Synthesis of 6-chloro-2-Aryl-1H-imidazo [4, 5-b] pyridine derivatives: Antidiabetic, antioxidant, β-glucuronidase inhibiton and their molecular docking studies. Bioorg. Chem.. 2016;65:48-56.
- [Google Scholar]
- Synthesis of benzimidazole derivatives as potent β-glucuronidase inhibitors. Bioorg. Chem.. 2015;61:36-44.
- [Google Scholar]
- Synthesis, α-Glucosidase inhibitory, cytotoxicity and docking studies of 2-Aryl-7-methylbenzimidazoles. Bioorg. Chem.. 2016;65:100-109.
- [Google Scholar]
- Synthesis of novel derivatives of 4-methylbenzimidazole and evaluation of their biological activities. Eur. J. Med. Chem.. 2014;84:731-738.
- [Google Scholar]
- The identification of potent and selective imidazole-based inhibitors of B-Raf kinase. Bioorg. Med. Chem. Lett.. 2006;16:378-381.
- [Google Scholar]
- Effects of vitamin C supplementation on antioxidants and lipid peroxidation markers in elderly subjects with type 2 diabetes. Arch. Gerontol. Geriatrics. 2009;48:67-72.
- [Google Scholar]
- Synthesis and in vitro antimicrobial activity of some novel substituted benzimidazole derivatives having potent activity against MRSA. Euro. J. Med. Chem.. 2009;44(3):1024-1033.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jksus.2018.04.003.
Appendix A
Supplementary data







