Translate this page into:
Synergistic toxicity of NiO nanoparticles and benzo[a]pyrene co-exposure in liver cells: Role of free oxygen radicals induced oxidative stress
⁎Corresponding author. mahamed@ksu.edu.sa (Maqusood Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Current attention has been given on health effects of combined exposure of nanoscale materials and organic pollutants. Nickel (II) oxide nanoparticles (NiO NPs) displays exceptional properties and is being used in various areas such as batteries, diesel–fuel additives, and biomedicals. Benzo[a]pyrene (BaP) is a ubiquitous pollutant. Cigarette smoke, diesel exhaust, and grilled foods are main sources of BaP exposure. Therefore, combined exposure of NiO NPs and BaP to humans is unavoidable. There is a dearth of knowledge on combined effects of NiO NPs and BaP in humans. This study was aimed to investigate co-exposure effects of NiO NPs and BaP in human liver cells (HepG2) and primary rat hepatocytes. We observed that individual and co-exposure of NiO NPs and BaP induced cytotoxicity, lactate dehydrogenase leakage, lipid peroxidation, depletion of mitochondrial membrane potential, and activation of caspases (-3 and -9) in both types of cells. Individual and co-exposure of NiO NPs and BaP further accelerated the generation of free oxygen radicals (reactive oxygen species and hydrogen peroxide) and depletion of antioxidants (glutathione and various antioxidant enzymes). Remarkably, NiO NPs and BaP exerted synergistic toxicity to both HepG2 cells and primary rat hepatocytes. Moreover, combined toxicity of NiO NPs and BaP in both cells was mediated through free oxygen radicals induced oxidative stress. This work warrants further research on risk assessment of co-exposure effects NiO NPs and BaP in an appropriate in vivo model.
Keywords
Combined toxicity
NiO nanoparticles
Benzo[a]pyrene
Liver cells
Human health
Cytotoxicity
ROS
1 Introduction
Humans and other environmental organisms are being exposed to a mixture of environmental contaminants. However, recent studies mainly focus on health effects of single contaminants representing a crucial information gap in understanding the health hazard of environmental exposure (Bellavia et al., 2019). Indeed, some current reports indicated that co-exposure effects of nano-scale materials and pre-existing environmental pollutants could be significantly different from their individual effects (Ahamed et al., 2020a, 2020b).
Nickel (II) oxide nanoparticles (NiO NPs) have attracted great attention for diverse applications due to their excellent chemical stability, magnetic, electrical, optical, and catalytic properties (Adinaveen et al., 2019). Engineered NiO NPs are being used in solar cells, catalysts, lithium-ion batteries, light emitting diodes, electrochemical sensors, and diesel–fuel additives (Diallo et al., 2018). Besides, NiO NPs also present in condensed aerosols produced by traditional metallurgical and arc-welding technologies (Sutunkova et al., 2019). Possible biomedical application (e.g. antimicrobial agent) of NiO NPs was also previously reported (Behera et al., 2019). These applications may increase the chances of NiO NPs exposure and possible risk to human and the environmental health.
A number of studies on different types of cell lines demonstrated that NiO NPs cause cytotoxicity, severe DNA damage, mitochondrial dysfunction, cell cycle arrest, oxidative stress and induction of apoptosis (Chang et al., 2020; Liu et al., 2017). Particularly, our previous study showed that NiO NPs induced cytotoxicity through free oxygen radical generation and stimulate apoptosis in human liver cells (HepG2) by bax/bcl2 activation (Ahamed et al., 2013). Toxicity of NiO NPs was also reported in non-mammalian organism e.g. Daphnia magna and Drosophila melanogaster (De Carli et al., 2018; Gong et al., 2016). Multi-organs toxicity of NiO NPs was also reported (Hussain et al., 2020). These studies suggested that liver is one of the target organs of NiO NPs. Furthermore, oxidative stress, mitochondrial dysfunction, and caspase activation were possible mechanisms of NiO NPs toxicity (Marzban et al., 2020). Currently, it is an important issue to study the effects of NiO NPs in combination with pre-existing pollutants on humans and the environmental health.
Benzo[a]pyrene is among the common environmental contaminants that humans are being exposed. BaP is a member of polycyclic aromatic hydrocarbons (PAHs) that generated in the environment by incomplete combustion of organic matters (Sun et al., 2020). BaP is categorized as a human group 1 carcinogen by the IARC (Einem Lindeman et al., 2011). Cigarette smoke, diesel exhaust particles as well as smoked and grilled food contained high level of BaP (Kazerouni et al., 2001). For non-smoker, diet is the main source of BaP exposure (Wang et al., 2020). Earlier report demonstrated that the total average dietary intake of BaP for humans is 8–9 ng/day (Alomirah et al., 2011). This indicates that humans are getting exposure to a low dose of BaP over a lifetime. BaP enters human body mainly via inhalation and ingestion, and transported to other body organs through blood and lymph (Ba et al., 2015). After internalization into cells, BaP undergoes metabolic activation and generates free oxygen radicals that causes toxicity in almost all vital organs including lung, liver, and kidneys (Deng et al., 2018). Previous research on BaP was mainly focussed on single exposure. Studies on co-exposure effects of BaP with other environmentally relevant materials (e.g. NPs) on human health are scarce.
Due to wide-spread application of NiO NPs and ubiquitous BaP, co-exposure of both materials to humans is unavoidable. However, combined effects of NiO NPs and BaP and their toxicity mechanisms have not been addressed before. We aimed to investigate the combined effects of NiO NPs and BaP in human liver cels (HepG2) and primary rat hepatocytes. Possible mechanism of combined toxicity of NiO NPs and BaP was also explored through free oxygen radicals induced oxidative stress.
2 Materials and methods
2.1 NiO nanoparticles and benzo[a]pyrene
Nickel (II) oxide (NiO) NPs and benzo[a]pyrene (BaP) were obtained from Sigma-Aldrich (St. Louis, MO, USA). X-ray diffraction (XRD) (PanAnalytic X'Pert Pro, Malvern Instruments, UK) with Cu-Kα radiation (λ = 0.15405 nm, at 45 kV and 40 mA) was employed to assess crystallinity and phase purity of NiO NPs. Morphology and size of NiO NPs was assessed by field emission scanning electron microscope (FESEM, JSM-7600F, JEOL, Inc., Tokyo, Japan) and field emission transmission electron microscope (FETEM, JEM-2100F, JEOL).
2.2 Cell culture and exposure protocol
Human liver (HepG2) cell line was obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA). Primary rat hepatocytes were isolated from collagenase perfusion technique as described by Moldeus and co-workers (Moldéus et al., 1978). Cells were cultured in Dulbecco’s modified eagle's medium (DMEM) (Invitrogen, Carisbad, CA, USA) with the supplementation of streptomycin (100 µg/ml)-penicillin (100 U/ml) (Invitrogen) and 10% fetal bovine serum (FBS, Invitrogen). Cell were maintained in a humidified incubator at 37 °C with 5% CO2 supply. At ∼80% confluence, cells were harvested with trypsin (Invitrogen) and sub-cultured for toxicity studies.
Stock solution (1 mg/ml) of NiO NPs was prepared in distilled water and BaP was dissolved in dimethyl sulfoxide (DMSO). Stock solutions were further diluted in culture medium as per the requirement of the experiments. Individual cytotoxicity of NiO NPs and BaP were examined by following exposure of different concentrations of concentrations of NiO NPs (0, 1, 5, 10, 25, 50, 100, and 200 µg/ml) and BaP (0, 1, 5, 10, 25, 50, and 100 µM) for 24 h. For combined toxicity studies cells were exposed for 24 h to NiO NPs (25 µg/ml) and/or BaP (10 µM). Basis of selection of dosages of NiO NPs and BaP is described in results section (Fig. 2). In some experiments, N-acetyl cysteine (NAC, 2 mM) was pre-exposed (30 min before) to cells with or without NiO NPs and/or BaP.
2.3 Biochemical studies
Cell viability was determined using modified MTT assay (Ahamed et al., 2011; Mosmann, 1983). Lactate dehydrogenase (LDH) enzyme leakage was assessed as described earlier (Welder, 1992). Intracellular ROS was assayed using a fluorescent probe 2‘-7‘-dichlorodihydrofluorescein diacetate (H2DCFDA, Sigma-Aldrich) (Siddiqui et al., 2013). ROS level was quantitatively measured by a micro-plate reader (Synergy-HT, BioTek Winooski, VT, USA). Intracellular hydrogen peroxide (H2O2) level was estimated employing a kit from Sigma-Aldrich. Glutathione (GSH) (Ellman, 1959) and malondialdehyde (MDA) (Ohkawa et al., 1979) were determined as described earlier. Activity of several antioxidant enzymes; superoxide dismutase (SOD) (Cayman chemical kit, Michigan. USA), catalase (CAT) (Sinha, 1972), and glutathione peroxidase (GPx) (Rotruck et al., 1973) were assayed as reported previously. Mitochondrial membrane potential (MMP) was determined using a fluorescent probe tetramethylrhodamine methyl ester (TMRM) as described previously (Ahamed et al., 2022). MMP level was quantitatively assessed by a microplate reader (Synergy-HT, BioTek). The mRNA expression of caspase-3 and -9 genes were assessed by real-time PCR (ABI PRISM 7900HT Sequence detection system) (Applied Biosystem, Foster city, CA, USA) as explained in previous work (Ahamed et al., 2011). Caspase-3 and -9 enzymes activity was assayed using BioVIsion kits (Milpitas, CA, USA). Protein content was measured using Bradford‘s method (Bradford, 1976).
2.4 Statistical analysis
One-way analysis of variance (ANOVA) and Dunnett’s multiple comparison tests were used for statistical analysis. The p < 0.05 was assigned as statistically significant difference between two groups. Data depicted as mean ± SD of five individual experiments (n = 5).
3 Results
3.1 Characterization of NiO NPs
Fig. 1A represents the XRD spectra of NiO NPs. Presence of strong and sharp diffraction peaks at 2θ values 37.58, 43.56, 63.16, 75.68, and 79.61 corresponding to (1 1 1), (2 0 0), (2 2 0), (3 1 1), and (2 2 2) crystal planes of NiO, respectively (JCPDS Card No. 04-0385). The sharpness of diffraction peaks indicates the high crystallinity of NiO NPs. Impurity peaks were not detected XRD spectra. The average crystallite size of NiO NPs calculated from Scherrer‘s formula was around 29 nm. Fig. 1B and C depict the typical SEM and TEM images of NiO NPs, respectively. These images suggested polygonal morphology and smooth surfaces of NiO NPs. Average particle size measured from random selection of >100 particles from TEM image was around 27 nm, which was according to XRD data. High resolution TEM image (Fig. 1D) demonstrates clear lattice fringes with a spacing of 0.241 nm, corresponds to (1 1 1) plane of NiO phase.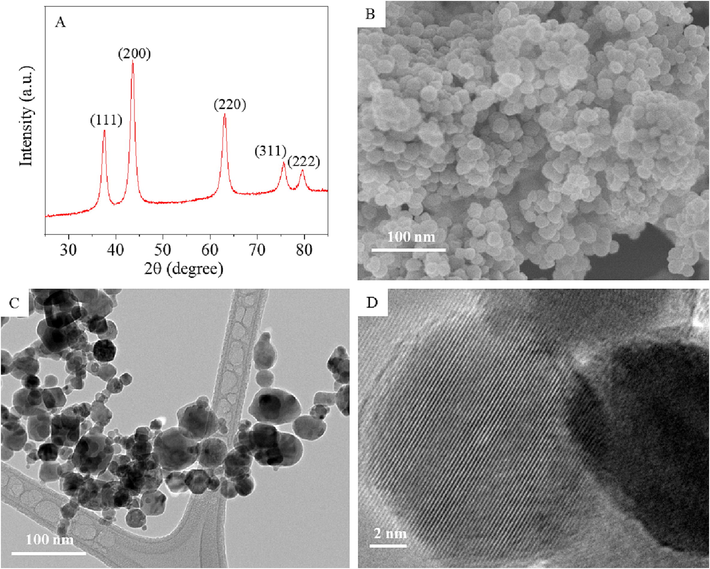
Characterization of NiO NPs. XRD spectra (A), SEM micrograph (B), low-resolution TEM micrograph (C), and high-resolution TEM micrograph (D).
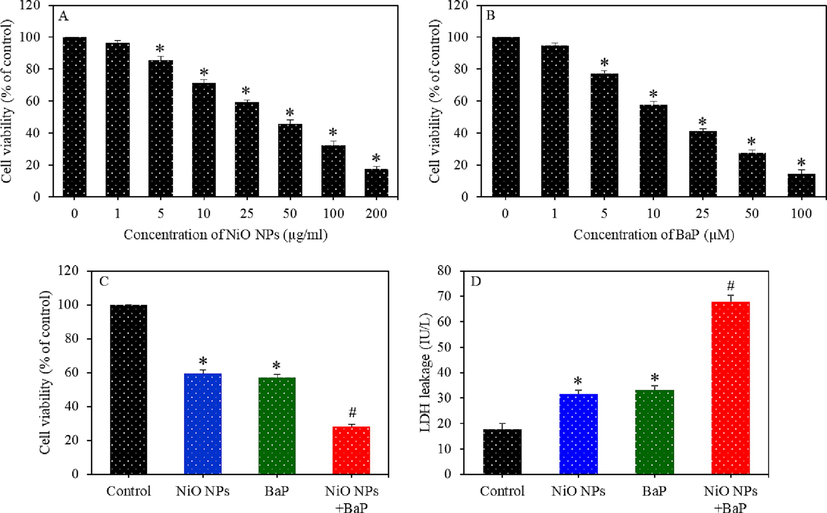
Dose-dependent cytotoxicity of HepG2 cells exposed to different concentrations of NiO NPs (0–200 µg/ml) (A) and BaP (0–100 µg/ml) (B) for 24 h. Cytotoxicity of HepG2 cells exposed to NiO NPs (40 µg/ml) and/or BaP (10 µM) for 24 h (C). LDH leakage in HepG2 cells exposed to NiO NPs (40 µg/ml) and/or BaP (10 µM) for 24 h (D). *p < 0.05 compared to the control group. #p < 0.05 compared to the NiO NPs group or BaP group.
3.2 Dose-dependent cytotoxicity of NiO NPs and BaP in HepG2 cells
First of all, a screening test was performed to obtain suitable concentrations of NiO NPs and BaP for co-exposure experiments. In brief, HepG2 cells were individually treated with different concentrations of concentrations of NiO NPs (0, 1, 5, 10, 25, 50, 100, and 200 µg/ml) and BaP (0, 1, 5, 10, 25, 50, and 100 µM) for 24 h. After the completion of treatment time, MTT cell viability assay was conducted to examine the cytotoxicity of these two materials. Results demonstrated that both NiO NPs and BaP induced dose-dependent cytotoxicity in HepG2 cells (Fig. 2A and B). On the basis of these screening data, one moderate cytotoxic concentration of NiO NPs (25 µg/ml, 59% cell viability) and one moderate concentration of BaP (10 µM, 57% cell viability) were chosen to investigate their individual and combined toxicity in liver cells.
3.3 Synergistic cytotoxicity of NiO NPs and BaP in HepG2 cells
Cell viability of HepG2 cells treated for 24 h to NiO NPs (25 µg/ml) and/ or BaP (10 µM) is presented in Fig. 2C. Results showed that cell viability in NiO NPs, BaP, and co-exposure (NiO NPs + BaP) groups were 59%, 57%, and 28%, respectively. These results suggested that NiO NPs and BaP co-exposure synergistically enhanced the cytotoxicity in HepG2 cells. LDH enzyme leakage assay demonstrated that individual exposure of NiO NPs and BaP significantly increased the LDH leakage in comparison to the control group (p < 0.05). Furthermore, in co-exposure group (NiO NPs + BaP), LDH leakage was significantly higher than those of individual group of NiO NPs or BaP (p < 0.05) (Fig. 2D). This data indicated the synergistic effects of NiO NPs and BaP on cytotoxicity parameters of HepG2 cells.
3.4 Synergistic oxidative stress response of NiO NPs and BaP in HepG2 cells
Several biomarkers of oxidative stress were examined in HepG2 cells following exposure to NiO NPs and/or BaP for 24 h. As we can in Fig. 3A, individual exposure of NiO NPs and BaP significantly induced ROS generation (p < 0.05). Besides, co-exposure of NiO NPs and BaP synergistically induced ROS generation (p < 0.05). In comparison to control, significantly higher levels of H2O2 and MDA (one of the final products of membrane lipid peroxidation) were also observed upon individual exposure of NiO NPs or BaP (p < 0.05) (Fig. 3B and C). Again, H2O2 and MDA levels were significantly higher in co-exposure group (NiO NPs + BaP) than those of individual group of NiO NPs or BaP (p < 0.05).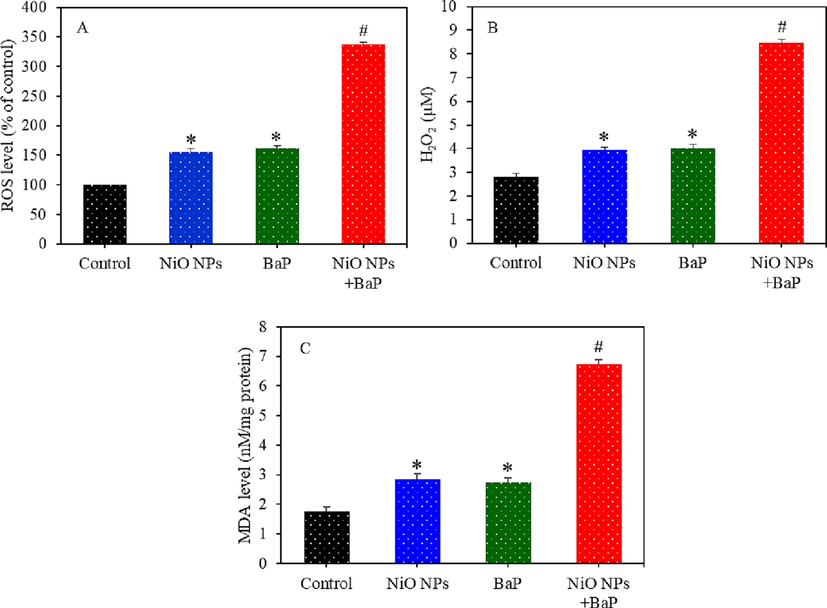
Pro-oxidants generation in HepG2 cells exposed to NiO NPs (40 µg/ml) and/or BaP (10 µM) for 24 h. ROS level (A), H2O2 level (B), and MDA level (C). *p < 0.05 compared to the control group. #p < 0.05 compared to the NiO NPs group or BaP group.
Individual and combined effects of NiO NPs and BaP on antioxidant levels of HepG2 cells were further examined. Fig. 4A-C demonstrated that level of antioxidant molecule GSH and activity of several antioxidant enzymes (e.g. GPx, SOD, and CAT) were lower in NiO NPs or BaP treated cells as compared to control group (p < 0.05). Furthermore, co-exposure of NiO NPs and BaP synergistically decreased these antioxidant levels than those of individual exposure of NiO NPs or BaP (p < 0.05). This section of results suggested that NiO NPs and BaP synergistically induced oxidative stress in HepG2 cells.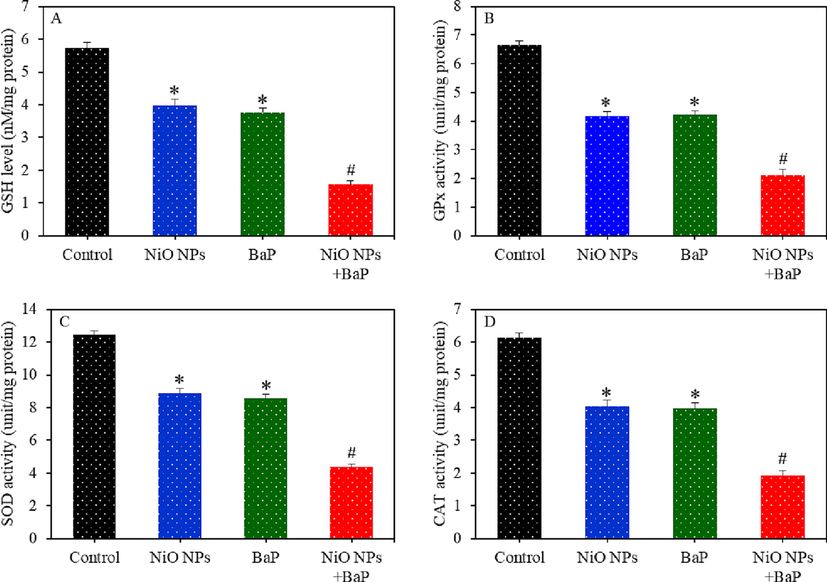
Antioxidants depletion in HepG2 cells exposed to NiO NPs (40 µg/ml) and/or BaP (10 µM) for 24 h. GSH level (A), GPx enzyme activity (B), SOD enzyme activity (C), and CAT enzyme activity (D). *p < 0.05 compared to the control group. #p < 0.05 compared to the NiO NPs group or BaP group.
3.5 Synergistic apoptotic response of NiO NPs and BaP in HepG2 cells
Apoptosis study following exposure to NiO NPs and/or BaP were assessed in HepG2 cells by examining the regulation caspase-3 and -9 genes along with MMP level. Real-time PCR data showed that NiO NPs and BaP individually upregulated the mRNA level of caspase-3 and -9 genes (Fig. 5A). Besides, co-exposure of BONPs and BaP exerted synergistic effects on upregulation of these two apoptotic genes. Enzymatic activity of caspase-3 and -9 enzymes (protein level) was further assessed to support mRNA results. Fig. 5B demonstrated higher enzymatic activity of caspase-3 and -9 upon individual or co-exposure of NiO NPs and BaP. Moreover, co-exposure of NiO NPs and BaP had synergistic effects on these enzymes. Fig. 5C showed that individual exposure of NiO NPs and BaP significantly depleted MMP level (p < 0.05) and co-exposure of BONPs and BaP had synergistic effects on MMP depletion (p < 0.05).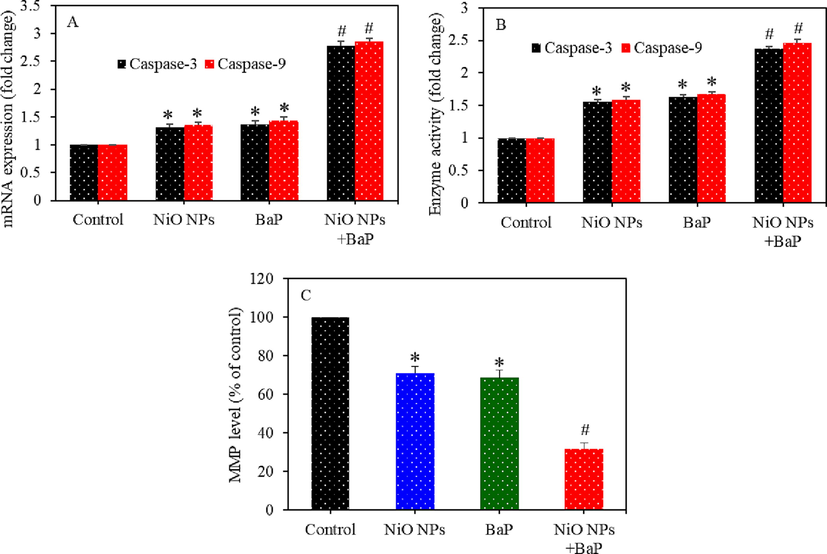
Apoptosis induction in HepG2 cells exposed to NiO NPs (40 µg/ml) and/or BaP (10 µM) for 24 h. mRNA level of caspase-3 and -9 genes (A), activity of caspase-3 and -9 enzymes (B), and MMP level (C). *p < 0.05 compared to the control group. #p < 0.05 compared to the NiO NPs group or BaP group.
3.6 Synergistic toxicity of NiO NPs and BaP in primary rat hepatocytes
Individual and combined effects of NiO NPs and BaP was further investigated in primary rat hepatocytes. Cells were exposed for 24 h to NiO NPs (25 µg/ml) and/ or BaP (10 µg/ml) and cytotoxicity, oxidative stress, and apoptosis biomarkers were measured. Fig. 6A showed that NiO NPs and BaP co-exposure synergistically reduced cell viability in primary rat hepatocytes. Cell viability reduction following exposure to NiO NPs, BaP, and NiO NPs + BaP was 56%, 54%, and 26%, respectively. LDH leakage in NiO NPs and BaP groups was significantly higher as compared to the control group (p < 0.05). Interestingly, LDH leakage in co-exposure group (NiO NPs + BaP) was significantly higher as compared to individual group of NiO NPs or BaP (p < 0.05) (Fig. 6B). Fig. 6C showed that intracellular ROS generation was significantly higher in NiO NPs or BaP group in comparison to control group (p < 0.05). Intracellular GSH level was significantly lower in primary rat hepatocytes exposed to NiO NPs or BaP than those of untreated control group (p < 0.05) (Fig. 6D). Remarkably, effects of combined exposure of NiO NPs and BaP on ROS generation and GSH depletion were synergistic.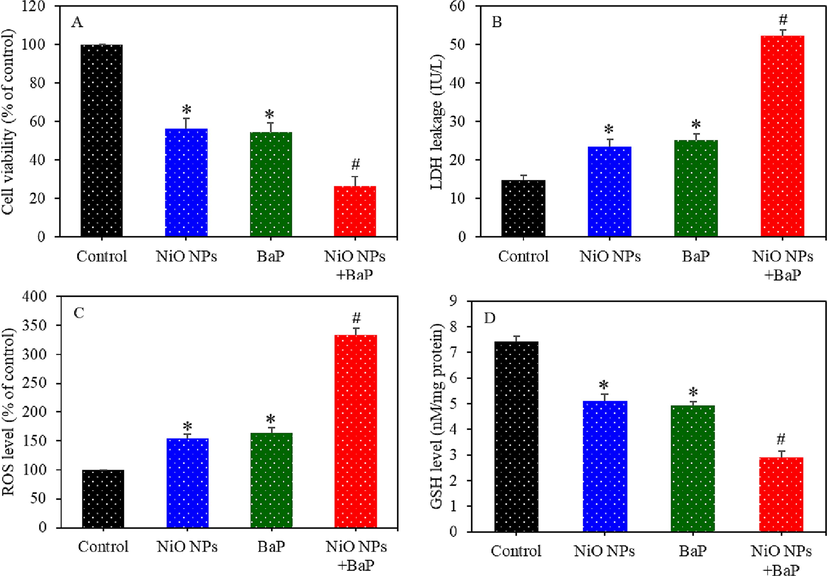
Cytotoxicity and oxidative stress response of primary rat hepatocytes exposed to NiO NPs (40 µg/ml) and/or BaP (10 µM) for 24 h. Cell viability (A), LDH leakage (B), ROS level (C), and GSH level (D). *p < 0.05 compared to the control group. #p < 0.05 compared to the NiO NPs group or BaP group.
Expression (mRNA) of caspase-3 and -9 genes were upregulated in primary rat hepatocytes upon individual or co-exposure of NiO NPs and BaP as compared to control group (p < 0.05) (Fig. 7A). This data was further supported by higher activity of caspase-3 and -9 enzymes upon individual or co-exposure of NiO NPs and BaP (p < 0.05) (Fig. 7B). Fig. 7C demonstrated that individual or combined exposure of NiO NPs and BaP significantly depleted the MMP level of primary rat hepatocytes as compared to control (p < 0.05). Interestingly, effects of combined exposure of NiO NPs and BaP on apoptotic markers were synergistic.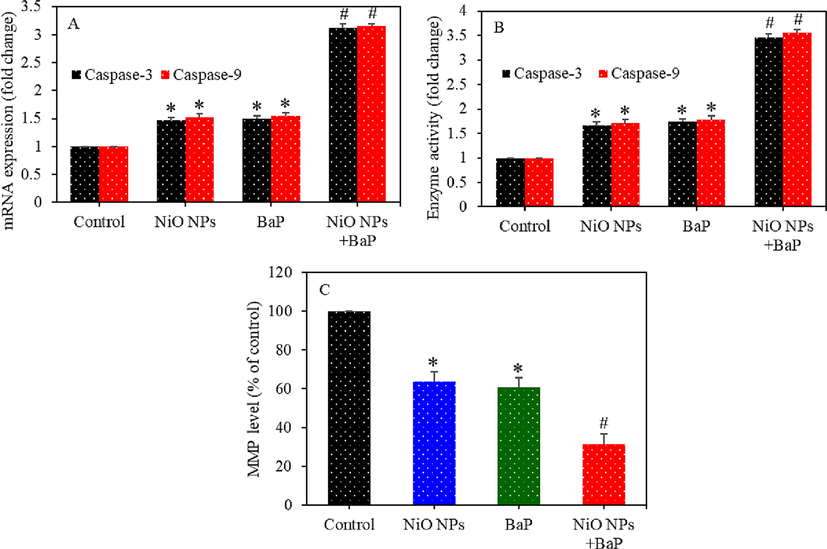
Apoptosis induction in primary rat hepatocytes exposed to NiO NPs (40 µg/ml) and/or BaP (10 µM) for 24 h. mRNA level of caspase-3 and -9 genes (A), activity of caspase-3 and -9 enzymes (B), and MMP level (C). *p < 0.05 compared to the control group. #p < 0.05 compared to the NiO NPs group or BaP group.
3.7 Oxidative stress mediated cytotoxicity of NiO NPs and BaP co-exposure in HepG2 cells and primary rat hepatocytes
Role of ROS in individual or combined exposure induced toxicity of NiO NPs and BaP was investigated in HepG2 cells and primary rat hepatocytes. Both types of cells were treated for 24 h to NiO NPs (25 µg/ml) and/ or BaP (10 µg/ml) with or without NAC (2 mM) pre-treatment (30 min). Results showed that NAC pre-treatment remarkably reverted the cytotoxicity exerted by NiO NPs, BaP, or NiO NPs + BaP in both HepG2 cells (Fig. 8A) and primary rat hepatocytes (Fig. 8B). This data suggested that cytotoxicity exerted by individual or combined exposure of NiO NPs and BaP was mediated through free radicals induced oxidative stress.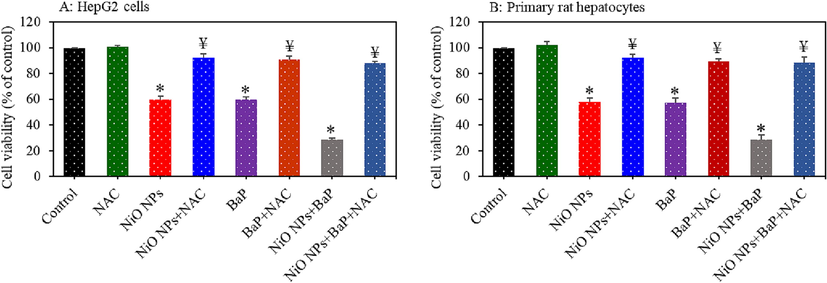
Role of ROS in cytotoxicity of individual or co-exposure to NiO NPs and BaP in HepG2 cells and primary rat hepatocytes. Cells were exposed to NiO NPs (40 µg/ml) and/or BaP (10 µM) for 24 h with or without NAC (2 mM) pre-treatment. Cytotoxicity in HepG2 cells (A) and primary rat hepatocytes (B). *p < 0.05 compared to the control group. ¥p < 0.05 compared to the NiO NPs group or BaP group.
4 Discussion
The liver is the main organ that metabolizes exogenous materials including carbohydrates, proteins, drugs, and toxins. Hence, environmental exposure of pollutants/toxins might lead to liver injury, dysfunction, and even organ failure (Siddiqui et al., 2013; Ahamed et al., 2013). Co-exposure of NiO NPs and BaP to humans is unavoidable because of their consistent release in the environment. This is the first study that examined the individual and combined toxicity of NiO NPs and BaP in HepG2 cells and primary rat hepatocytes. Results showed that individual and combined exposure of NiO NPs and BaP induce cytotoxicity, LDH leakage, caspases (-3 and -9) activation, MMP depletion, pro-oxidants generation, and antioxidants depletion in both HepG2 cells and primary rat hepatocytes. Interesting finding of this work was that NiO NPs and BaP acts synergistically in exerting the toxicity to both types of liver cells.
Our data suggested that NiO NPs potentiate the BaP-induced toxicity in liver cells. Earlier studies also reported that nano-scale materials could exacerbate the toxicity of BaP. Asweto et al. found that joint exposure of silica NPs and BaP cause more severe toxicity on immunity and cardiovascular development of zebrafish embryo as compared to single exposure of silica NPs and BaP (Asweto et al., 2018). Fullerene C60 increased the toxicity BaP in hepatocytes of zebrafish (Ferreira et al., 2014). Some other studies observed that metal oxide NPs worsen the toxicity of organic chemicals upon co-exposure. For instance, TiO2 NPs enhanced the teratogenicity of tributyltin (TBT) in abalone embryos (Zhu et al., 2011).
Adsorption of organic contaminants on the higher surface area of NPs might play crucial role in combined toxicity of NPs and organic pollutants (Liu et al., 2018). In this condition NPs can serve as a carrier for transportation of organic polluatants into the cells. The NPs-organic complexes might subsequently be released once internalized in the cells. Hence, bioaccumulation and toxicity of organic chemicals might enhance by the NPs through Trojan horse mechanism (Deng et al., 2017). Fang and co-workers found that TiO2 NPs serve as a carrier for bisphenol A (BPA) in Zebrafish and exert reproductive toxicity (Fang et al., 2016). Another study also reported that TiO2 NPs increased the bioaccumulation of BDE-209 in Zebrafish producing greater developmental neurotoxicity (Wang et al., 2014).
It is crucial to explore the underlying mechanisms of combined toxicity of environmentally relevant NPs and ubiquitous contaminants. In this study, we further explore the possible mechanisms of combined toxicity of NiO NPs and BaP in both HepG2 cells and primary rat hepatocytes. Results showed that individual and combined exposure of NiO NPs and BaP induced intracellular ROS and H2O2 levels in both types of liver cells. MDA is one of the final products of membrane lipid peroxidation and higher production of ROS leads to lipid peroxidation (Ahamed et al., 2013). We observed that MDA level was higher upon individual or combined exposure of NiO NPs and BaP in liver cells. Moreover, antioxidant molecule GSH depletion and lower activity of several antioxidant enzymes (e.g. GPx, SOD, and CAT) in liver cells following individual or co-exposure of NiO NPs and BaP. Free oxygen radicals serve as signalling molecules in apoptotic pathway and GSH depletion is also linked with apoptosis (Ahamed et al., 2020a). Mitochondria contains key regulator of caspases, a family of proteases that play critical role in apoptosis (Balakireva and Zamyatnin, 2019). Caspase-3 and -9 are suggested to be crucial in apoptotic response. Besides, MPP loss is an early indicator of apoptosis (Chang et al., 2020). In this study, we found the activation of caspase-3 and -9 genes and depletion of MMP in liver cells upon individual or co-exposure of NiO NPs and BaP. Interesting finding was that both NiO NPs and BaP acts synergistically in inducing oxidative stress and apoptosis.
5 Conclusion
This study demonstrates the formerly unrecognized combined toxicity of NiO NPs and BaP in liver cells. Individual exposure of NiO NPs and BaP exert cytotoxicity, membrane damage, activation of caspase genes (-3 and -9), MMP depletion, pro-oxidants generation, and antioxidants depletion in both HepG2 and primary rat hepatocytes. Besides, NiO NPs or BaP induced toxicity was mediated through ROS-induced oxidative stress. Interestingly, combined exposure of NiO NPs and BaP acts synergistically in causing toxicity to liver cells. These results warranted further study on risk assessment of combined effect NiO NPs and BaP in suitable animal model.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, "Ministry of Education" in Saudi Arabia for funding this research (IFKSUOR3-414-1).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photocatalytic and optical properties of NiO added Nephelium lappaceum L. peel extract: An attempt to convert waste to a valuable product. Heliyon. 2019;5
- [CrossRef] [Google Scholar]
- Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology. 2011;283:101-108.
- [CrossRef] [Google Scholar]
- Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2) Chemosphere. 2013;93
- [CrossRef] [Google Scholar]
- TiO2 nanoparticles potentiated the cytotoxicity, oxidative stress and apoptosis response of cadmium in two different human cells. Environ. Sci. Pollut. Res.. 2020;27:10425-10435.
- [CrossRef] [Google Scholar]
- Influence of silica nanoparticles on cadmium-induced cytotoxicity, oxidative stress, and apoptosis in human liver HepG2 cells. Environ. Toxicol. 2020:22895.
- [CrossRef] [Google Scholar]
- Dietary antioxidant curcumin mitigates CuO nanoparticle-induced cytotoxicity through the oxidative stress pathway in human placental cells. Molecules. 2022;27:7378.
- [CrossRef] [Google Scholar]
- Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control. 2011;22:2028-2035.
- [CrossRef] [Google Scholar]
- Gene profiles to characterize the combined toxicity induced by low level co-exposure of silica nanoparticles and benzo[a]pyrene using whole genome microarrays in zebrafish embryos. Ecotoxicol. Environ. Saf.. 2018;163:47-55.
- [CrossRef] [Google Scholar]
- Effects of benzo[a]pyrene exposure on human hepatocellular carcinoma cell angiogenesis, metastasis, and NF-κB signaling. Environ. Health Perspect.. 2015;123:246-254.
- [CrossRef] [Google Scholar]
- Cutting out the gaps between proteases and programmed cell death. Front. Plant Sci.. 2019;10
- [CrossRef] [Google Scholar]
- Oxidative stress generated at nickel oxide nanoparticle interface results in bacterial membrane damage leading to cell death. RSC Adv.. 2019;9:24888-24894.
- [CrossRef] [Google Scholar]
- Approaches for incorporating environmental mixtures as mediators in mediation analysis. Environ. Int. 2019
- [CrossRef] [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [CrossRef] [Google Scholar]
- Nano nickel oxide promotes epithelial-mesenchymal transition through transforming growth factor β1/smads signaling pathway in <scp>A549</scp> cells. Environ. Toxicol.. 2020;35:1308-1317.
- [CrossRef] [Google Scholar]
- Evaluation of the genotoxic properties of nickel oxide nanoparticles in vitro and in vivo. Mutation Res. - Genetic Toxicol. Environ. Mutagen.. 2018;836:47-53.
- [CrossRef] [Google Scholar]
- Acute benzo[a]pyrene treatment causes different antioxidant response and DNA damage in liver, lung, brain, stomach and kidney. Heliyon. 2018;4:e00898.
- [Google Scholar]
- Nanoparticle interactions with co-existing contaminants: joint toxicity, bioaccumulation and risk. Nanotoxicology 2017
- [CrossRef] [Google Scholar]
- Structural, optical and photocatalytic applications of biosynthesized NiO nanocrystals. Green Chem. Lett. Rev.. 2018;11:166-175.
- [CrossRef] [Google Scholar]
- The resveratrol analogue, 2,3′,4,5′-tetramethoxystilbene, does not inhibit CYP gene expression, enzyme activity and benzo[a]pyrene-DNA adduct formation in MCF-7 cells exposed to benzo[a]pyrene. Mutagenesis. 2011;26:629-635.
- [CrossRef] [Google Scholar]
- Enhanced bioconcentration of bisphenol A in the presence of nano-TiO2 can lead to adverse reproductive outcomes in zebrafish. Environ. Sci. Tech.. 2016;50:1005-1013.
- [CrossRef] [Google Scholar]
- Co-exposure of the organic nanomaterial fullerene C60 with benzo[a]pyrene in Danio rerio (zebrafish) hepatocytes: Evidence of toxicological interactions. Aquat. Toxicol.. 2014;147:76-83.
- [CrossRef] [Google Scholar]
- Acute and chronic toxicity of nickel oxide nanoparticles to Daphnia magna: The influence of algal enrichment. NanoImpact. 2016;3–4:104-109.
- [CrossRef] [Google Scholar]
- Exposure to variable doses of nickel oxide nanoparticles disturbs serum biochemical parameters and oxidative stress biomarkers from vital organs of albino mice in a sex-specific manner. Biomarkers 2020
- [CrossRef] [Google Scholar]
- Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem. Toxicol.. 2001;39:423-436.
- [CrossRef] [Google Scholar]
- Nano NiO induced liver toxicity: Via activating the NF-κB signaling pathway in rats. Toxicol. Res.. 2017;6:242-250.
- [CrossRef] [Google Scholar]
- Mechanisms involved in the impact of engineered nanomaterials on the joint toxicity with environmental pollutants. Ecotoxicol. Environ. Saf.. 2018;162:92-102.
- [CrossRef] [Google Scholar]
- Biochemical, Toxicological, and Histopathological outcome in rat brain following treatment with NiO and NiO nanoparticles. Biol. Trace Elem. Res.. 2020;196:528-536.
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [CrossRef] [Google Scholar]
- Selenium: Biochemical role as a component of glatathione peroxidase. Science. 1973;179:588-590.
- [CrossRef] [Google Scholar]
- Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS One. 2013;8
- [CrossRef] [Google Scholar]
- Long-term exposure to benzo[a]pyrene affects sexual differentiation and embryos toxicity in three generations of marine medaka (oryzias melastigma) Int. J. Environ. Res. Public Health. 2020;17:970.
- [CrossRef] [Google Scholar]
- Toxic effects of low-level long-term inhalation exposures of rats to nickel oxide nanoparticles. Int. J. Mol. Sci.. 2019;20
- [CrossRef] [Google Scholar]
- Bioconcentration and metabolism of BDE-209 in the presence of titanium dioxide nanoparticles and impact on the thyroid endocrine system and neuronal development in zebrafish larvae. Nanotoxicology. 2014;8:196-207.
- [CrossRef] [Google Scholar]
- Arsenic and benzo[a]pyrene co-exposure acts synergistically in inducing cancer stem cell-like property and tumorigenesis by epigenetically down-regulating SOCS3 expression. Environ Int. 2020;137:105560.
- [CrossRef] [Google Scholar]
- A primary culture system of adult rat heart cells for the evaluation of cocaine toxicity. Toxicology. 1992;72:175-187.
- [CrossRef] [Google Scholar]
- TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Environ. Sci. Tech.. 2011;45:3753-3758.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102750.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







