Translate this page into:
Susceptibility pattern of multi-drug resistance Pseudomonas aeruginosa isolates from tertiary care hospital in Riyadh, KSA
⁎Corresponding author at: Department of Botany and Microbiology, College of Science, King Saud University, P. O. Box 2455, Riyadh, Saudi Arabia. imoussa1@ksu.edu.sa (Ihab M. Moussa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Objectives
Pseudomonas aeruginosa is important pathogens commonly cause nosocomial infections. The occurrence of multi-resistant organisms (MROs) of Pseudomonas aeruginosa strains have been increased worldwide and limiting the therapeutic options. The MRO of Pseudomonas aeruginosa phenotype can be mediated by a variety of resistance mechanisms and highly versatile property to mutate. Therefore the our study aimed to evaluate the resistance pattern of Pseudomonas aeruginosa collected from Riyadh tertiary care hospital, Kingdom of Saudi Arabia.
Methods
During the period from 2019 to 2021 clinical samples were collected from microbiology lab at King Khalid University Hospital and analysed for the antibiotic susceptibility pattern.
Results
Suggested that the rates of resistance for the three years were higher for isolates collected from patients older than 50 years if its compared with the strains collected from young age. A total of 1024 Pseudomonas aeruginosa isolates were collected during the last three years, the prevalence rate were 44.6%, 32.6% and 22.7% during the period of 2019, 2020, and 2021 respectively. Meanwhile, the highest percentages of multi drug resistance Pseudomonas aeruginosa strains were recovered from body fluids; about 38 (47.5%) out of 80 Pseudomonas aeruginosa isolates were MRO Pseudomonas aeruginosa. The rate of resistances showed that Imipenem was significantly higher in resistant among the clinical isolates (77.8%), then Meropenem (61%), Aztreonam (42%) and Ceftazidime (36%) than other antibiotics. Most of isolates were sensitive to colistin except (2.7%) were resistance. Moreover, antibiotic resistant bacteria have been observed with increasing frequency over the past three years.
Conclusions
The current study reports that the susceptibility among P. aeruginosa isolates have been decreased in KSA, perhaps due to the massive use of antibiotics, the lack of adherence to approved infection control practices by hospitals, or due to the changes to the public health infrastructure.
Keywords
Pseudomonas aeruginosa
Antibiotic susceptibility
Clinical isolates
Multi-drug resistant
1 Introduction
Pseudomonas aeruginosa has been responsible for wide range of ICU acquired infections in critically ill patients (Gajdács et al., 2020, Tamma et al., 2021) due to the presence of constitutive resistance to many antibiotics and antiseptics and its ability to acquire further resistance mechanism against multiple classes of antibiotics. Pseudomonas aeruginosa is a common pathogen in hospitals and particularly in intensive care units (Höfte 2021). It involves in various life-threatening infection in ICU such as endocarditis and septicemia, urinary tract infections, cystitis, pneumonia, surgical wound infections (Pachori et al., 2019). Various mechanism involve in drug resistance of Pseudomonas aeruginosa, like presence of over expressed efflux pump, of acquisition of resistance gene through plasmids and transposons, or through mutation in genes encoding porins, efflux pumps, penicillin-binding proteins, and chromosomal β-lactamase, all contributing to resistance to β-lactams, carbapenems, aminoglycosides, and fluoroquinolones (Normark & Normark 2002). Carbapenem-resistant Pseudomonas aeruginosa (CRPA) was first reported in 2001 from a cancer patient in Texas (Walters et al., 2019). Klebsiella pneumoniae carbapenemase (KPC) and New Delhi metallo-β-lactamase (NDM) have also been identified in healthcare-associated outbreaks (Epson et al., 2014, Ham et al., 2021, Leung et al., 2013). Recent data from a convenience sample of CRPA tested through the Antibiotic Resistance Laboratory Network found that 1.9% of isolates produced a carbapenemase (Walters et al., 2019). Pseudomonas aeruginosa with difficult-to-treat resistance (DTR) was proposed in 2018 and defined as nonsusceptibility to all of the following: piperacillin–tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem–cilastatin, ciprofloxacin, and levofloxacin (Kadri et al., 2018, Bader et al., 2020, Pang et al., 2019).
In this regard, a concern for P. aeruginosa infections is in the global emergence of multidrug resistant (MDR) and extensively drug resistant (XDR) strains, which limit the selection of effective antimicrobial therapies (Recio et al., 2020, Martis et al., 2014). In addition, Nosocomial infections caused by this organism are often hard to treat because the intrinsic resistance of the specie and its remarkable ability to acquire further mechanisms of β-lactamases enzymes to multiple groups of antimicrobial agents (García-Betancur et al., 2021).
In addition, to a significant population flow from the Middle East, the Kingdom is also annually a host for more than 4 million Muslim pilgrims from over 180 countries worldwide in the Hajj and Umrah seasons. These factors could potentially make Saudi Arabia a hot spot for the collection and spread of multidrug-resistant strains around the world. Bacterial resistance to antimicrobial agents is on the rise in the Kingdom (Memish et al., 2015).
In current study, we are aiming to analyse the prevalence of Pseudomonas aeruginosa infection for last 3 years 2019–2021 from Riyadh tertiary care hospital. Moreover, identifying the pattern of multi-drug resistance clinical isolates of P. aeruginosa (MRO). This study could lead insight of understanding the treatment options and control of spread of MRO P. aeruginosa infection in Saudi Arabia.
2 Methodology
2.1 Sampling and data collection
A total of 1024 Pseudomonas aeruginosa isolates from different clinical specimens; wound infections, blood, respiratory tract infections, body fluid and urine) and a total of 221 MRO Pseudomonas aeruginosa were collected from clinical microbiology lab, King Khalid University hospital (KKUH) during the period of 2019 to 2021. The isolates were cultured on blood agar plates and incubated at 37 °C for 24 h. The bacterial colonies were inoculated in Luria-Bertani (LB) broth and incubated in a shaking rack at 37 °C for 24 h.
2.2 Phenotypic analysis
Phenotypic identification using conventional culture methods, colony characteristics, pigment production, grape-like odor, oxidase positivity, motility, Gram-negative character of the bacilli, and to grow at 42 °C. (Memish et al., 2015).
2.3 Antibiotic susceptibility testing
All isolates were evaluated for susceptibility performed by microscan for the (MROs) in different sites of isolation from 2019 to 2021. The antibiotic susceptibility testing was done for all the isolates using the automated Vitek®2 system. The antibiotic susceptibility profiles of the isolates were determined using antimicrobial agents, imipenem (IPM) (0.002–32 ng/ml), meropenem (MER)(0.002–32 ng/ml), ceftazidime (CAZ) (0.016–256 ng/ml), amikacin (AN) (0.016–256 ng/ml), tobramycin (TM) (0.016–256 ng/ml), ciprofloxacin (CIP) (0.002–32 ng/ml), colistin (CS) (0.016–256 ng/ml) and aztreonam (ATM) (0.016–256 ng/ml). Ceftalozan, ceftazidime-avibactam, (4 mg/liter) by an agar dilution method. The results were compared to that of standard strain and interpreted as sensitive, intermediate resistant or resistant, based on CLSI guidelines. (Clinical and Laboratory Standards Institute. 2016).
3 Results
3.1 Phenotypic analysis
Colonies of Pseudomonas aeruginosa appeared as smooth, mucoid, greenish color with beta hemolysis and grape-like smell when grown on blood agar with pigment production, oxidase positive and motile.
3.2 Distribution of P. Aeruginosa among different body sites
1024 Pseudomonas aeruginosa were isolated from different clinical specimens during the last three year from 2019 to 2021. 221 isolates were multi drug resistance MROS Pseudomonas aeruginosa, most of the strains recovered from wound infections (32.4%) while the lowest rate of strains were recovered from respiratory tract infections (9.4%) as shown in Table 1.
Clinical specimens
Years
Total
2019
2020
2021
Wound
150
95
87
332 (32.4%)
Blood Culture
37
43
16
96 (9.4%)
Respiratory tract
122
99
34
255 (25%)
Body fluid
44
15
21
80 (7.8%)
Urine
22
15
11
48 (4.6%)
Total
457
334
233
1024
Percentage
44.6%
32.6%
22.7%
–
3.3 Demographic data
The mean age of the patients included in the study was 37.5 years. The higher numbers of the strains were collected from patients above 50 years old, while the lower no of the strains were collected from young patients even less than 1 year old. Out of 1024 isolates of P. aeruginosa; 221 strains were found to be multi drug resistant P. aeruginosa (MROs). Data suggested that older patients greater than 50 comprised the highest percentage (46%), adult aged 25–50 were (39%), then patients aged 1–25 years were (17%), followed by patient aged less than 1 years were (1.8%) as shown in Fig. 1. The higher rate of isolation occur from samples collected from wound (WO) n = 332 and the lowest number of the strains were recovered from urine n = 48 as shown in Table 2.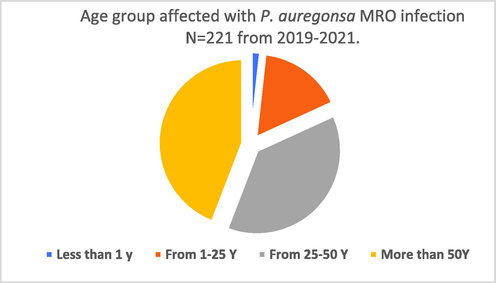
Age distribution of patients suffering from P. aeruginosa (MROs) infection during the period of 2019–2021.
Sites of isolation
Total No of
P. aeruginosa
Total No of
P. aeruginosa (MRO)
% P. aeruginosa (MRO)
Wound (WC)
332
64
19.2%
Blood (BC)
96
10
10.4%
Respiratory (RR)
255
98
43.5%
Body fluid (BF)
80
38
47.5%
Urine (UR)
48
11
22.9%
Total
1024
221
21.58%
3.4 P. aeruginosa screening by microscan
The antibiotic susceptibility of all isolates was evaluated by microscan to detect the (MROs) from the strains collected from different clinical specimens during the period of 2019–2021. The total number of P. aeruginosa (MROs) were N = 221. Most MROs P. aeruginosa isolates were collected from wound infections (64 isolates, 28.5%), followed by blood culture (10 isolates, 10.4%), then respiratory tract infections (98 isolates, 44.3%), Body fluid (38 isolates, 17%) and urine samples (11 isolates, 5%) as shown in Table 2.
The rate of isolation of P. aeruginosa (MROs) throughout the examination period revealed that the strains recovered from respiratory tract infections were significantly higher (47%) in the strains collected during the period of 2019 than that strains recovered during the period of 2020 and during the period of 2021; 28 (44%) and 21 (21%) respectively. Although, the number of the strains recovered from blood culture were significantly low (5.6%) in 2019, 2 (3%) during the period of 2020, and 2 (3.7%) during the period of 2021, are shown in Table 3 and Fig. 2.
Site of isolation
Urine (UR)
Body Fluid (BF)
Respiratory tract (RR)
Blood culture (BC)
Wound (WC)
Years
No
%
No
%
No
%
No
%
No
%
Total “2019”(104)
21
20%
6
5.7%
49
47%
24
23%
4
3.8%
Total” 2020″(63)
23
36.5%
2
3%
28
44%
6
9.5%
4
6.3%
Total”2021″(54)
20
37%
2
3.7%
21
38%
8
14.8%
3
5.5%

Incidences of P. aeruginosa (MROs) isolated from different clinical specimens during the period of 2019–2021.
3.5 Susceptibility testing
The outcomes of the antibiotic sensitivity pattern demonstrated that 77.8% and 61% of the isolates showed resistance against imipenem and meropenem, respectively. Table 4 represents the complete outcomes of the antibiotic resistance pattern for all MROs isolates, the data clearly showed that susceptibility test perform by microscan for all the strains collected from 2019 to 2021 revealed that (MROs) were resistance against Imipenem (77.8%), Meropenem (61%), Aztreonam (42%) and Ceftazidime (36%) which had been significantly higher than other antibiotics. All isolates sensitive to colistin except (2.7%) of the strains were sensitive, moreover, its of value to said that antibiotic resistant bacteria have been observed with increasing frequency over the past three years shown in Table 4 and Fig. 3. Additionally we evaluated the effect of Colistin on 221 clinical isolates of MRO P. aeruginosa and it was found that only 6 clinical isolates showed Colistin resistance making 2.7%.
Antibiotic
Years
Total Rate of “R” from
2019–2021
2019
2020
2021
Amikacin
15.3%
7.6%
12.6%
33.3%
Gentamicin
20%
13.4%
15.8%
37%
Tobramycin
18%
11.5%
15.8%
33.3%
Piperacillin
30%
16.3%
30%
59.2%
Aztreonam
42%
23%
42.8%
77.7%
Ceftazidime
36%
18.2%
36.5%
70.3%
Cefepime
30%
17.3%
31.7%
53.7%
Ciprofloxacin
11.3%
13.4%
19%
55.5%
Levofloxacin
25.3%
11.5%
20%
57.4%
Imipenem
77.8%
78.8%
74.6%
79.6%
Meropenem
61%
62.5%
68.2%
50%
Colistin
2.7%
2.8%
4.7%
–
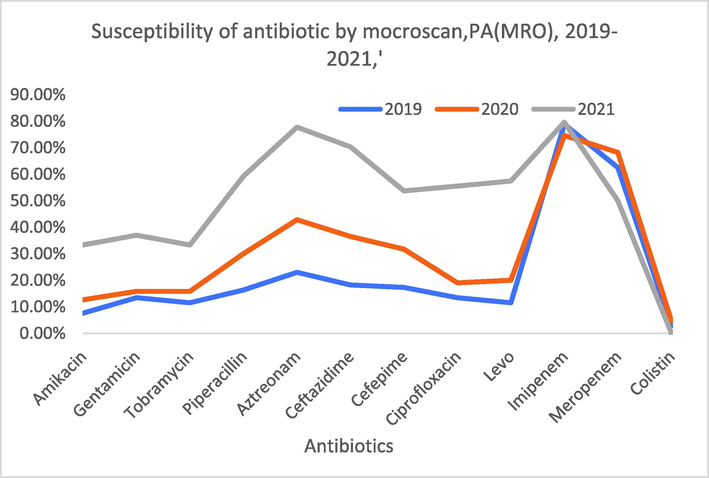
Susceptibility performs by microscan for the (MROs) Organisms from 2019 to 2021.
4 Discussion
In this study, 1024 isolates of P. aeruginosa from clinical samples received from year 2019–2021 from KKHU hospital Riyadh, the percentage prevalence of infection with P. aeruginosa seems to be declining yearly with percentage prevalence of 44.6%, 32.6%, and 22.7% in 2019, 2020 and 2021 respectively that is the sign that infection is being controlled from spreading. However, this study is generalized based on specimens from all clinical units of the hospital that is considered as larger study sample. Most isolates in this study were obtained from urine samples, accounting for 51.1% of the total. Unfortunately, it could not be determined which of the urine samples were catheter specimens, post catheterisation specimens or mid-stream urine samples.
In addition, it was also analyzed that the highest percentage of multi drug resistant P. aeruginosa strains were associated with Wound (WO) (28.5%), respiratory tract is second most common specimens (44.3%) that showed association with MRO P. aeruginosa strains. Whereas, 17% of body fluids, 10.4% of Blood Culture and 5% of Urine isolates showed lowest percentage MRO. Our findings consent with the findings conducted in Riyadh Saudi Arabia were collected from microbiology lab hospitals in Jazan region during 2017–2018, which documented that most of the MRO P. aeruginosa strains were associated with wound (20.2%) and respiratory tract infections (24%) (Kam et al., 2020; Fatima et al., 2019, Hashem et al., 2016, Tamma et al., 2021).
In the present study the most common MRO phenotypes were resistance against carbapenem antibiotics including imipenem & meropenem with 77.8% and 61% respectively. This is much higher than studies conducted by Hashem et al., (2016), who revealed 26.5% to (imipenem & meropenem) resistance in P. aeruginosa.
Moreover, In our study pattern of susceptibility demonstrated the resistances from 2019 to 2021 (MROs) of ceftazidime, cefepime, Amikacin piperacillin and aztreonam and were 36%, 30%, 15.3%, 30% and 42% respectively. Reported 70% resistance to ceftazidime, 75% to piperacillin, 59% to piperacillin/tazobactam, 74% to amikacin, 81% to cefepime, and 69% to aztreonam (Behera et al., 2008, Khan et al., 2016, Nivitha 2016).
In recent years colistin has been used extensively to treat multidrug resistance P. aeruginosa infections therefor we also evaluated the effect of Colistin on 221 clinical isolates of MRO P. aeruginosa. 6 clinical isolates showed Colistin resistance which is in contrary to results stated in study carried out in Jazan hospitals region during 2017–2018. The potential for encountering antimicrobial resistance is an important concern for clinicians treating patients with con– firmed or suspected P. aeruginosa infections. Resistance in P. aeruginosa has been shown to lead to increased morbidity and mortality (Carmeli et al., 1999, Harris et al., 1999).
5 Conclusion
MRO P. aeruginosa is responsible for serious nosocomial infections among the patients admitted in the hospital, it appears that prevalence of over all infection is decreasing yearly but in contrast, antibiotic susceptibility among P. aeruginosa isolates is decreasing in the KSA, perhaps because of increasing or massive use of antibiotics and the lack of adherence to approved infection control practices by hospitals.
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSURG-2-1677).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad. Med.. 2020;132(3):234-250.
- [Google Scholar]
- High prevalence of carbapenem resistant Pseudomonas aeruginosa at a tertiary care centre of north India. Are we under-reporting? Indian J. Med. Res.. 2008;128(3):324-326.
- [Google Scholar]
- Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother.. 1999;43(6):1379-1382.
- [Google Scholar]
- Carbapenem-resistant Klebsiella pneumoniae producing New Delhi metallo-β-lactamase at an acute care hospital, Colorado, 2012. Infect. Control Hosp. Epidemiol.. 2014;35(4):390-397.
- [Google Scholar]
- L-asparaginase produced from soil isolates of Pseudomonas aeruginosa shows potent anti-cancer activity on HeLa cells. Saudi Journal of Biological Sciences. 2019;26(6):1146-1153.
- [Google Scholar]
- Characterization of resistance in gram-negative urinary isolates using existing and novel indicators of clinical relevance: a 10-year data analysis. Life. 2020;10(2):16.
- [Google Scholar]
- Update on the epidemiology of carbapenemases in Latin America and the Caribbean. Expert Rev. Anti Infect. Ther.. 2021;19(2):197-213.
- [Google Scholar]
- Gram-negative bacteria harboring multiple carbapenemase genes, United States, 2012–2019. Emerg. Infect. Dis.. 2021;27(9):2475.
- [Google Scholar]
- Epidemiology and clinical outcomes of patients with multiresistant Pseudomonas aeruginosa. Clin. Infect. Dis.. 1999;28(5):1128-1133.
- [Google Scholar]
- Carbapenem susceptibility and multidrug-resistance in Pseudomonas aeruginosa isolates in Egypt. Jundishapur journal of microbiology. 2016;9(11)
- [Google Scholar]
- Höfte, M. 2021. The use of Pseudomonas spp. as bacterial biocontrol agents to control plant disease. In Book. Burleigh Dodds.
- Kadri, S. S., Adjemian, J., Lai, Y. L., Spaulding, A. B., Ricotta, E., Prevots, D. R., et al.. 2018. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clinical Infectious Diseases, 67(12), 1803-1814.
- Systematic analysis of disease specific immunological signatures in patients with febrile illness from Saudi Arabia. Clin. Transl. Immunol.. 2020;9(8):e1163.
- [Google Scholar]
- Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann. Saudi Med.. 2016;36(1):23-28.
- [Google Scholar]
- Persistence of related bla-IMP-4 metallo-beta-lactamase producing Enterobacteriaceae from clinical and environmental specimens within a burns unit in Australia-a six-year retrospective study. Antimicrob. Resist. Infect. Control. 2013;2(1):1-8.
- [Google Scholar]
- Colistin in multi-drug resistant Pseudomonas aeruginosa blood-stream infections: a narrative review for the clinician. J. Infect.. 2014;69(1):1-12.
- [Google Scholar]
- Molecular characterization of carbapenemase production among gram-negative bacteria in Saudi Arabia. Microb. Drug Resist.. 2015;21(3):307-314.
- [Google Scholar]
- Nivitha, M. 2016. Identification Of Non Fermenting Gram Negative Bacilli From Clinical, Environmental Samples, their Antimicrobial Resistance and Detection Of blaVIM/blaIMP genes in Imipenem resistant isolates (Doctoral dissertation, Chennai Medical College Hospital and Research Centre, Trichy).
- Evolution and spread of antibiotic resistance. J. Intern. Med.. 2002;252(2):91-106.
- [Google Scholar]
- Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes & diseases. 2019;6(2):109-119.
- [Google Scholar]
- Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv.. 2019;37(1):177-192.
- [Google Scholar]
- Predictors of mortality in bloodstream infections caused by Pseudomonas aeruginosa and impact of antimicrobial resistance and bacterial virulence. Antimicrob. Agents Chemother.. 2020;64(2) e01759-19
- [Google Scholar]
- Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing Enterobacterales (ESBL-E), carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa) Clin. Infect. Dis.. 2021;72(7):e169-e183.
- [Google Scholar]
- Carbapenem-resistant Pseudomonas aeruginosa at US emerging infections program sites, 2015. Emerg. Infect. Dis.. 2019;25(7):1281.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102702.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







