Translate this page into:
Supercritical fluid extraction of torch ginger: Encapsulation, metabolite profiling, and antioxidant activity
⁎Corresponding author. jawaid@upm.edu.my (M. Jawaid)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Objectives
The objective of this study was first to perform the supercritical fluid extraction (SFE) and encapsulationof torch ginger (Etlingera elatior) inflorescences into a functional powder. Second objective was to evaluate the powder’s characteristics, metabolite profiles, and antioxidant activity.
Methods
Torch ginger inflorescences were extracted via SFE technique, and the obtained extract was encapsulated by a spray-drying process with maltodextrin as an encapsulating agent. Subsequently, the powder was evaluated for its physical characteristics, determination of metabolite profiles by using a Fourier Transform Infrared Spectrophotometer (FTIR) and Gas Chromatography-Mass Spectrometry (GC–MS), and antioxidant activity.
Results
Spray drying encapsulation process managed to yield around 59.8% of torch ginger extract powder (TGEP) by using 10% of extract, which the obtained yield was twice higher than in another study. TGEP showed inconsistent agglomeration behaviour in particle size examination with distinct sizes concentrating at 2.2 μm and 17.4 μm, respectively. Brunauer-Emmett-Teller (BET) analysis of TGEP unveiled a considerably high surface area (1.13 m2/g), pore volume (0.218 cm3/g), and pore size (384.6 nm). The metabolites profile of TGEP was studied and characterized using two spectroscopic analyses. Analysis by the FTIR showed the presence of O–H, C–H, C = C, C = O, CO-O-CO, C-N, and C-O functional groups in the sample. Subsequently, the result of the GC–MS characterization revealed about 59 metabolites that predominantly fatty acids (30.5%), terpenes and derivatives (20.3%), fatty acid esters (16.9%), and alcohols (8.47%) were present in TGEP. The powder also demonstrated a high antioxidant activity based on the evaluation of its total phenolic content (23.3 ± 0.662 mg GAE/g TGEP), EC50 as determined from 2,2-diphenyl-1-picrylhydrazyl radical scavenging capacity assay (1.31 ± 0.002 mg/mL), and ferric reducing antioxidant power (2919.5 ± 19.9 μM TE/g TGEP), which were better than previous studies.
Conclusion
Therefore, this study unveiled TGEP as a functional powder with a high content of bioactive compounds with excellent bioactivity.
Keywords
Etlingera elatior
Encapsulated torch ginger extract powder
Supercritical fluid extraction
Spray drying
Encapsulation
Metabolite
1 Introduction
Torch ginger or scientifically known as Etlingera elatior is a perennial herbaceous plant belong in the Zingiberaceae family which is endemic to the Southeast Asian region. It grows in a large colonies and has a pink, ovoid-shaped, inflorescence with a unique fragrance (NParks, 2019). In the Southeast Asian gastronomy, torch ginger is synonymous in various cuisine such as Asam Laksa in Malaysia, Pecel in Indonesia, and added to traditional Thai meat dishes in Thailand (Rachkeeree et al., 2018).
Torch ginger has been receiving major traction among researchers and various studies have been conducted to unveil its promising medicinal value. Torch ginger has been reported to contains various phytochemical constituents specifically secondary metabolites that serve a multitudes of biological functions. Secondary metabolites in the class of lipids, phenolics, and terpenes are often found in torch ginger’s extract and its metabolites profile was well documented in literature (Ghasemzadeh et al., 2015; Marzlan et al., 2020; Wijekoon et al., 2011; Wijekoon et al., 2013). As mentioned earlier, these classes of secondary metabolites have been scientifically proven to render various biological functions namely, as antioxidant, antimicrobial, and antibiotic (Hussein & El-Anssary, 2018; Lachumy et al., 2010). Furthermore, torch ginger also distinctively known as an aromatic flower and this aroma is fundamental in the Southeast Asian gastronomy (Oh et al., 2019; Raji et al., 2017). Hence, apart from elevating the sensory experience of food, the inclusion of torch ginger in daily diet is presumed to elicit the functional importance of bioactive compounds, which are vital for optimal human health (Abdelwahab et al., 2010; Lachumy et al., 2010).
In recent decade, supercritical fluid extraction (SFE) has been well reported as a safe and efficient extraction technique for various plant-based natural products in broad array of application. SFE utilise supercritical carbon dioxide (SCO2) as a solvent in which this unique state delivers a gas-like diffusivity and liquid-like solubility throughout the extraction process (Sunol et al., 2019). These properties are important to induce the penetration of solvent deep into the plant matrices which it will increase the rate of mass transfer between the extracted material and the solvent used (Arumugham et al., 2021). Additionally, the use of CO2 also provides an added advantage to this technique as the Food and Drug Administration (FDA) has classified the substance as Generally Recognized as Safe (GRAS) (FDA, 2020). Hence, this unique mechanism of SFE promotes the extraction, isolation, and retention of plant’s beneficial compounds, such as terpenes, flavonoids and phenolics in the obtained extract (Caballero et al., 2020).
The encapsulation technique has ushered in a multifaceted benefit to the plant extract. This technique has been claimed to protect bioactive compounds against oxidation, creates a thermal barrier, preserve the physical structure of the compounds, and conserve the organoleptic attributes of the compound (Mooranian et al., 2014). The aforementioned benefits are made possible via this technique as the bioactive compounds are trapped in a capsule-like structure with a shell made up of biomaterials or synthetic polymer that serves as a protective layer (Onsaard & Onsaard, 2019). Therefore, researchers and industry players have preferred the encapsulation technique to produce bioactive compound-rich extract suitable for various applications.
Several studies have been conducted on developing plant-extract-based powder from various herbal plant species in literature (Rajabi et al., 2015; Simon-Brown et al., 2016). However, to the authors’ knowledge, only one study on developing torch ginger extract powder using the encapsulation technique exists in the literature, which has been published by Anuar et al. (2021). Consequently, due to insufficient studies on torch ginger-extract powder, information about its phytochemical composition and bioactivity remain scarce in the literature. In this study, the torch ginger extract powder (TGEP) was developed, the retention of bioactive compounds in the powder was evaluated, and its bioactivity was tested. Therefore, the objective of this study was first to perform the supercritical fluid extraction (SFE) and encapsulation of torch ginger (Etlingera elatior) inflorescences into a functional powder. Meanwhile, the second objective was to evaluate the powder’s characteristics, metabolite profiles, and antioxidant activity.
2 Materials and methods
2.1 Torch ginger sample and chemicals
Torch ginger (Etlingera elatior) inflorescences were procured from a farm in Kuala Pilah, Negeri Sembilan, Malaysia. The sample was harvested and delivered to the authors’ laboratory within the same day. Subsequently, the sample was stored in the laboratory's refrigerator for storage under refrigerated conditions (±4 °C). Analytical grade organic solvents and chemicals were used in the experiments for an optimum precision. The N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) used for the analysis was bought from Thermo Fisher Scientific (Waltham, MA, United States of America). Denatured ethanol (99% purity) and acetic acid (glacial, ≥99% assay) were obtained from HmbG Chemicals (Hamburg, Germany). Additionally, hydrochloric acid (fuming 37%), gallic acid, Folin-Ciocateu phenol reagent, and ferric chloride hexahydrate were purchased from Merck (Darmstadt, Germany). Meanwhile, anhydrous pyridine (99.8%), methoxyamine hydrochloride (98%), sodium acetate (anhydrous), sodium carbonate (anhydrous), gallic acid (TraceCERT®), 2,2-Diphenyl-1-picrylhydrazyl radical (DPPH), and (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were procured from Sigma-Aldrich (St. Louis, MO, United States of America).
2.2 Preparation
2.2.1 Pre-processing of torch ginger
The inflorescences were separated from its stalk and later, they were cut into smaller pieces. Subsequently, the sample was lyophilized by using the Labconco FreeZone Benchtop freeze dryer (Missouri, United States of America) that was operated at −40 °C and the vacuum level was set at 133 × 10-3 mBAR. The drying process was conducted until the sample was sufficiently dried (±10% moisture content) which it took approximately four days to complete. Using a RT-CR30S 3HP cutting mill with a cyclone powder collector (Rong Tsong Precision Technology Co., Dawei Rd., Taichung, Taiwan), the dried sample was pulverized into powder with particles approximately 0.22 mm in size. Following that, the remaining debris were removed from the powder by sieving it using a 200 × 50 mm sieve with the aperture size of 0.220 mm and the powder was stored in an airtight container.
2.2.2 Supercritical fluid extraction of torch ginger
Supercritical fluid extraction (SFE) technique was performed to extract and isolate essential oil from torch ginger by using a laboratory scale extraction plant (Deven Supercritical Pvt. Ltd., Phatak Baug, Navi Peth, Pune, India). The extraction process was carried out according to method used in our previous study with some modifications (Naziruddin et al., 2022). In brief, a filter bag with a pore size of 45.0 µm was filled with approximately 200 g of torch ginger powder and subsequently positioned within the high-pressure extraction vessel of the SFE unit. Stream of liquid CO2 (99.8% purity, 1.2 kg/h) was flowed into the chiller for it to be cooled at 5 °C and it was later pressurised by a high-pressure pump before entering the extraction vessel. Inside the extraction vessel, liquid CO2 was converted to a supercritical state (SCO2) upon being pressurised to 28 MPa at 50 °C. Consequently, the SCO2 penetrated the sample’s microporous matrix to induce the extraction and isolation of the desired compounds. The entire extraction process took about six hours to complete, by which the yield was collected every consecutive hour and dispensed into an amber glass bottle. The bottle was tightly capped and hermetically sealed by wrapping it with a sheet of parafilm for storage at 4 °C.
2.2.3 Spray drying encapsulation of torch ginger extract
Prior to the process, a mixture made up of torch ginger extract (10%), water (80%), maltodextrin as an encapsulating agent (7%), and emulsifiers (glyceryl monostearate and sodium stearoyl lactylate) each at 1.5% was prepared. Subsequently, the prepared mixture was evenly mixed and homogenised for 15 min at 6000 rpm by using a Silverson L5M-A laboratory mixer (Silverson Machines, Inc., East Longmeadow, Massachusetts, United States of America). The aforementioned machine was operated with a short stop at every consecutive 5-minute to allow the cool down of the rotor blades. The mixture was further homogenised for 30 min by applying speed at 5800 rpm using a GEA Lab Homogeniser PandaPLUS 2000 (GEA Group Aktiengesellschaft, Düsseldorf, Germany). The formed emulsion was dried using a Büchi B-290 spray dryer (Büchi Labortechnik AG, Flawil, Switzerland) which equipped with an atomiser nozzle (0.5 mm in diameter) at 15 MPa. The spray drying process was conducted under the following conditions: inlet air temperature at 130 °C, outlet air temperature at 50 ± 1 °C, flow rate fixed at 150 L/h, and feed suspension rate set at 180 mL/h. Upon completion, the obtained powder was transferred into an amber glass bottle and tightly sealed. The bottle was stored in a refrigerator for storage at a refrigerated condition (±4 °C). Schematic diagram of the supercritical fluid extraction (SFE) process and the encapsulation of torch ginger extract by spray drying is showed in Fig. 1.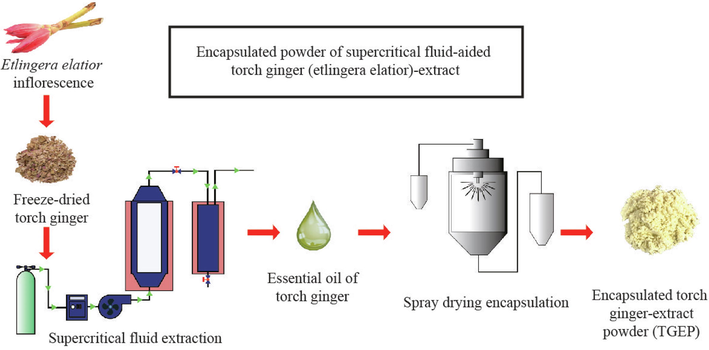
A schematic representation illustrating the SFE and the encapsulation process of TGEP.
Yield of the encapsulated torch ginger extract powder (TGEP) obtained from the process was determined according to the equation used by Navarro-Flores et al. (2020) which given as follows:
2.3 Analysis of powder characteristics
2.3.1 Particle size analysis and BET analysis
The particle size distribution of TGEP was studied using a Mastersizer 2000 particle size analyser equipped with Scirocco 2000 sample dispersion unit (Malvern Instruments Ltd., Malvern, United Kingdom). Prior to analysis, the refractive index of TGEP was determined at 1.52 by using a PAL-RI refractometer (ATAGO Co., Ltd., Tokyo, Japan). TGEP was precisely weighed at 2.0 g, and it was loaded into the hopper attached to the Scirocco 2000 sample dispersion unit, and the pressure was set at 4 bar. In addition, the specific surface area and porosity distribution of TGEP were investigated with Brunauer-Emmett-Teller (BET) analysis through a Micromeritics ASAP 2000 equipment. Before analysis, the sample was degassed for 30 min at 60 °C under a continuous nitrogen gas flow.
2.3.2 FTIR spectroscopy analysis
The metabolite screening of TGEP was performed using a Spectrum 100 Fourier Transform Infrared Spectrophotometer (FTIR) (PerkinElmer Inc., Waltham, United States of America) by using method used in our previous study (Naziruddin et al., 2021). The scanning range of the FTIR was set from 4000 to 600 cm−1 with a scanning resolution of 4 cm−1. By grinding, potassium bromide (KBr) was combined with TGEP and the powder mixture was pelletised before analysis.
2.4 Metabolites profiling by GC–MS
Prior to Gas Chromatography-Mass Spectrometry (GC–MS) analysis, TGEP was derivatized according to the method used by Robinson et al. (2005). Briefly, 25.0 mg of TGEP was placed inside a 2.0 mL centrifuge tube, and about 50.0 μL of anhydrous pyridine was then added into it. The mixture was subjected to sonication for a duration of 10 min at 30 °C by using an Elmasonic S 30 (H) ultrasonic device (Elma Schmidbauer GmbH, Singen, Germany). Subsequently, about 100 μL of methoxyamine HCl (20 mg/mL in pyridine) was pipetted into the solution and it was vortexed for one minute. Two consecutive incubation was conducted by which the solution was initially incubated for 2 h at 60 °C and it was again incubated for 30 min at 60 °C upon the addition of 300 μL of MSTFA. Lastly, the solution was filtered using a 0.22 μm nylon syringe filter, and the filtered liquid was then transferred to an amber vial to be left at room temperature overnight.
The GC–MS method used in our previous study was followed for the identification of metabolites in TGEP and some modifications were made to improve the detection (Naziruddin et al., 2022). The derivatized fraction of TGEP was analysed by using a TSQ Quantum XLS GC–MS system (Thermo Scientific, United States of America). TGEP’s aliquot was injected (1 μL injection volume) into an Agilent J&W DB-5MS column (length: 30 m, inner diameter: 0.25 mm, and film thickness: 0.25 mm) (Agilent Technologies, California, United States of America) in split-less mode and the carrier gas used was helium at 1.0 mL/min. The column was first held at 80 °C for 5 min and afterwards increased at 8 °C/min to 200 °C. Subsequently, the temperature of the oven was gradually raised to 280 °C at a ramp rate of 4 °C/min, and maintained at that level for 15 min. The temperature of the ion source and interface were regulated at 280 °C and 250 °C, respectively. The GC–MS analysis was performed in a total ion chromatography (TIC) mode and the full scan data was collected within a mass scan range of 40 to 600 m/z. To identify the compounds present, the acquired mass spectra for each chromatographic peak were compared with a retention time index and mass spectral libraries for GC–MS that were created by the National Institute of Standards and Technology (NIST). The data version used for this analysis was NIST17 (NIST, 2017).
2.5 Analysis of antioxidant activity
2.5.1 Quantification of the total phenolic content
Total Phenolic Content (TPC) in TGEP was quantified using the Folin-Ciocalteu method (Xiao et al., 2020). TGEP (precisely weighed at 0.5 mg) was dissolved in 1 mL ethanol and shaken for 1 min. Meanwhile, ethanolic gallic acid (GA) calibration solutions were prepared at five concentration levels which ranging from 6.25 × 10-3 mg/mL – 0.1 mg/mL. Briefly, approximately 0.1 mL of the extract was pipetted into a test tube and mixed with 0.5 mL of 50% Folin-Ciocalteu reagent. Subsequently, the solution was mixed using a vortex mixer (OHAUS Corporation, New Jersey, United States of America) for 3 min and about 7.9 mL of distilled water was added to the tube. The solution was allowed to set at room temperature for 5 min. Following that, 7.5% sodium carbonate solution was added to made the final volume of 10 mL and later it was incubated in a dark room (±28 °C) for 2 h. Throughout the incubation, the tube was periodically shaken at every 30 min to ensure it was fully reacted. Both sample and calibration standard solutions were determined for its absorbance at the wavelength of 765 nm by using a ultraviolet–visible (UV–Vis) spectrophotometer of the GENESYS™ 10S model (Thermo Fisher Scientific, Waltham, United States of America). The TPC’s result was expressed as milligram (mg) of gallic acid equivalents (GAE) per gram (g) of TGEP (mg GAE/g TGEP). Calibration curve of the gallic acid standard was constructed and its linear equation was used to estimate the TPC value of TGEP.
2.5.2 DPPH radical scavenging capacity assay
The DPPH assay was performed based on previously reported methods with minor alterations (Trucillo et al., 2018). Sample was prepared by dissolving TGEP in ethanol at five different concentrations (0.1 mg/mL, 0.05 mg/mL, 0.025 mg/mL, 0.0125 mg/mL, and 6.25 × 10-3 mg/mL in ethanol). Meanwhile, all five levels of GA calibration solutions were also prepared in ethanol which ranging from 0.05 mg/mL to 3.125 × 10-3 mg/mL in concentration. Precisely weighed DPPH at 0.0197 g was dissolved in 500 mL ethanol to make a 10-4 M solution. Briefly, about 1 mL of the diluted TGEP was mixed with 3.0 mL DPPH solution in a test tube and intensely shaken for 1 min. The test tube was allowed to incubate for 30 min in a dark environment at ambient temperature. A UV–Vis spectrophotometer (similar model used for TPC assay) was used to measure the absorbance at a wavelength of 517 nm for the sample, standard, and control (diluted DPPH in ethanol absolute). Scavenging capacity (%) of the sample and standards were estimated by solving the Eq. (2) as follows:
Where abs. A is the absorbance of sample or standards and abs. B is the control’s absorbance. Reduction of the initial DPPH radical concentration by 50% based on its respective TGEP’s concentration was represented by EC50 value.
2.5.3 Ferric reducing antioxidant power (FRAP) assay
FRAP assay was conducted by applying methods described by Benzie and Strain (1996) and Tomasina et al. (2012) with slight modifications. Freshly prepared FRAP reagent solution was prepared by combining 300 mM acetate buffer (pH 3.6), 10 mM TPTZ solution (in 40 mM hydrochloric acid), and 20 mM ferric chloride hexahydrate (FeCl3) with a volume (mL) ratio of 25: 2.5: 2.5. Ethanolic TGEP’s extract was prepared by dissolving the sample (1 mg) in 1 mL of ethanol. Meanwhile, Trolox calibration solutions in the concentration of 19.977 μM, 9.988 μM, 4.994 μM, 2.497 μM, and 1.251 μM were also prepared in ethanol. The assay was performed by mixing 8.7 mL of FRAP reagent with 0.3 mL TGEP’s ethanolic extract and it was put aside to incubate at 50 °C for 1 h. Using a UV–vis spectrophotometer, the absorbance at 593 nm was measured to monitor the reduction of ferric iron (Fe3+) to ferrous iron (Fe2+) by the antioxidants present in both TGEP and the standard. Results were expressed as micromolar (μM) of Trolox equivalents (TE) per g of TGEP (μM TE/g TGEP). Calibration curve based on the Trolox standards was constructed and the obtained equation was used to calculate the FRAP value.
3 Results and discussion
3.1 Yield of the encapsulated torch ginger-extract powder
In the process, about 400 g of the prepared emulsion with a total solid mass of 80.0 g (50:50 torch ginger’s extract and encapsulating agent) was fed into the spray dryer, which produced 47.8 g of TGEP. Hence, based on the determination by Eq. (1), the process managed to yield around 59.8% of TGEP. The obtained yield in this study was found to be substantially higher than the yields reported by Anuar et al. (2021) which were in the range of 15 % – 36 % based on various formulations tested.
3.2 Particle size analysis and BET analysis
Fig. 2 displays the distribution of particle size for encapsulated torch ginger-extract powder. The median diameter, D0.5 for TGEP was measured at 6.188 ± 0.771 μm. Meanwhile, the sample revealed two distinct particle sizes concentrating at 2.2 μm and 17.4 μm, respectively. This non-uniform size distribution was likely due to the formation of large agglomerates as a result of spray drying at low inlet air temperature (≤140 °C) (Both et al., 2020). Additionally, Siccama et al. (2021) also mentioned that the presence of high residual moisture content and low glass transition temperature of the spray dried powder might increase its stickiness which leads to agglomeration. Nonetheless, the produced TGEP showed relatively small size of particles which was less than 50 μm.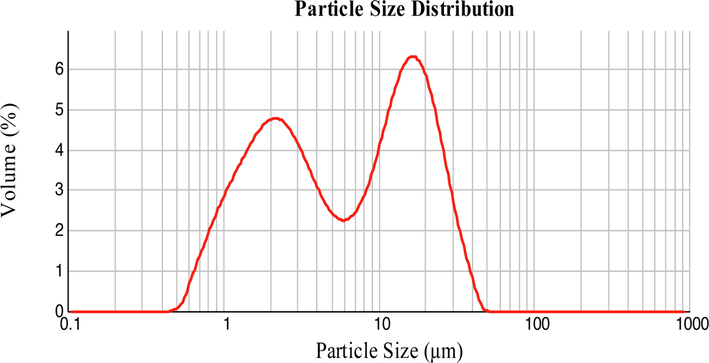
Particle size distribution of TGEP.
In BET analysis (Table 1), TGEP was examined with the presence of considerably high surface area, pore volume, and pore size features. Surface area purportedly affected the functionality of powders: solubility, flowability, rehydration, and wetting characteristics. Furthermore, high surface area of functional powder also contributed to a high degree of solvation which is paramount for the absorption of retained compounds (Burgain et al., 2017; Koç & Kaymak-Ertekin, 2014). The outcomes’ trend of BET analysis were in agreement with results reported by Zhang et al. (2018), as the spray-dried powder obtained from their research exhibited higher surface area (ranging from 1.54 to 2.18 m2/g) with greater porosity. The authors also reported that the physical characteristics of spray-dried powder often affected by the spray drying inlet air temperature and type of atomiser. Zhang et al. (2018) also reported that the inlet air temperature in between 120 °C and 160 °C can induced the increased of powder’s surface area due to formation of particles with dryer and harder coating. The aforementioned inlet air temperature reflected to this study as the spray drying process was conducted at 130 °C. Hence, similar powder characteristics reported by Zhang et al. (2018) were expected.
Sample
Surface Area (m2/g)
Pore Volume (cm3/g)
Pore Size (nm)
TGEP
1.131
0.218
384.6
3.3 FTIR analysis
The FTIR spectrum of TGEP is displayed in Fig. 3, and the peaks in the fingerprint region were assigned based on their corresponding functional groups in Table 2. A wide band was observed extending from 3000 cm−1 to 3600 cm−1, which corresponded to the O–H stretching vibration of alcohols and carboxylic acids in the sample. Several researchers have been reported the present of alcohols, such as 1-Dodecanol, Tetradecanol, and 1-Undecanol in the torch ginger extract. Meanwhile, carboxylic acid that predominantly fatty acids such as Hexacosanoic acid, Decanoic acid, and Hexadecanoic acid also has been reported to present in torch ginger extract (Al-Mansoub et al., 2021; Anzian et al., 2020; Marzlan et al., 2020). The spectra showed two intense sharp peaks at 2853 cm−1 and 2925 cm−1 which were in relation to the C–H stretching vibration of alkyl group. Additionally, the presence of alkene in TGEP can be identified by a medium peak formed at 1463 cm−1 which corresponded to C–H bending of methylene group and a 1652 cm−1 shoulder band which representing the double bonds (C = C) vibrational stretching of alkenyl group. Alkane and alkene which belong to the class of hydrocarbons also has been reported by Al-Mansoub et al. (2021), as the author managed to identify the presence of Cyclododecane, Heptadecane, 1-Decene, and 1-Tetradecene in the ethanolic torch ginger extract. The sample also exhibited a sharp peak at 1727 cm−1 which indicating the C = O stretching of carbonyl group such aldehyde, ester, and carboxylic acid. This finding was in agreement with results reported by Marzlan et al. (2020), as the authors identified esters such as Lauryl acetate, Myristyl myristate, and (E)-9-Tetradecen-1-ol acetate in torch ginger oil extracted by supercritical fluid extraction. Anhydride group also potentially present in TGEP as indicated by the intense peak formed at 1025 cm−1 which attributing to the CO-O-CO stretching of the functional group. Subsequently, a sharp peak present at 1200 cm−1 signalled the C-N stretching of amine, while a broad peak formed at 1250 cm−1 to 1310 cm−1 might be corresponded to the C-O stretching of aromatic ester. Lastly, a noticeable peak formed at 750 cm−1 could be associated to C–H bending of 1,2-disubstituted and monosubstituted compounds that present in TGEP. Hence, the results proved that the presence of chemically-diverse compounds in TGEP was valid.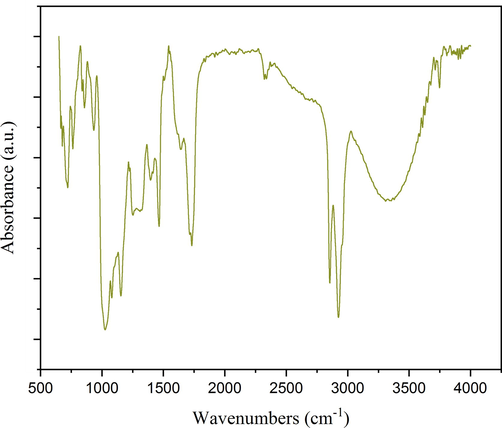
FTIR spectrum of TGEP.
Wavenumbers (cm−1)
Band assignment
3000 cm−1 to 3600 cm−1
O–H stretching vibration of alcohols and carboxylic acids
2853 cm−1 and 2925 cm−1
C–H stretching vibration of alkyl group
1463 cm−1
C–H bending of methylene group
1652 cm−1
Double bonds (C = C) vibrational stretching of alkenyl group
1727 cm−1
C = O stretching of carbonyl group
1025 cm−1
CO-O-CO stretching of anhydride group
1200 cm−1
C-N stretching of amine
1250 cm−1 to 1310 cm−1
C-O stretching of aromatic ester
750 cm−1
C–H bending of 1,2-disubstituted and monosubstituted compounds
3.4 Metabolite profiling and identification by GC–MS
GC–MS chromatogram of the detected metabolites in TGEP was showed in Fig. 4. Broad array of masses was acquired (scan range 40 – 600 m/z) within the scan time of 5.0 to 55.0 min and presence of metabolites were monitored at various retention times (RTs). The metabolites’ identities were confirmed by comparing the generated spectral pattern with those of established spectral library developed by National Institute of Standards and Technology (NIST), data version NIST17 (NIST, 2017). Additionally, the detected metabolites were classified by referring to the comprehensive database of FooDB which was developed by the renounce research teams specialising in metabolomics (TMIC, 2021).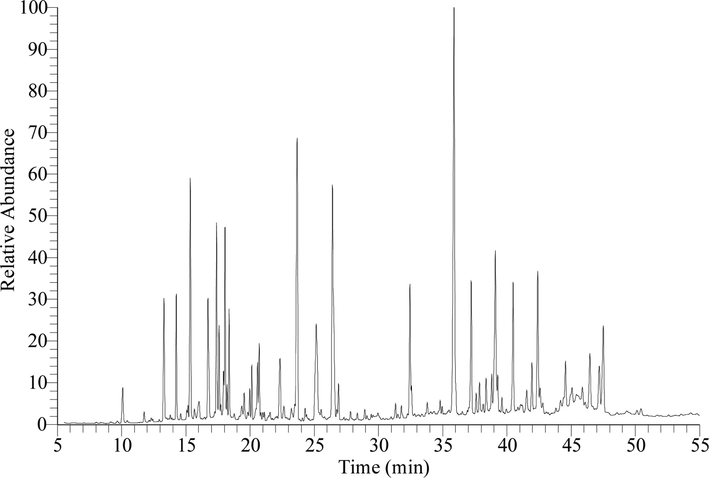
The chromatogram obtained by GC–MS (TIC) displays the metabolites that have been identified in TGEP.
Altogether, about 59 metabolites belong to the 19 different classes of compounds were identified in TGEP as listed in Table 3. The number of metabolites able to be detected in this study is substantially higher and more diversified than other reported torch ginger’s metabolite studies. In literature, studies conducted by Marzlan et al. (2020) and Anzian et al. (2020) reported the numbers of metabolite found in torch ginger’s essential oil at 20 and 33 respectively. In brief, most of the detected metabolites were belong to four major classes which were fatty acids (30.5%), terpenes and derivatives (20.3%), fatty acid esters (16.9%), and alcohols (8.47%).
Nr.
Metabolite
Retention time (min)
Molecular formula
Molecular weight (g/mol)
Probability (%)
Acetate Esters
1.
1-Tetradecyl acetate
20.14
C16H32O2
256
23.72
2.
Lauryl acetate
17.39
C14H28O2
228
36.04
Acid anhydride
3.
2,5-Furandione, 3-dodecyl-
32.45
C16H26O3
266
10.42
Alcohols
4.
1-Dodecanol
15.35
C12H26O
186
13.35
5.
11-Tetradecen-1-ol, (E)-
18.22
C14H28O
212
11.34
6.
cis-9-Tetradecen-1-ol
18.22
C14H28O
212
10.46
7.
cis-11-Tetradecen-1-ol
18.22
C14H28O
212
10.06
8.
1-Heptatriacotanol
29.96
C37H76O
536
24.73
Aldehyde
9.
Dodecanal
10.09
C12H24O
184
44.48
Amine
10.
2,6-Octadien-1-amine,
3,7-dimethyl-11.76
C10H19N
153
12.92
Carboxylic esters
11.
4-Azido-2-nitrobutyric
acid,
2,6-di-t-butyl-4-methoxyph
enyl ester34.96
C19H28N4O5
392
21.06
Coumaric acid ester
12.
Hexadecyl-(E)-p-coumarate, trimethylsilyl ether
38.38
C28H48O3Si
460
36.63
Dithiane
13.
2-[3-(1-Ethoxyethoxy)prop
yl][1,3]dithiane24.29
C11H22O2S2
250
28.96
Fatty acids
14.
Dodecanoic acid,
3-hydroxy-10.43
C12H24O3
216
12.20
15.
Dodecanoic acid
16.04
C12H24O2
200
63.76
16.
Dodecanoic acid, TMS
derivative17.59
C15H32O2Si
272
56.20
17.
Undecanoic acid, TMS
derivative18.06
C14H30O2Si
258
10.35
18.
Tetradecanoic acid
19.55
C14H28O2
228
39.02
19.
Myristic acid, TMS
derivative20.71
C17H36O2Si
300
68.45
20.
Tridecanoic acid
22.34
C13H26O2
214
30.30
21.
Pentadecanoic acid
22.34
C15H30O2
242
18.22
22.
Palmitelaidic acid, TMS
derivative23.22
C19H38O2Si
326
58.40
23.
Petroselinic acid, TMS
derivative23.22
C21H42O2Si
354
11.55
24.
Palmitic Acid, TMS
derivative23.68
C19H40O2Si
328
45.52
25.
Tridecanoic acid, TMS
derivative11.42
C16H34O2Si
286
11.42
26.
Pentadecanoic acid, TMS
derivative10.98
C18H38O2Si
314
10.98
27.
Octadecanoic acid
25.54
C18H36O2
284
11.19
28.
9,12-Octadecadienoic acid(Z,Z)
-, TMS derivative26.42
C21H40O2Si
352
22.10
29.
Stearic acid, TMS
derivative26.90
C21H44O2Si
356
37.56
30.
Heptadecanoic acid, TMS
derivative26.90
C20H42O2Si
342
25.76
31.
2-Oleoylglycerol, 2TMS
derivative35.50
C27H56O4Si2
500
16.18
Fatty acid esters
32.
9-Octadecenoic acid (Z)-,
oxiranylmethyl ester25.54
C21H38O3
338
12.67
33.
9(E),11(E)-Conjugated
linoleic acid, trimethylsilyl
ester26.42
C21H40O2Si
352
36.47
34.
Butanoic acid,
4-cyano-2-nitro-,2,6-bis(1,1-dimethylethyl)
-
4-methoxyphenyl ester34.96
C20H28N2O5
376
13.18
35.
cis-9-Tetradecenoic acid,
heptyl ester43.13
C21H40O2
324
43.13
36.
cis-9-Tetradecenoic acid,
isobutyl ester35.88
C18H34O2
282
10.33
37.
Tetradecanoic acid,
2-oxo-, ethyl ester37.61
C16H30O3
270
51.47
38.
Hexadecanoic acid,
octadecyl ester39.29
C34H68O2
508
23.56
39.
Hexadecanoic acid,
tetradecyl ester39.29
C30H60O2
452
16.16
40.
Hexadecanoic acid,
hexadecyl ester39.29
C32H64O2
480
11.41
41.
Oleic acid, eicosyl ester
46.10
C38H74O2
562
14.32
Hydrocarbon
42.
17-Pentatriacontene
41.55
C35H70
490
14.77
Ketones
43.
Cyclododecanol
10.09
C12H24O
184
16.24
44.
(Z)-18-Octadec-9-enolide
25.16
C18H32O2
280
14.66
45.
15-Isopropenyl-3-(trimethylsilyl)
oxacyclopentadecan-2
-one26.42
C20H38O2Si
338
26.42
Phytoestrogen and derivative
46.
Estra-1,3,5(10)-trien-17β-ol
25.54
C18H24O
256
14.99
Xanthophyll
47.
.psi.,.psi.-Carotene,
1,1′,2,2′-tetrahydro-1,1′-dimethoxy-45.88
C42H64O2
600
7.79
Terpenes and derivatives
Monoterpenes
48.
3-Cyclohexene-1-methanol
, 5-hydroxy-α,α,4-trimethyl-13.79
C10H18O2
170
14.18
49.
trans-3(10)-Caren-2-ol
13.79
C10H16O
152
13.79
Monoterpene derivative
50.
Sobrerol 8-acetate
13.79
C12H20O3
212
14.18
Sesquiterpenes
51.
1,4,7,-Cycloundecatriene,
1,5,9,9-tetramethyl-, Z,Z,Z-15.17
C15H24
204
33.15
52.
Humulene
15.17
C15H24
204
24.07
53.
Formic acid,
3,7,11-trimethyl-1,6,10-do
decatrien-3-yl ester15.68
C16H26O2
250
11.47
Triterpenes
54.
ç-Sitosterol
46.47
C29H50O
414
15.68
55.
β-Sitosterol
46.67
C29H50O
414
30.42
56.
Uvaol, 2O-TMS
47.19
C36H66O2Si2
586
10.48
57.
β-Sitosterol, TMS
derivative47.51
C32H58OSi
486
10.54
58.
Stigmast-5-ene,3β-(trimethylsiloxy)
-,(24S)
-47.51
C32H58OSi
486
52.32
Terpene alcohol
59.
7,8-Epoxylanostan-11-ol,
3-acetoxy-41.08
C32H54O4
502
17.48
Among these major classes of metabolites, alcohols particularly 1-Dodecanol was reported to render a potent antibacterial activity against several bacterial species (Marzlan et al., 2022; Marzlan et al., 2020). Additionally, the pharmacological activities of terpenoids were also well-known and heavily studied in the literature. Uvaol, a triterpene, was reported by Agra et al. (2016) to be effective as an active ingredient for the treatment of inflammation caused by allergic reaction.
Torch ginger notoriously known for its distinctive aroma which is a vital characteristic of Southeast Asian’s cuisine. Expert described that torch ginger embodies a sweet, tangy, and lemongrass-like aroma profile (Khor et al., 2017). Although the key aromatic compounds that responsible for torch ginger’s aroma have never been reported in literature, such study was already being conducted for ginger (Zingiber officinale Roscoe) and galangal (Kaempferia galanga L.) which also belong in the Zingiberaceae family. The researchers identified the presence of metabolite composition that consist of aldehyde, alcohol, hydrocarbon, ketone, terpene, and ester to be accountable for the distinctive aroma of both herbs (Hasegawa et al., 2016; Pang et al., 2017). In spite of the differences in species, similar composition as previously mentioned was also found in TGEP and the aroma profiles exude by the compounds could be assumed similar to torch ginger. Pang et al. (2017) mentioned that the presence of primary odorants in ginger namely, monoterpenes and sesquiterpenes could be associated to the woody, minty, citrusy, and herbal-like aroma. The researchers also attributed the sweet notes (balsamic and floral) of ginger to the presence of metabolites in the class of alcohol, aldehyde, terpene and terpene derivative. Additionally, humulene, a sesquiterpene found in TGEP was also reportedly presence in galangal and it was presumed to emits the unique galangal-like aroma (Hasegawa et al., 2016). The formerly defined aroma profile of ginger and galangal are noticeably similar to the general description of torch ginger’s aroma. Therefore, it is presumed that the key aromatic compounds of torch ginger were retained in the TGEP, which would be beneficial for the application in food.
3.5 Antioxidant activity
The antioxidant capacity of TGEP was evaluated based on the performance of TPC, DPPH, and FRAP as showed in Table 4. The quantified value of TPC for TGEP was found to be higher than other reported values in literature which were in the range of 2.12 – 19.4 mg GAE/g (Anzian et al., 2017; Yan & Asmah, 2010). The TPC values of the aforementioned studies were based on the quantification in the fresh and dried forms of torch ginger. Therefore, it indicates that the phenolic compounds present in torch ginger retained at an exceptional level in TGEP. Additionally, this claimed also supported by the presence of Hexadecyl-(E)-p-coumarate which is a phenolic acid identified in TGEP by the GC–MS. Values are means of triplicate determination ± SD.
TPC (mg GAE/g TGEP)
EC50 of DPPH radical scavenging ability (mg/mL)
FRAP (μM TE/g TGEP)
23.3 ± 0.662
1.31 ± 0.002
2919.5 ± 19.9
The free radical scavenging capacity of TGEP was predicted based on its antioxidants ability to reduce DPPH radical. The activity of scavenging DPPH radicals was measured by the EC50 value in which it indicates the effective TGEP’s concentration needed to reduce the DPPH radical’s absorbance by 50%. The value of EC50 for TGEP was determined at 1.31 ± 0.002 mg/mL, which was substantially lower than such value reported by Nurain et al. (2013) at 3.47 ± 0.420 mg/mL based on the determination in ethanolic torch ginger’s extract. This low value of EC50 for TGEP signified its high antioxidant activity which could potentially linked to its high TPC value. Additionally, the quantified FRAP value of TGEP also was found to be substantially higher as compared to such values reported by Bunleu and Buavaroon (2018) and Wijekoon et al. (2011) which were in the range of 9.0 – 130 μM Fe(II)/g. The excellent performance of TGEP’s antioxidants in the assay demonstrated its high reactivity against the Fe3+ - TPTZ and effectively reduced it to Fe2+ - TPTZ. Hydroxyl and carbonyl-rich compounds in plants have been associated with excellent reducing capabilities and stabilisers (Mohamad et al., 2014; Pradeep et al., 2022). Based on Table 3, TGEP possessed abundant compounds with sufficient hydroxyl and carbonyl groups present, such as dodecanal (aldehyde), xanthophyll, and β-Sitosterol (terpene) (Mahavy et al., 2022; Tovey, 2019). The oxidation–reduction abilities of these compounds allow the binding of metals and inactivate them via chelation (Azri et al., 2019).
The outstanding performance of TGEP in the aforementioned antioxidant assay could be linked to the existence of various metabolites in the class of terpenoids as profiled in Table 3. Terpenoid has been vastly studied in literature and the evidences for its potency as an antioxidant were well recorded. Terpenoids, namely humulene and uvaol found in TGEP have been reported in multiple studies able to induce the reduction of oxidative stress by effectively control the autoxidation reaction (Allouche et al., 2010; Gunawan et al., 2016). Additionally, the abundance of TGEP’s metabolites with a hydroxyl, methoxy, and carboxylic acid groups could potentially contribute to the high antioxidant capacity as these functional group were reported to render a vital effect on the antioxidant ability (Chen et al., 2020).
3.6 Potential future as a plant-based functional food additive
The development of functional food additives derived from plants has aroused the interest of consumers for a much healthier alternative and reduced the dependency on its artificial counterparts (Domínguez et al., 2021). However, various shortcomings in applying plant extract at the industrial scale might induce complexity in the manufacturing process and not be economically sound. Plant extract must be handled with the utmost care as it is highly volatile, hydrophobic, and prone to stability issues when exposed to environmental stressors (e.g., extreme temperature, light) (Kfoury et al., 2016; Rezaei & Nasirpour, 2019). The encapsulation technique has been known to offset these problems as it can trap the plant’s bioactive compounds in an encapsulating agent and makes it more stable (Muñoz-Shugulí et al., 2021). Based on the evaluations, TGEP has demonstrated the retention of various metabolites with different bioactive functions and maintained its antioxidant capability. This technique also captures the key aromatic compounds of torch ginger, which would be vital for its application as a food additive.
4 Conclusion
Present study revealed the extraction, encapsulation and comprehensive metabolite profiling of torch ginger-extract powder. Spray drying encapsulation process managed to yield around 59.8% of TGEP by incorporating 10% supercritical fluid-torch ginger extract into encapsulating agent mixture, which the obtained yield was twice higher than other study. Based on the performed analyses, the developed powder showed the present of varying valuable bioactive compounds. From the particle size analysis, TGEP revealed aggregated feature, which shown by two distinct particle sizes concentrating at 2.2 μm and 17.4 μm, respectively. BET analysis of TGEP unveiled a considerably high surface area (1.13 m2/g), pore volume (0.218 cm3/g), and pore size (384.6 nm) which were purportedly affected by the spray drying inlet air temperature. The FTIR analysis revealed the presence of O–H, C–H, C = C, C = O, CO-O-CO, C-N, and C-O functional groups in the sample. Meanwhile, based on the GC–MS analysis, about 59 metabolites that predominantly fatty acids (30.5%), terpenes and derivatives (20.3%), fatty acid esters (16.9%), and alcohols (8.47%) were identified in TGEP. TGEP also demonstrated an excellent antioxidant capacity as indicated by low EC50 value at 1.31 ± 0.002 mg/mL (determined from the DPPH radical scavenging capacity), ferric reducing antioxidant power assay (2919.5 ± 19.9 μM TE/g TGEP), and high value of total phenolic content (23.3 ± 0.662 mg GAE/g TGEP) in comparison to the previous studiesTherefore, this study has indicated that the developed encapsulated torch ginger extract powder able to retain the beneficial bioactive compounds which makes it a promising functional powder.
Funding
The authors also extend their gratitude to Researchers Supporting Project number (RSP2023R117) King Saud University, Riyadh, Saudi Arabia for funding this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their gratitude to Researchers Supporting Project number (RSP2023R117), King Saud University, Riyadh, Saudi Arabia.
References
- Chemical composition, antioxidant and antibacterial properties of the essential oils of Etlingera elatior and Cinnamomum pubescens Kochummen. J. Sci. Food Agric... 2010;90(15):2682-2688.
- [CrossRef] [Google Scholar]
- Uvaol attenuates pleuritis and eosinophilic inflammation in ovalbumin-induced allergy in mice. Eur. J. Pharmacol... 2016;780:232-242.
- [CrossRef] [Google Scholar]
- Antioxidant and antiatherogenic activities of pentacyclic triterpenic diols and acids. Food Chem. Toxicol... 2010;48(10):2885-2890.
- [CrossRef] [Google Scholar]
- Chemical composition, antiproliferative and antioxidant attributes of ethanolic extract of resinous sediment from Etlingera elatior (Jack.) inflorescence. Braz. J. Pharm. Sci.. 2021;57
- [CrossRef] [Google Scholar]
- Spray Drying Microencapsulation of Kantan Extract (Etlingera Elatior) with Various Wall Materials. IOP Conference Series: Earth and Environmental Science.. 2021;736(1):012007
- [CrossRef] [Google Scholar]
- Anzian, A., Rashidah, S., Nazamid, S., Safraa binti Che Wan Sapawi, C. W. N., & Meor Hussin, A. S. b., 2017. Chemical composition and antioxidant activity of Torch Ginger (Etlingera elatior) flower extract. Food and Applied Bioscience Journal. 5(1), 32-49. https://doi.org/10.14456/fabj.2017.4.
- Anzian, A., Muhialdin, B. J., Mohammed, N. K., Kadum, H., Marzlan, A. A., Sukor, R., & Meor Hussin, A. S., 2020. Antibacterial Activity and Metabolomics Profiling of Torch Ginger (Etlingera elatior Jack) Flower Oil Extracted Using Subcritical Carbon Dioxide (CO2) Evidence-Based Complementary and Alternative Medicine. 2020, 4373401. https://doi.org/10.1155/2020/4373401.
- Arumugham, T., K, R., Hasan, S. W., Show, P. L., Rinklebe, J., & Banat, F., 2021. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications – A review. Chemosphere. 271, 129525. https://doi.org/10.1016/j.chemosphere.2020.129525.
- Etlingera elatior-Mediated Synthesis of Gold Nanoparticles and Their Application as Electrochemical Current Enhancer. Molecules.. 2019;24(17):3141.
- [CrossRef] [Google Scholar]
- The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem... 1996;239(1):70-76.
- [CrossRef] [Google Scholar]
- Particle morphology and powder properties during spray drying of maltodextrin and whey protein mixtures. Powder Technol... 2020;363:519-524.
- [CrossRef] [Google Scholar]
- Antioxidant Activities, Acute Toxicity and Chemical Profiling of Torch Ginger (Etlingera elatior Jack.) Inflorescent Extract. Pharmacognosy Journal.. 2018;10(5):979-982.
- [CrossRef] [Google Scholar]
- Surface chemistry and microscopy of food powders. Prog. Surf. Sci... 2017;92(4):409-429.
- [CrossRef] [Google Scholar]
- Supercritical fluid extraction for enhancing polyphenolic compounds production from olive waste extracts. J. Chem. Technol. Biotechnol... 2020;95(2):356-362.
- [CrossRef] [Google Scholar]
- Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep... 2020;10(1):2611.
- [CrossRef] [Google Scholar]
- Domínguez, R., Pateiro, M., Munekata, P. E. S., McClements, D. J., & Lorenzo, J. M., 2021. Encapsulation of Bioactive Phytochemicals in Plant-Based Matrices and Application as Additives in Meat and Meat Products. Molecules. 26(13), 3984. https://www.mdpi.com/1420-3049/26/13/3984.
- FDA, 2020. Part 184 Direct Food Substances Affirmed as Generally Recognized as Safe. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1240 (accessed 13 June 2021).
- Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) R.M.Sm grown in different locations of Malaysia. BMC Complementary and Alternative Medicine.. 2015;15(1):335.
- [CrossRef] [Google Scholar]
- The Response to Oxidative Stress α-Humulene Compounds Hibiscus Manihot L Leaf on The Activity of 8-Hydroxy-2-Deoksiquanosin Levels Pancreatic β-Cells in Diabetic Rats. Biomedical and Pharmacology Journal.. 2016;9(2)
- [Google Scholar]
- Aroma Profile of Galangal Composed of Cinnamic Acid Derivatives and Their Structure-Odor Relationships. Nat Prod Commun.. 2016;11(10):1463-1469.
- [CrossRef] [Google Scholar]
- Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In: Builders P.F., ed. Herbal Medicine. London, UK: IntechOpen Limited; 2018.
- [Google Scholar]
- Determination of formation constants and structural characterization of cyclodextrin inclusion complexes with two phenolic isomers: carvacrol and thymol. Beilstein J. Org. Chem... 2016;12:29-42.
- [CrossRef] [Google Scholar]
- Phytochemical, Antioxidant and Photo-Protective Activity Study of Bunga Kantan (Etlingera elatior) Essential Oil. Journal of Applied Pharmaceutical Science.. 2017;7
- [CrossRef] [Google Scholar]
- Koç, B., & Kaymak-Ertekin, F. (2014). The effect of spray drying processing conditions on physical properties of spray dried maltodextrin. Original Paper presentation at the meeting of the Foodbalt 2014. 9th Baltic Conference on Food Science and Technology, Riga, Latvia.
- Pharmacological activity, phytochemical analysis and toxicity of methanol extract of Etlingera elatior (torch ginger) flowers. Asian Pac. J. Trop. Med... 2010;3(10):769-774.
- [CrossRef] [Google Scholar]
- The Xanthophyll Carotenoid Lutein Reduces the Invasive Potential of Pseudomonas aeruginosa and Increases Its Susceptibility to Tobramycin. Int. J. Mol. Sci... 2022;23(13):7199.
- [CrossRef] [Google Scholar]
- Optimized supercritical CO2 extraction conditions on yield and quality of torch ginger (Etlingera elatior (Jack) R.M. Smith) inflorescence essential oil. Ind. Crop. Prod... 2020;154:112581
- [CrossRef] [Google Scholar]
- Incorporating torch ginger (Etlingera elatior Jack) inflorescence essential oil onto starch-based edible film towards sustainable active packaging for chicken meat. Ind. Crop. Prod... 2022;184
- [CrossRef] [Google Scholar]
- Plant Extract as Reducing Agent in Synthesis of Metallic Nanoparticles: A Review. Adv. Mat. Res... 2014;832:350-355.
- [CrossRef] [Google Scholar]
- Microencapsulation as a novel delivery method for the potential antidiabetic drug, Probucol. Drug Des. Devel. Ther... 2014;8:1221-1230.
- [CrossRef] [Google Scholar]
- Encapsulation of plant extract compounds using cyclodextrin inclusion complexes, liposomes, electrospinning and their combinations for food purposes. Trends Food Sci. Technol... 2021;108:177-186.
- [CrossRef] [Google Scholar]
- Navarro-Flores, M. J., Ventura-Canseco, L. M. C., Meza-Gordillo, R., Ayora-Talavera, T. d. R., & Abud-Archila, M., 2020. Spray drying encapsulation of a native plant extract rich in phenolic compounds with combinations of maltodextrin and non-conventional wall materials. Journal of Food Science and Technology. 57(11), 4111-4122. https://doi.org/10.1007/s13197-020-04447-w.
- Sage biomass powders by supercritical fluid extraction and hydro-distillation techniques: a comparative study of biological and chemical properties. Biomass Convers. Biorefin. 2021
- [CrossRef] [Google Scholar]
- Development of encapsulated sage extract powder: Inter-comparison with commercially available powder for physical properties and metabolites composition. J. Supercrit. Fluids.. 2022;184
- [CrossRef] [Google Scholar]
- NIST, 2017. NIST/EPA/NIH Mass Spectral Library with Search Program, (Data Version: NIST v17, Software Version: 2.3). Gaithersburg, Maryland, USA: National Institute of Standards and Technology (NIST).
- NParks, 2019. Flora: Etlingera elatior. https://www.nparks.gov.sg/florafaunaweb/flora/1/9/1990 (accessed 22 September 2021).
- Comparative Study of Aqueous and Ethanolic Aromatic Malaysian Herbs Extracts Using Four Antioxidant Activity Assays. Int. J. Agric. Res... 2013;8(2):55-66.
- [CrossRef] [Google Scholar]
- The development of Nyonya cuisine in the Malay Archipelago: Penang and Malacca Nyonya cuisine. Journal of Ethnic Foods.. 2019;6(1):17.
- [Google Scholar]
- Microencapsulated Vegetable Oil Powder. In: Salaün F., ed. Microencapsulation - Processes, Technologies and Industrial Applications. London, UK: IntechOpen Limited; 2019.
- [Google Scholar]
- Identification of Ginger (Zingiber officinale Roscoe) Volatiles and Localization of Aroma-Active Constituents by GC–Olfactometry. J. Agric. Food Chem... 2017;65(20):4140-4145.
- [CrossRef] [Google Scholar]
- Uncovering the Phytochemical Basis and the Mechanism of Plant Extract-Mediated Eco-Friendly Synthesis of Silver Nanoparticles Using Ultra-Performance Liquid Chromatography Coupled with a Photodiode Array and High-Resolution Mass Spectrometry. ACS Sustain. Chem. Eng... 2022;10(1):562-571.
- [CrossRef] [Google Scholar]
- Nutritional Compositions and Phytochemical Properties of the Edible Flowers from Selected Zingiberaceae Found in Thailand. Front. Nutr... 2018;5
- [CrossRef] [Google Scholar]
- Retention of saffron bioactive components by spray drying encapsulation using maltodextrin, gum Arabic and gelatin as wall materials. Food Hydrocoll... 2015;51:327-337.
- [CrossRef] [Google Scholar]
- Past and present practices of the Malay food heritage and culture in Malaysia. Journal of Ethnic Foods.. 2017;4(4):221-231.
- [Google Scholar]
- Evaluation of Release Kinetics and Mechanisms of Curcumin and Curcumin-β-Cyclodextrin Inclusion Complex Incorporated in Electrospun Almond Gum/PVA Nanofibers in Simulated Saliva and Simulated Gastrointestinal Conditions. BioNanoScience.. 2019;9(2):438-445.
- [CrossRef] [Google Scholar]
- The potential of metabolite profiling as a selection tool for genotype discrimination in Populus. J. Exp. Bot... 2005;56(421):2807-2819.
- [CrossRef] [Google Scholar]
- The effect of partial replacement of maltodextrin with vegetable fibres in spray-dried white asparagus powder on its physical and aroma properties. Food Chem... 2021;356:129567
- [CrossRef] [Google Scholar]
- Microencapsulation of ginger (Zingiber officinale) extract by spray drying technology. LWT Food Sci. Technol... 2016;70:119-125.
- [CrossRef] [Google Scholar]
- 20 - SUBSTITUTION OF SOLVENTS BY SAFER PRODUCTS. In: Wypych G., ed. Handbook of Solvents (Third Edition). Ontario: Chemtec Publishing; 2019. p. :1455-1634.
- [Google Scholar]
- TMIC, 2021. FooDB. https://foodb.ca/ (accessed 30 September 2021).
- Analysis of two methods to evaluate antioxidants. Biochem. Mol. Biol. Educ... 2012;40(4):266-270.
- [CrossRef] [Google Scholar]
- Chapter 8 - Duodenal Ulcer and Diet in Sub-Saharan Africa. In: Segal I., ed. Digestive Diseases in Sub-Saharan Africa. Academic Press; 2019. p. :53-65.
- [Google Scholar]
- Supercritical assisted process for the encapsulation of olive pomace extract into liposomes. J. Supercrit. Fluids.. 2018;135:152-159.
- [CrossRef] [Google Scholar]
- Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J. Food Compos. Anal... 2011;24(4):615-619.
- [CrossRef] [Google Scholar]
- Chemical Composition and Antimicrobial Activity of Essential Oil and Solvent Extracts of Torch Ginger Inflorescence (Etlingera elatior Jack.) Int. J. Food Prop... 2013;16(6):1200-1210.
- [CrossRef] [Google Scholar]
- Guidelines for antioxidant assays for food components. Food Frontiers.. 2020;1(1):60-69.
- [CrossRef] [Google Scholar]
- Comparison of total phenolic contents and antioxidant activities of turmeric leaf, pandan leaf and torch ginger flower. Int. Food Res. J... 2010;17:417-423.
- [Google Scholar]
- Maltodextrin: A consummate carrier for spray-drying of xylooligosaccharides. Food Res. Int... 2018;106:383-393.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102700.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







