Translate this page into:
Sugarcane juice mediated synthesis of copper oxide nanoparticles, characterization and their antibacterial activity
⁎Corresponding author. drthaminum@gmail.com (A. Thaminum Ansari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
In this work, an environmental benign, sugarcane juice (SCJ) was applied as an eco-friendly stabilizing agent to synthesize copper oxide nanoparticles (CuO NPs). To produce CuO NPs, 2, 5 and 10 ml of juice were added during the synthesis. The produced CuO NPs were characterized using FTIR, which confirmed the transformation of functional groups through Cu–O. The XRD analysis confirmed the monoclinic crystalline structure and purity of the material. SEM images confirm the nanoparticles formation. Quantitative estimation of Cu, O and C present in samples were carried out by EDS. Square, rectangular, cubic cylindrical and spherical shaped particles observed from the TEM micrographs. Furthermore, Micro-raman and X-ray photoelectron spectroscopy (XPS) were taken to assure the formation of CuO NPs. The results revealed that the SCJ is a good stabilizing agent which reduces the size of particles significantly at higher concentrations and altered shapes to spherical. Hence, it sugarcane juice could be applied as a green stabilizing agent to fabricate the CuO NPs. Antibacterial activity of CuO NPs was assessed against some pathogenic bacteria.
Keywords
Sugarcane juice
Green synthesis
CuO nanoparticles
Antibacterial activity
1 Introduction
Research interest in nanoparticles has rapidly improved because of their distinctive physical-chemical properties. Nanomaterials are entirely differing from the bulk materials in terms of diffusivity, electrical resistivity and electrical conducting property. The strength of the nanomaterials and chemical reactivities of nanomaterials differs from the of the bulk materials (Sahooli et al., 2012; Khashan et al., 2016). Metal oxide nanoparticles drawn the attention of researchers due to its wide application in the area of catalysis, sensor and in medical applications. The metal oxide nanoparticles have been proved as disinfectant in the medical field, antimicrobial agent, fillers in cosmetics and as catalysts in industrial field. Metal oxide possessed good semiconducting properties and hence they have been used microelectronics (Katwal et al., 2015). Cupric oxide (CuO) is well known and very smart semiconducting materials which possessed constricted band gap of 1.2 eV (Chen et al., 2013). Outstanding characteristics such as thermal stability, optical properties and electrical conductivity of copper oxide inspired the researcher to work on this material for photocatalytic activity and electrical conductivity (Devi et al., 2014; Bhattacharjee and Ahmaruzzaman, 2016; Eibl, 1993). The application of CuO is mostly acknowledged solar energy harvesting and energy storage batteries (Rakhshani, 1986; Wang, 2009). The CuO NPs also used in antimicrobial devices. There is a relationship between the efficiency and particle size of the CuO. The morphological aspects and structure of the CuO NPs found to be improved by applying various techniques (Ren et al., 2009). To achieve the desired morphology, various synthesis methods have been employed. Some of the common methods are sonochemical, sol-gel method, chemical precipitation, electrochemical, laser ablation and organic modifiers or surfactant mediated synthesis (Safa et al., 2016; Kayani et al., 2015; Ananth et al., 2015).
CuO has drawn the curiosity of researchers due to its biological properties. Antimicrobial and biocidal properties, which led utilize in the biomedical applications (Nations et al., 2015). Electrical conducting, optical and electrical properties of CuO find its applications as supercapacitors, infrared filters, storage devices and sensors (Devi et al., 2014; Dagher et al., 2014). It is interesting to know that the size of the particles playing key role in the constructing optical, catalytic, electrical and biological properties (Katwal et al., 2015). Apart from the cosmetic and pharmacological applications, CuO NPs also used as paints and coatings (Ruiz et al., 2015).
The optical property is one of the important properties of CuO NPs, which drastically affect the size, morphology and also the temperature (Zhang et al., 2014; Yang et al., 2014). The dimensions of the particles and the morphology are two major feature influencing magnetic properties of CuO NPs (Rao et al., 2009). The electrical conductivity of the nanoparticles influenced by the size of the particles (Azimi et al., 2015). The experimental procedure also controls the properties of nanomaterials. Beyond the known industrial applications, CuO NPs used in anticancer formulations and detection of viruses in the human body as a part of diagnosis of diseases (Ahamed et al., 2014). However, in chemical synthesis, byproducts produced in all the methods. Chemical synthesis of copper oxide nanoparticles might affect and produce toxic to the environment. To avoid the harmfulness posed by chemicals or byproducts, eco-friendly methods gaining the importance to synthesis the nanoparticles. The plant extract acted as reducing as well as a stabilizing agent in the synthesis of metal and metal oxide nanoparticles Besides, green synthesis is a simple, cheap and environmentally friendly (Sharma et al., 2015).
SCJ mediated synthesis CdFe2O4, silver based chitosan nanocomposite, novel Schiff base caped silver nanocomplexes, SnO2 quantum dots and Cl@AgCl a solar light active material and proved their dye degradation property against many organic dyes. The synthesized nanomaterials evaluated for their antibacterial activity and degradation of various dyes. They reported that the SCJ act as reducing, capping and stabilizing agent (Patil et al., 2018; Paulkumar et al., 2017; Elemike et al., 2016; Zaman et al., 2015; Kulkarni et al., 2014).
Sugarcane (Saccharum officinarum) is a rich source of sugar present in the juice. SCJ contains reducing sugar and non-reducing sugars (glucose & sucrose). It contains chloride, calcium and sodium ion. This juice is also well known for the presence of food supplements such as malic acid, oxalic acid, citric acid D-gluconic acid. Therefore, an attempt was made to use SCJ as stabilizing agent that can be used to produce the nanoparticles by varying the concentration. The aim of the present work was to synthesis CuO NPs using sugarcane juice as a stabilizing agent and capping agents and analyzes their morphology, size and shape including the bactericidal activity.
2 Materials and methods
2.1 Reagents and materials
Fresh sugarcane juice was obtained from the local vendor and centrifuged at 10,000 rpm for 10 min. The clear juice was segregated from the residue and used as stabilizing agent to synthesis the copper oxide nanoparticles. Copper nitrate was purchased from LobaChemie, Mumbai, India.
2.2 CuO NPs synthesis
To synthesize CuO NPs, three different concentrations 2, 5 and 10 ml of juice was mixed with 100 ml of 0.1 M copper nitrate solution and stirred well. Then, the solution was kept on a magnetic stirrer and stirred at 80 °C for an hour. The blue color of reaction mixture turned to green by indicating the development of CuO. Thereafter, an excess amount of 0.5 M ammonium hydroxide was dropwise added to the above solution until the pH reached up to 10 with vigorous stirring. After the addition of ammonium hydroxide, the color of the solution turned to dark brown. The reaction mixture was continued on a magnetic stirrer for 8 h to form the gel. In order to remove the free nitrate ions and organic impurities and excess ammonia, the precipitate obtained was centrifuged and thoroughly washed with distilled water and placed in hot air oven at 80 °C for 8 h. The material was heated at 500 °C for 3 h in a muffle furnace to convert hydroxide into copper oxide.
2.3 Materials characterization
Crystalline property of the CuO NPs was analyzed using advanced diffractometer using Cu–Ka radiation (Model: X’Pert-PRO). Metal-oxygen bond formation was assessed by Fourier transform infrared (Jasco 6300 FT-IR spectrophotometer). The IR spectra of the samples were measured by KBr pellets. Formation of the CuO was identified by scanning electron microscope with the resolution of 15 nm (JEOL Model JSM – 6390LV). The magnification of the instrument was 5 × 3, 00,000 (both in high and low vacuum modes). The surface morphology of particles was studied using transmission electron microscope (TEM) with resolution (Point: 0.23 nm, Lattice: 0.14 nm) (Jeol/JEM 2100). The percentage copper, oxygen and carbon present in the samples were analyzed by energy dispersive study (EDS). Raman spectra of the nanoparticles was studied by Raman scattering using the 514 nm line with Argon laser source. We also employ microscopic Raman spectroscopy to characterize the cupric particles obtained by X-ray irradiation. Electronic states of CuO NPs were studied using X-ray Photoelectron Spectroscopy (XPS) with Auger Electron Spectroscopy (AES) Module: Model/Supplier: PHI 5000 Versa Prob II, FEI Inc. s.
2.4 Antibacterial activity
Antibacterial activity of CuO NPs was tested using the agar well diffusion method against Escherichia coli, Pseudomonas aeruginosa, Staphylooccus aureus and Bacillus subtilis (Elango et al., 2018). Bacterial culture (Mueller Hinton agar) 20 ml was transferred into Petri plates. The wells were punched into the agar plate. The bacteria culture was swabbed on the agar plates. CuO NPs, 100 mg/ml dispersed in DMSO were weighed down into the each well. The petri plates were incubated at 37 °C. The zone of inhibition was noted after 24 h of incubation and expressed in mm dia and compared to the reference substance (Ciprofloxacin). The tests were performed in triplicates. 25 μg of Ciprofloxacin and 100 µg of CuO NPs were used to test the bactericidal activity.
3 Results and discussion
3.1 FTIR studies
The functional group transformation is identified using the stretching frequencies obtained using FT-IR spectra. Cu–O bond formation, metal-O stretching and presence of hydroxyl, and moisture content were characterized using FT-IR spectral techniques. The FT-IR spectra of CuO NPs produced by green route are presented in Fig. 1(a-d). A peak observed at 609 cm−1 due to stretching vibrations of Cu–O bond in CuO NPs. Two peaks shown at 799 and 855 cm−1 are revealed the M−O stretching vibrations of CuO NPs (Thekkae Padil et al., 2013; Xu et al., 2007). The absorption peaks observed at 1635 and 3346 cm−1 contribute to the bending and stretching vibrations of moisture content present on the surface of CuO NPs. The peaks noticed near 500–600 cm−1 indicate the metal–oxygen vibration frequencies of copper and oxygen (Kuppusamy et al., 2017; Taghavi Fardood et al., 2016). Components of sugarcane juice are high molecular weight polysaccharide and protein moieties. To assure this study, the FTIR examination of the sugarcane juice was performed (Fig. 1e). There are three characteristic peaks at wave numbers 827–1045, 1517 and 1631 cm−1 present in both the samples analyzed. In general, the peak intensities for the polysaccharide fraction are higher except at 1517 and 1631 cm−1. It showed peaks at 3263 (high molecular weight), 2933, 1633, 1048, 1411, 1346 and 1267 cm−1 corresponds to OH, C–H stretching, C⚌O, O–C⚌O, C–H stretching of the aromatic ring, phenolic OH and C–C stretching of various molecules. Small peaks noticed at 103 and 887–827 cm−1 attributed to β-glycosidic linkage between sugars. The observed peaks correspond to characteristic protein and polysaccharide bands (Guilherme et al., 2015; Camargo et al., 2012).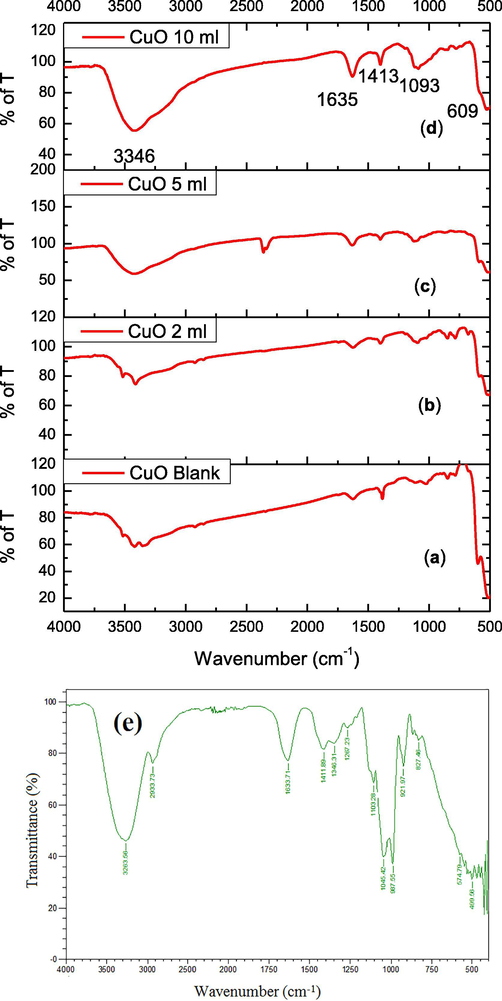
(a-e). FTIR spectra (a) CuO NPs (b) CuO NPs with 2 ml (c) CuO NPs with 5 ml (d) CuO NPs with 10 ml juice) (e) FTIR spectrum of sugarcane juice.
3.2 XRD analysis
The XRD system was utilized to establish and authenticate the crystalline nature of the CuO NPs. The XRD spectra of the CuO NPs are shown in Fig. 2(a-d). The XRD report revealed a sequence of diffraction peaks at 2θ of X-axis at 32.11°, 35.62°, 38.87°, 48.85°, 53.49°, 58.46°, 61.71° and 66.5° matching for the (1 1 0), (1 1 1), (2 0 0), (−2 0 2), (0 2 0), (2 0 2), (−1 1 3) and (0 2 2) planes, correspondingly. The XRD spectrum evidently suggested the crystalline nature and purity of the CuO. All the diffraction peaks are identical to the mono-clinic phase structure. Further, it was cross checked with existing JCPDS data (80–1916) (Kuppusamy et al., 2017). No impurities other than CuO NPs were noticed in the XRD peaks representing the high phase purity. The average crystallite size of CuO NPs synthesized using 2, 5 and 10 ml juice and blank CuO were found to be 29.89, 23.61, 23.93 and 22.80 nm.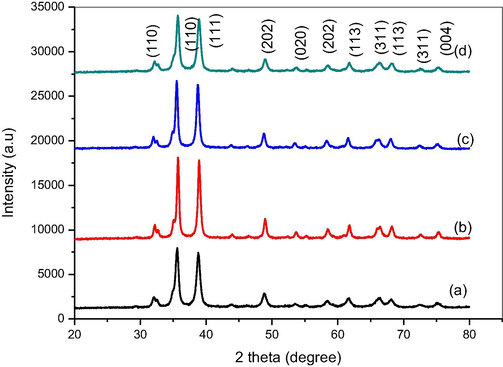
XRD pattern (a) CuO NPs (b) CuO NPs with 2 ml juice (c) CuO NPs with 5 ml (d) CuO NPs with 10 ml.
3.3 SEM and EDS study
Fig. 3A(a-b) show the SEM images of CuO synthesized without SCJ. As seen in the figure, SEM images showed lengthy particles with irregular shapes. The quantity of Cu and O confirmed by energy dispersive (EDS) study as shown in Fig. 3A(c). The SEM images and elemental analysis of Cu NPs synthesized using 2, 5 and 10 ml juice is shown in Fig. 3B(a-c), Fig. 3C(a-c) and Fig. 3D(a-c). The SEM images exhibited agglomerated particles as seen in the figure. Though the specific shapes were not observed from the SEM images, but it confirmed the formation of nanoparticles. The morphology of CuO NPs synthesized using 2 and 5 ml juice appeared to be similar in morphology. However, CuO NPs synthesized using 10 ml juice found to be spherical in shape. The EDS analysis revealed the percentage of Cu and O present in the samples. The Cu and O existed in the blank CuO found to be 75.66 and 15.25% (Fig. 3A(c). The CuO with 2 ml showed 55.54% of Cu and 44.46% of O (Fig. 3B(c). The percentage of C content was not observed in this sample. The elemental composition of CuO NPs synthesized using 5 and 10 ml showed C in addition to Cu and O. The percentage of Cu, O and C present in CuO with 5 ml juice found to be 56.60, 32.13 and 34.25% (Fig. 3C(c). The CuO NPs produced with 10 ml juice showed 63.91, 22.11 and 7.99% of Cu, O and C atoms (Fig. 3D(c). The C content must be derived from the sucrose present in the juice. The significant decrease in C content in CuO NPs synthesized using 10 ml juice is unclear. Because, all the samples were heat treated at 500 °C for 3 h.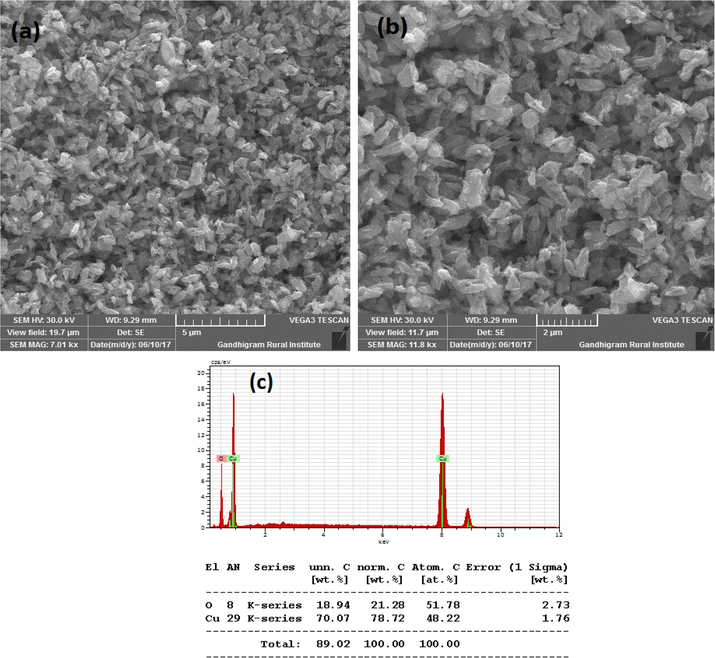
(a-c). SEM images and EDS of CuO NPs synthesized without juice.
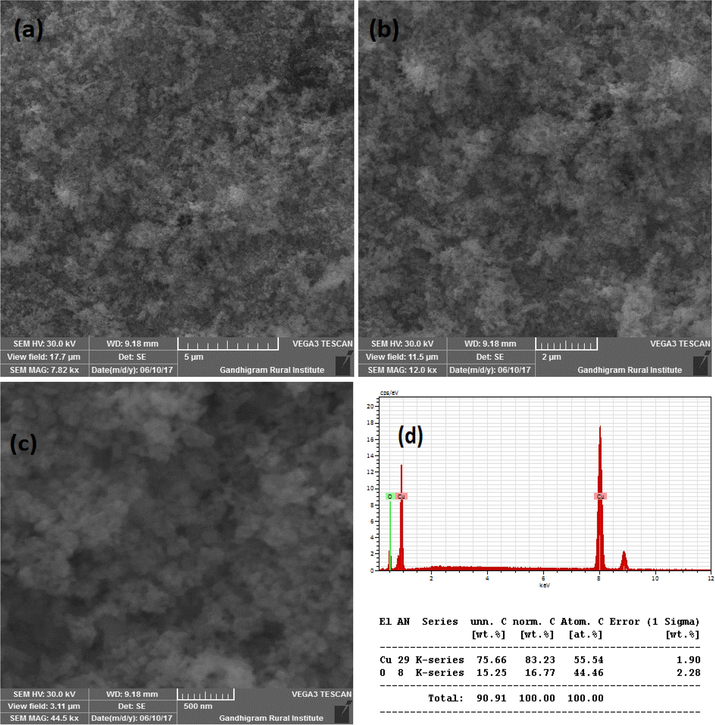
(a-d). SEM images and EDS of CuO NPs with 2 ml juice.
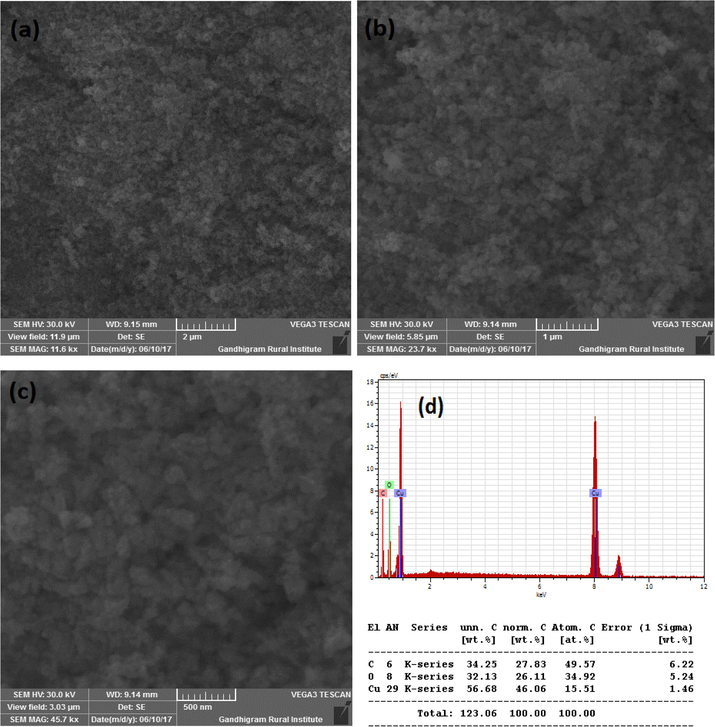
(a-d). SEM images and EDS of CuO NPs synthesized with 5 ml juice.
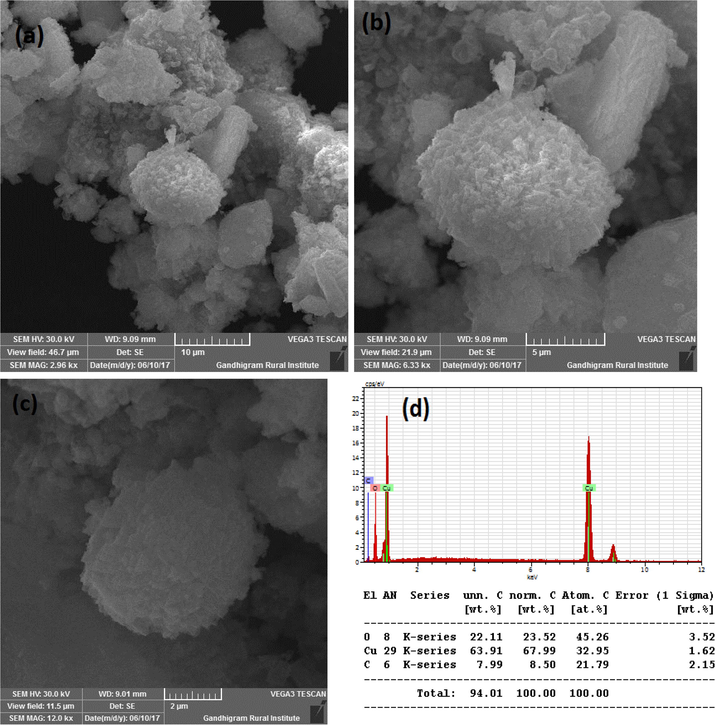
(a-d). SEM images and EDS of CuO NPs with 10 ml juice.
3.4 TEM analysis
As shown in Fig. 4A(a-d), TEM micrographs of CuO NPs synthesized without juice exhibited agglomerated particles. The average size of the particles calculated was 84.40 nm. It exhibited irregular shapes as seen in the images. The CuO NPs produced with 2 ml juice showed well separated particles. It exhibited spherical, square, cube, plate, rectangular with some irregular shapes when 5 ml of juice was used (Fig. 4B(a-f)). For 5 ml, nanoparticles showed similar shapes as shown by 2 ml juice (Fig. 4C(a-d)). The average size of the particles synthesized using 2, 5 and 10 ml of juice measured from the TEM images were 60.50, 40.40 and 29.50) nm, respectively. The gradual addition of the juice (2, 5 and 10 ml) rapidly reduced the size of the particles (60.50, 40.40 and 29.50 nm). The existence of inter-planar spacing of 0.23 nm of CuO with 2 and 5 ml representing the perfect orientation (2 0 0) of the nanoparticles. CuO NPs produced by 10 ml juice showed spherical shaped particles might be due to the attractive force between the molecules and the CuO NPs (Fig. 4D(a-f). It is well known that sugarcane juice contains glucose and fructose as major components and other organic acids as minor components. It has been proved that the sugar molecule and organic acids can act as a surface modifier, stabilizing and capping agents, which proved to control the size, shape and morphology (Sharma et al., 2015; Bhattacharjee and Ahmaruzzaman, 2016). The formation of CuO nanoparticles is proposed by the following mechanism as given in equations (1) and (2). Copper nitrate is reduced into copper hydroxide when ammonium hydroxide was added. In the presence of sugarcane juice, copper hydroxide was calcinated at 400 °C to form copper oxide. We believed that the interaction between glucose and fructose present in the sugarcane juice and copper hydroxide led to the formation of various shapes of particles. As the volume of juice increases, spherical shaped particles appeared predominantly as shown in (Fig. 4D(a-f). Stability of the colloids was monitored in the laboratory. After three months, particles settled down completely.
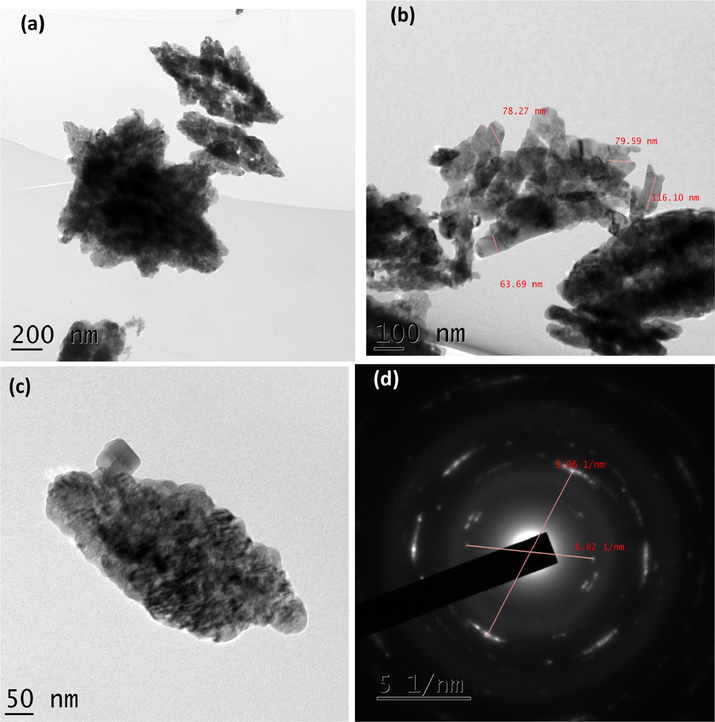
(a-d). TEM images and SAED pattern of CuO NPs.
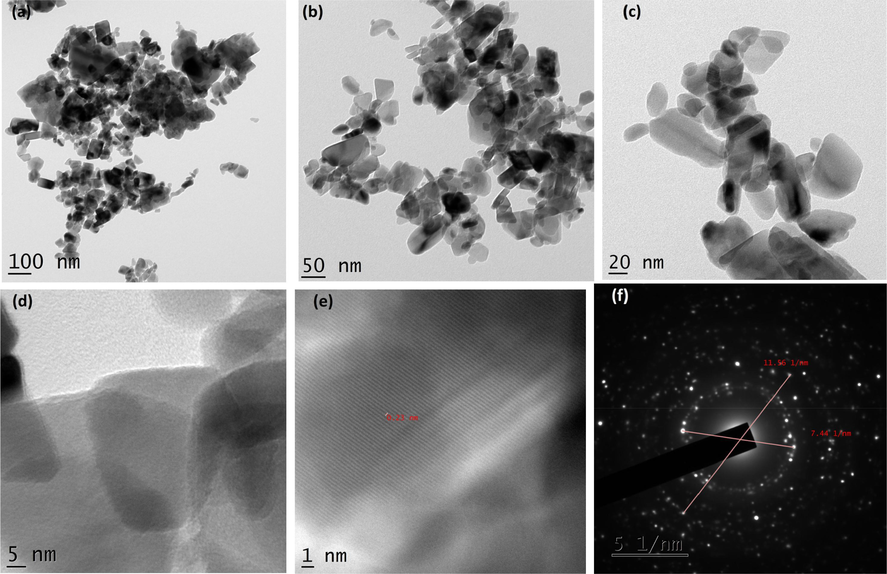
(a-f). TEM images and SAED pattern of CuO with 2 ml juice.
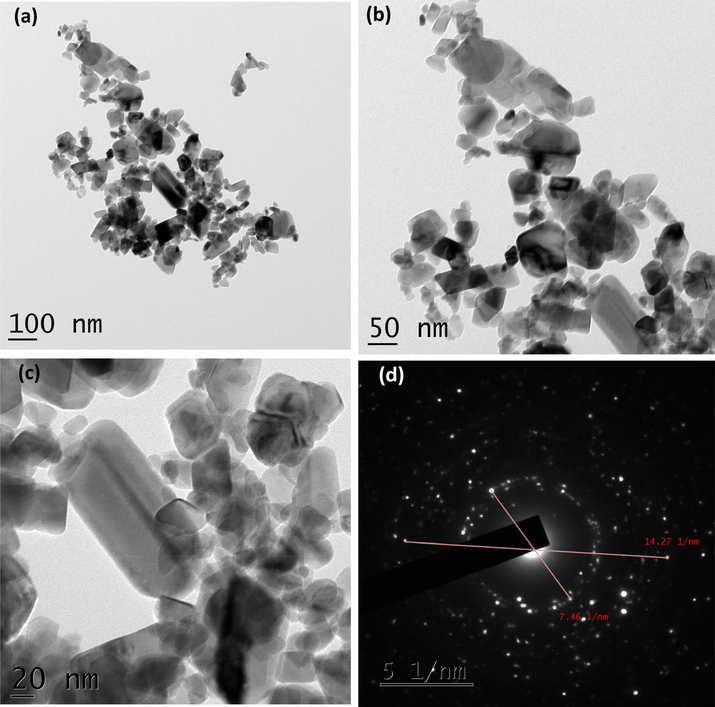
(a-d). TEM images and SAED pattern of CuO with 5 ml juice.
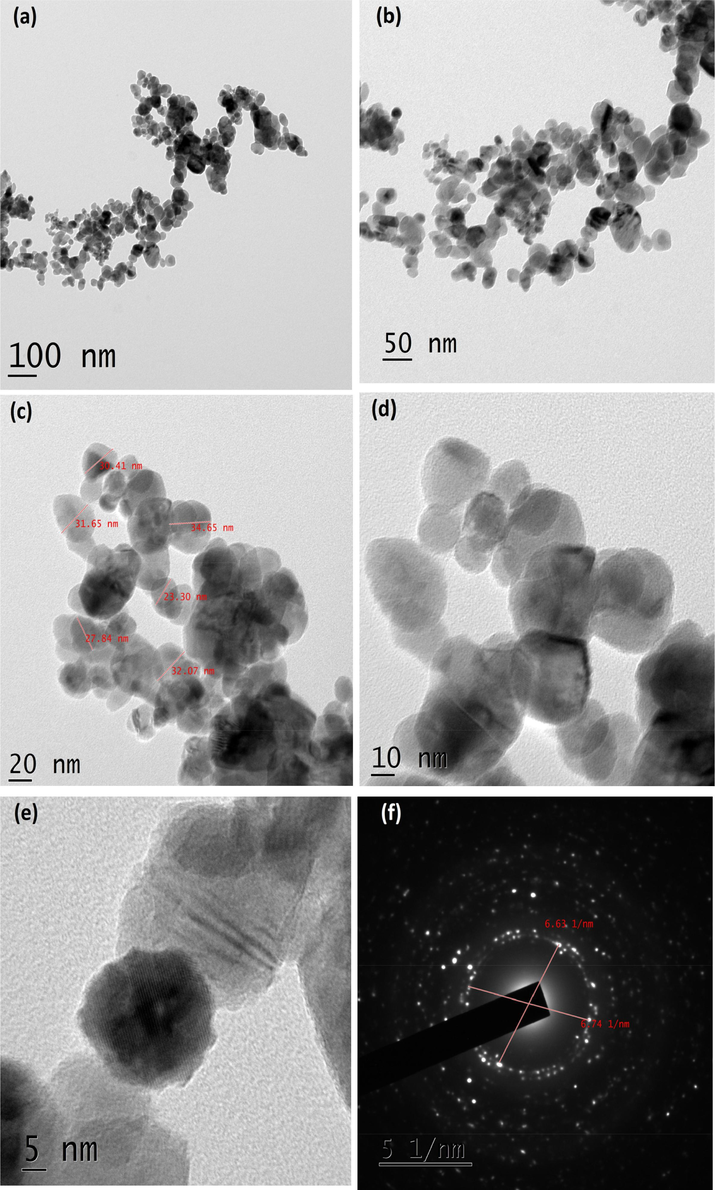
(a-f). TEM images and SEAD pattern of CuO NPs with 10 ml juice.
3.5 Raman spectra
Raman spectroscopy is a main tool to identify the local atomic arrangements and vibrations of the metal oxides, which helps to analyze the structural nature of the micro and nanoparticles. Raman spectra of plain CuO NPs and one CuO NPs synthesized using 10 ml of sugarcane juice are representative samples are shown Fig. 5(a,b). Raman spectra of pure CuO NPs showed peaks at 280, 332 and 616 cm−1. Sugarcane juice stabilized CuO NPs showed peaks at 285, 335 and 620 cm−1. The peak assignment at 280–285 cm−1 to the Ag mode of vibration, while the other two peaks at 332–335 and 616–620 cm−1 assigned to the Bg modes (Irwin et al., 1990). The oxygen atoms only shift with a dislocation in the b-direction for Ag and perpendicular to the b-axis for Bg Raman modes (Gan et al., 2004). It was proven that the Raman shift and bandwidth alter with decreasing the size of the particles (Swarnkar et al., 2011; Rashad et al., 2013).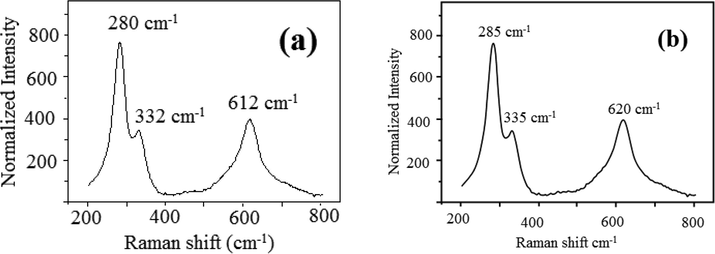
(a, b). Raman spectra (a) Pure CuO (b) CuO NPs with 10 ml juice.
3.6 XPS analysis
The survey scan of XPS for pure CuO and CuO NP synthesized using sugarcane juice is presented Fig. 6(a). The elements such as Cu, C, and O are all indexed. The peaks corresponding to Cu 3d, 3p, 3s, 2p and Auger, O 1s are apparently noticed. C 1s peak is also noticed. Peak for argon is not found, revealing that no argon present in the samples. The XPS spectra consequent to Cu 2p and O 1s are shown in Fig. 6 (a-d). The binding energy of 283.47 eV reveals the C 1s peak. The relevant major peaks of Cu 2p3/2 for pure CuO and CuO NPs synthesized using 2, 5 and 10 ml of juice lie at 932.17 and 941.63 eV. Fig. 6 (a-d) with well-built fitting 952.98 and 961.58 eV for Cu 2p1/2 (Gao et al., 2010). The calculated gap between the Cu 2p3/2 and Cu 2p1/2 is 19.95 eV, which is in concurrence with the normal value of 20.0 eV for CuO (Lin et al.,2004). This gives strong evidence of an 3d9 shell resultant to the Cu+ state. Further, Fig. 6 (a-d) shows the XPS spectra of O 1 s. Also, a peak observed at lower binding energy region is assigned to O2− of CuO nanoparticles. A major peak at the lower peak at 529.4 eV is ascribed to Cu–O (Nagajyothi et al., 2017).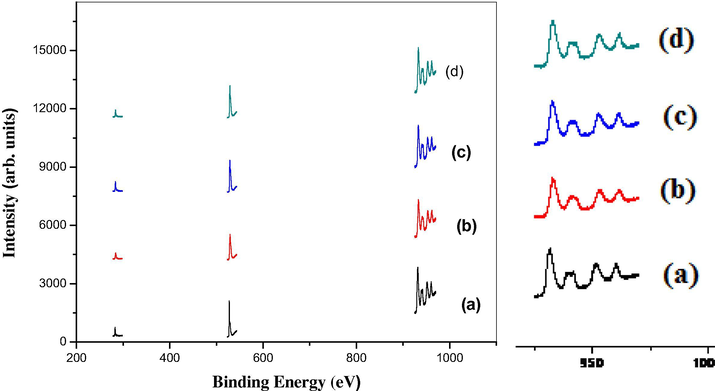
(a-d). XPS spectr (a) Pure CuO, (b) CuO with 2 ml, (c) CuO with 5 ml, (d) CuO with 10 ml juice.
3.7 Bactericidal activity
25 μg of Ciprofloxacin and 100 µg of CuO NPs were used to test the bactericidal activity.
The zone of inhibition shown by CuO NPs synthesized using 10 ml juice against Escherichia coli, Pseudomonas aeruginosa, Staphylooccus aureus and bacillus subtilis was 5, 8 and 9 mm (Fig. 7(A-D)). All bacteria showed more or less inhibition, especially 10 ml juice. Whereas, 2 and 5 ml juice stabilized CuO NPs showed 5, 7 and 9 mm zone of inhibition. The zone of inhibition of shown by the NPs is comparable (12 mm) with reference compound (Ciprofloxacin).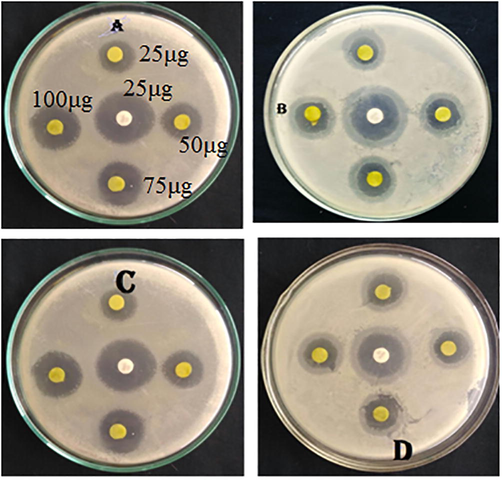
(A-D). Antibacterial activity of CuO NPs: Zone of inhibition of (A) Escherichia coli (B) Pseudomonas aeruginosa (C) Staphylooccus aureus and (D) bacillus subtilis.
Various mechanisms have been widely proposed by the researcher for the antibacterial activity of the nanoparticles. Penetration of smallest particles into the cell walls of the bacteria and binding of nanoparticles with the nucleic acids of bacteria also causes severe toxic (Elango et al., 2017). It is well known sugarcane juice contains monosaccharides (glucose and fructose), doscaccharides (sucrose), minerals and amino acids. According to literature, sugarcane juice contains 81–87% and 3–6% of reducing sugars and remaining constituents includes minerals and amino acids (Walfors, 1996). The presence of sugar moieties was confirmed by FT-IR spectrum of sugarcane juice. Various plant extracts mediated green synthesis of CuO NPs and their size including morphology from the literature is presented in Table 1. The green synthesis of CuO NPs using aqueous extract of various parts of plant extract revealed the spherical shaped particles. Range of size starting from 5 nm to 140 nm of CuO NPs fornd in literature (Table 1). We obtained spherical shaped CuO NPs at higher concentration. At lower concentration, in addition to spherical, various shapes such as square, cube, plate, rectangular with some irregular shapes obtained. The size and shapes of the CuO NPs synthesized using sugarcane was comparable with literature.
S. No
Name of the plant
Size of the Particle (nm)
Shape and Morphology
References
1.
Caloropis procera
46
cylindrical
Rayapa Reddy, 2017
2.
Oak Fruit Hull
34
quasi-spherical
Sorbiun et al., 2018
3.
Syzygium alternifolium
13–17
spherical
Yugandhar et al., 2017
4.
Gloriosa superba L
5–10
spherical
Raja Naika et al., 2015
5.
Solanum tuberosum
54
spherical
Alishah et al., 2016
6.
Banana peel
60
Spherical
Aminuzzaman et al., 2017
7.
Dry black beans
26
Spherical
Nagajyothi et al., 2017
8.
Aloe vera leaf
20
Spherical
Vijay Kumar et al., 2015
9.
Bauhinia tomentosa
22–40
Spherical
Sharmila et al., 2018
10.
Azadirachta indica
48
Cubical
Nagar and Devra, 2018
11.
Punica granatum
40
Spherical
Ghidan et al., 2016
12.
Rheum palmatum L
30
Spherical
Bordbar et al., 2016
13.
Chamomile flower
140
Sphere
Fatih Duman et al., 2016
4 Conclusions
The present study report the eco-friendly and convenient greener route synthesis of CuO nanoparticles using SCJ as a stabilizing agent. FTIR and XRD pattern of the samples proved the formation of CuO NPs and their crystalline structure. The SEM images confirmed formation of particles in nanoscale. The TEM images confirmed the existence spherical, square, cubic and rectangular shaped nanoparticles at lower concentrations. Sugarcane juice rapidly reduced the size of the particles in higher concentration. In addition, the above mentioned various shapes of particles disappeared at higher concentrations and produced spherical shaped particles. This work proved that the sugarcane is a suitable green stabilizing agent to produce copper oxide nanoparticles. Further, formation CuO NPs supported by the Raman spectra and XPS analysis. Synthesized CuO NPs exhibited significant bactericidal activity against Escherichia coli, Streptococcus aureus, Pseudomonas aeruginosa and bacillus subtilis. Synthesis of well dispersed copper oxide nanoparticles suggesting that the sugarcane juice acting as a capping well as stabilizing agent and put off the agglomeration, and provides stability to medium
Conflict of interest
No conflict of interest declared by the authors regarding this manuscript.
References
- Green synthesis of copper oxide (CuO) nanoparticles using banana peel extract and their photocatalytic activities. AIP Conference Proceedings. 2017;1828:020016
- [Google Scholar]
- Copper oxide nanomaterials: synthesis, characterization and structure-specific antibacterial performance. Chem. Eng. J.. 2015;262:179-188.
- [Google Scholar]
- Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J. Nanomater.. 2014;5:519-524.
- [Google Scholar]
- Green synthesis of starch-mediated CuO nanoparticles: preparation, characterization, antimicrobial activities and in vitro MTT assay against MCF-7 cell line. Rend. Fis. Acc. Lincei. 2017;28:65-71.
- [Google Scholar]
- CuO nanostructures: facile synthesis and applications for enhanced photodegradation of organic compounds and reduction of pnitrophenol from aqueous phase. RSC Adv.. 2016;6:41348-41363.
- [Google Scholar]
- Processing and characterization of composites of poly (3-hydroxybutyrateco-hydroxyvalerate) and lignin from sugar cane bagasse. J. Compos. Mater.. 2012;46:417-425.
- [Google Scholar]
- Low-temperature solution synthesis of CuO nanorods with thin diameter. Mater. Lett.. 2013;93:60-63.
- [Google Scholar]
- Synthesis of copper oxide nanoparticles by a novel method and its application in the degradation of methyl orange. Adv. Electr. Electron. Eng.. 2014;4:83-88.
- [Google Scholar]
- Synthesis and optical properties of colloidal CuO nanoparticles. J. Lumin.. 2014;151(2014):149-154.
- [Google Scholar]
- Chamomile flower extract-directed CuO nanoparticle formation for its antioxidant and DNA cleavage properties. Mater. Sci. Eng. C. 2016;60:333-338.
- [Google Scholar]
- Synthesis, structural characterization and antimicrobial activities of polyindole stabilized Ag-Co3O4 nanocomposite by the reflux condensation method. Mater. Chem Phys.. 2018;216:305-315.
- [Google Scholar]
- Synthesis, characterization of polyindole/Ag-ZnO nanocomposites and its antibacterial activity. J. Alloys Compds.. 2017;696:391-401.
- [Google Scholar]
- 2-Imino-(3,4-dimethoxybenzyl) ethanesulfonic acid Schiff base anchored silver nanocomplex mediated by sugarcane juice and their antibacterial activities. J. Appl. Res. Technol.. 2016;14:38-46.
- [Google Scholar]
- Application of a new method for absorption correction in high-accuracy, quantitative EDX microanalysis in the TEM: analysis of oxygen in CuO-based high-Tc superconductors. Ultramicroscopy. 1993;50:189-201.
- [Google Scholar]
- Evaluation of composition, characterization and enzymatic hydrolysis of pretreated sugar cane bagasse. Brazilian J. Chem. Eng.. 2015;32:23-33.
- [Google Scholar]
- Preparation and characterization of copper oxide thin films deposited by filtered cathodic vacuum arc. J. Phys. D: Appl. Phys.. 2004;37(2004):81-85.
- [Google Scholar]
- Vacancy-mediated magnetism in pure copper oxide nanoparticles. Nanoscale Res. Lett.. 2010;5:769-772.
- [Google Scholar]
- Green synthesis of copper oxide nanoparticles using Punica granatum peels extract: effect on green peach Aphid. Environ. Nanotechnol. Monit. Manage.. 2016;6:95-98.
- [Google Scholar]
- Treating of palm oil mill effluent using Commelina nudiflora mediated copper nanoparticles as a novel biocontrol agent. J. Clean. Prod.. 2017;141:1023-1029.
- [Google Scholar]
- Synthesis and antibacterial activity of CuO nanoparticles suspension induced by laser ablation in liquid. Arab. J. Sci. Eng.. 2016;41:301-310.
- [Google Scholar]
- Electrochemical synthesized copper oxide nanoparticles for enhanced photocatalytic and antimicrobial activity. J. Ind. Eng. Chem.. 2015;31:173-184.
- [Google Scholar]
- Characterization of copper oxide nanoparticles fabricated by the sol-gel method. J. Electron. Mater.. 2015;44:3704-3709.
- [Google Scholar]
- Ag@AgCl nanomaterial synthesis using sugarcane juice and its application in degradation of azo dyes. ACS Sustain. Chem. Eng.. 2014;2:1007-1013.
- [Google Scholar]
- Characterizing well-ordered CuO nanofibrils synthesized through gas-solid reactions. J. Appl. Phys.. 2004;95:5889-5895.
- [Google Scholar]
- Subchronic and chronic developmental effects of copper oxide (CuO) nanoparticles on Xenopus laevis. Chemosphere. 2015;135:166-174.
- [Google Scholar]
- Green synthesis: in-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem.. 2017;10:215-225.
- [Google Scholar]
- Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater. Chem. Phys.. 2018;213:44-51.
- [Google Scholar]
- Sugarcane juice facilitated eco-friendly synthesis of solar light active CdFe2O4 nanoparticles and its photocatalytic application. Eur. Phys. J. Plus. 2018;133:229.
- [Google Scholar]
- Silver based citosan bionanocomposite using stem extract of Saccharum officinarum and assessment of its antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol.. 2017;8:035019
- [Google Scholar]
- Short-term effects on antioxidant enzymes and long-term genotoxic and carcinogenic potential of CuO nanoparticles compared to bulk CuO and ionic copper in mussels Mytilus galloprovincialis. Mar. Environ. Res.. 2015;111:107-120.
- [Google Scholar]
- Evolution of size, morphology, and magnetic properties of CuO nanoparticless by thermal annealing. J. Appl. Phys.. 2009;105:093901
- [Google Scholar]
- Green synthesis, morphological and optical studies of CuO nanoparticles. J. Mol. Struct.. 2017;1150:553-557.
- [Google Scholar]
- Preparation, characteristics and photovoltaic properties of cuprous oxide-a review. Solid State Electron.. 1986;29:7-17.
- [Google Scholar]
- CuO and Co3O4 nanoparticles: synthesis, characterizations, and Raman spectroscopy. J. Nanomater.. 2013;2013 Article ID 714853, 6
- [Google Scholar]
- Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J. Taibah Univ. Sci.. 2015;9:7-12.
- [Google Scholar]
- Characterisation of copper oxide nanoparticles for antimicrobial applications. Int. J. Antimicrob. Agents. 2009;33:587-590.
- [Google Scholar]
- Investigating on photocatalytic performance of CuO micro and nanostructures prepared by different precursors. Desalin. Water Treatment. 2016;57(2016):6723-6731.
- [Google Scholar]
- Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys Compds.. 2015;632:321-325.
- [Google Scholar]
- Biogenic synthesis of CuO nanoparticles using Bauhinia tomentosa leaves extract: characterization and its antibacterial application. J. Mol. Struct.. 2018;1165:288-292.
- [Google Scholar]
- Synthesis and characterization of mono sized CuO nanoparticles. Mater. Lett.. 2012;81:169-172.
- [Google Scholar]
- Effect of aging on copper nanoparticles synthesized by pulsed laser ablation in water: structural and optical characterizations. Bull. Mater. Sci.. 2011;34:1363-1369.
- [Google Scholar]
- Green synthesis of zinc oxide and copper oxide nanoparticles using aqueous extract of oak fruit hull (Jaft) and comparing their photocatalytic degradation of basic violet 3. Int. J. Environ. Res.. 2018;12:29-37.
- [Google Scholar]
- Green synthesis and characterization of copper oxide nanoparticles using coffee powder. J. Nanostruct.. 2016;6:167-171.
- [Google Scholar]
- Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed.. 2013;8:889-898.
- [Google Scholar]
- Green synthesis of copper oxide nanoparticles using Aloevera leaf extract and its antibacterial activity against fish bacterial pathogens. BioNanoSci.. 2015;5:135-139.
- [Google Scholar]
- Walfors, S.N., 1996. Composition of cane juice. Proc.S. Afr. Sug. Technol. Ass, 70.
- CuO microflowers composed of nanosheets: synthesis, characterization, and formation mechanism. Mater. Res. Bull.. 2007;42:1723-1731.
- [Google Scholar]
- Synthesis and microwave modification of CuO nanoparticles: crystallinity and morphological variations, catalysis, and gas sensing. J. Colloid Interface Sci.. 2014;435(435):34-42.
- [Google Scholar]
- Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and activity. Appl. Nanosci.. 2017;7:417-427.
- [Google Scholar]
- Fabrication of CuO/C films with sisal-like hierarchical microstructures and its application in lithium ion batteries. J. Alloys Compds.. 2009;476:408-413.
- [Google Scholar]
- Photocatalytic-degradation and reduction of organic compounds using SnO2 quantum dots (via green route) under direct sunlight. RSC Adv.. 2015;5:66122-66133.
- [Google Scholar]
- CuO nanostructures: synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci.. 2014;60:208-337.
- [Google Scholar]







