Translate this page into:
Structural and optical properties of transition metal ion-doped ordered mesoporous TiO2 prepared by chelating-assisted evaporation induced self-assembly method
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This paper demonstrates the fabrication of highly ordered mesoporous titanium dioxide (OMT) with different transition metals (TM: Fe, Mn, and Ni) by employing a self-assembly surfactant template route. The ordered TM-loaded mesoporous TiO2 (TM-OMT) has uniform mesopores (3–5 nm pore diameter), and a crystalline framework is successfully prepared via soft template strategy by employing Pluronic® F127 triblock copolymer as a chelating and structural directing agent. The structural and optical characteristics of the TM-OMT hybrids have been examined by scanning electron microscopy (SEM), transmission electron microscope (TEM), XRD, N2-sorption isotherms, UV/visible diffuse reflectance spectroscopy, and X-ray photoelectron spectroscopy (XPS) reveals the successful incorporation of TM ions over the highly ordered mesostructured OMT. The XRD pattern evidenced that the obtained TM-doped OMT samples are pure anatase of the crystalline phase. By employing TEM analysis, the average particle size is estimated to be 2–2.7 nm, whereas diffraction patterns have evidenced the polycrystalline features of the mesoporous structure. The bandgap energy of TM-doped mesoporous decreases according to the particular metal and its amount due to the reduced particle size. This cost-effective method provides the exceptional potential to tune the optical features for TM-doped mesoporous TiO2 materials for its optoelectronic applications and show more significant promise for energy conversion technologies.
Keywords
Mesoporous
Titanium dioxide
Transition metal oxide
Electrocatalysis
Self-assembly
1 Introduction
The energy crisis has been a global concern today, and the advancement and exploitation of innovative energy sources represented by solar energy and hydrogen energy are impending. Since the discovery by Fujishima & Honda using rutile titanium dioxide (TiO2) materials for the H2 generation from photocatalytic water splitting (Fujishima and Honda, 1972). TiO2 is recognized as the most widely used material and a favorable candidate for dye-sensitized solar cells (DSSCs), polymer solar cells, heterojunctions-based solar cells, and photocatalytic applications (Fujishima et al., 2000). Particularly, DSSCs are a promising field of material sciences research due to their easy manufacturing, cost-effectiveness, and good photo-conversion efficiency (Li and Liu, 2010). More importantly, mesoporous TiO2 has received considerable recognization due to its great surface area (Calleja et al., 2004) and porosity (Zukalova et al., 2005). The high efficacy nano-structured cells such as DSSCs (Ahmad et al., 2017), hybrid SCs (Even et al., 2013), quantum dot SCs (Nozik, 2002) and hypro-organic plumage halide perovskites (PSCs) (Chung et al., 2016) have been produced by this structural framework.
Ordered mesoporous metal oxides tend to possess outstanding properties, ranging from an unusually high surface area, an easily adjustable pore size, high porosity, and large pore volume (Wagner et al., 2013). Mesoporous–templated TiO2 materials were first published by Antonelli et al. in 1995 (Antonelli and Ying, 1995). Afterward, several routes have been employed to manufacture mesoporous TiO2 with different mesophase structures such as 2D hexagonal, 3D cubic, and lamellar structure, involving sol–gel, solvothermal, sonochemical and hydrothermal approaches (Yu et al., 2007; Wang et al., 2004). Particularly, Soft-template methods of evaporation-induced self-assembly (EISA) methods built on amphiphilic block copolymers are effective and beneficial to fabricate mesoporous materials with different morphological features and tunable pore constructions and have the potential to apply on the commercial scale (Jo et al., 2013).
The metal doping in TiO2 materials helps the visible wavelength's intrinsic features with enhanced photoresponse (Xie et al., 2013; Liu et al., 2013). Numerous research works have been dedicated to metal doping over the past decades over mesoporous TiO2 (Reda et al., 2020; Khojasteh et al., 2016). Particularly, Ye et al. successfully fabricated the cobalt-doped mesoporous TiO2 materials via the EISA process, resulting in an enhanced optical feature (Wang et al., 2015). Also, Li et al. (Li et al., 2017) have developed Co-doped ordered mesoporous TiO2 for sensor applications using the EISA process. Earlier studies demonstrated that organic and inorganic species' co-assembly can be well-governed by applying ligands such as acetylacetone and acetic acid (Wang et al., 2015; Li et al., 2017). Enlightened by these research works, we used a soft-templating route to fabricate the highly ordered and crystalline (Fe, Ni, and Mn) doped mesoporous TiO2 by employing tetra butyl titanate as Ti precursor and the pluronic F127 as the template. We use acetic acid as a chelating agent through the EISA method to regulate the hydrolysis and condensation of TiO2 precursor by steadying the hydrolyzed Ti nanoentities through the chelation process. The optical, structural and morphological characterization of the obtained samples have been elucidated in detail.
2 Experimental details
2.1 Materials
Non-ionic surfactant Pluronic F127 (Mw = 12600, PEO106PPO70PEO106) and Titanium n-butoxide (Ti(OBu)4, 99%) were obtained from Aldrich Corp.
2.2 Synthesis of TM doped TiO2 mesoporous
The crystalline ordered TM-doped TiO2 mesoporous were obtained by chelating assisted EISA method in an ethanolic/Pluronic F127/HCl/acetic acid (HOAc)/Ti(OBu)4 mixed solution mixed through the template-carbonization approach. Typically, 2.0 g of pluronic F127 was dispersed in 20 ml of anhydrous ethanol and 2.4 g of conc. HCl (37%). Afterward, the obtained mixture was stirred continuously to create a transparent solution. Sequentially, 3.4 g of Ti(OBu)4 and 2.4 g of acetic acid was introduced with a constant stirring process to attain a homogeneous greenish-yellow solution and then transported to Petri dishes. It was then subjected to solidifying materials at 100 °C for 24 h to eradicate the solvents. The obtained green film was then annealed with a ramping rate of 1 °C min−1 to 350 °C in N2 and detained for 3 h, subsequent in the carbon-supported amorphous TM-doped TiO2 powders. Finally, the supported-carbon was removed and obtained TiO2 powder crystallization acquired through successive heat treatment with a ramp rate of 1 °C min−1 to 400 °C in the air for 3 h. The obtained products were assigned as M (x)-OMT, where M refers to the doping metal and x corresponding to the molar ratios of M/Ti in the synthetic process.
2.3 Material characterizations
XRD patterns were logged with a Bruker-Advance D8 instrument. The optical absorption spectra of materials were analyzed via spectrophotometer (Shimadzu UV-2600). TEM photographs were performed with a JEM- 2100F microscope (Japan). Nitrogen sorption isotherms were assessed via a NOVA 2200e analyzer. The specific surface areas of the mesoporous TiO2 materials were estimated by the Brunauer–Emmett–Teller (BET) approach.
3 Results and discussion
3.1 Synthesis of highly ordered TM-doped mesoporous TiO2
Fig. 1 presents the designed schematic way for the chelating-assisted soft-templating procedure to fabricate highly ordered mesoporous TM-doped TiO2 (TM/OMT). Firstly, the pluronic F127 was applied as a structural-directing agent, the acetic acid as a chelating agent, and the metals nitrate salts, TOBT as metal and Ti precursor, respectively (the details are presented in Supplementary Table S1). The F127 surfactant co-assembles with the acetic acid-stabilized Ti precursor to form a composite through the evaporation process. Subsequently, the hydrophilic PEO segments of F127 with associated metal salts precursor were co-assembled into rod-like micelles in an ethanolic medium. After solidification by treating at 100˚C for 24 h, the subsequent composite materials were rubbed and crushed into a powder form, which was then treated under annealing process at 350 °C in N2 for 3 h, and 400 °C in the air for 3 h.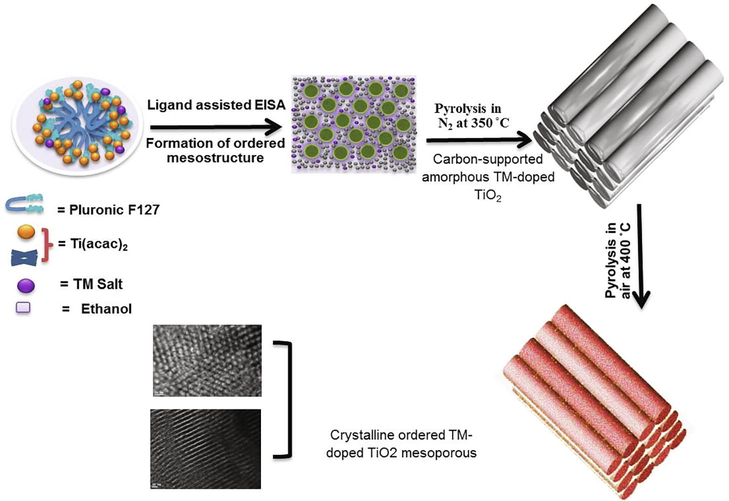
Schematic representation of the formation process for chelating-assisted soft-template fabrication of highly ordered TM-doped mesoporous TiO2.
The photographs in Fig. 2a show a sequence of TM-doped OMT materials with dissimilar colors that could be obtained by altering the molar ratio of transition metals salt to titanium source through the self-assembly template process. As can be seen theirs a substantial color changes in the pure OMT caused by metal doping. The TM-doped OMT materials exhibit different colors for each dopant: brownish color for Mn/OMT, brownish-yellow for Fe/OMT, and yellow for Ni/OMT materials. Fig. 2b displays the absorption spectra of the bare-OMT and TM-doped OMT samples. The equivalent Tauc plots of the obtained materials and their band gaps are presented in Fig. 2c and Table 1, demonstrating that the band gaps of the TM-doped OMT can be tailored by varying of TM/Ti molar ratio. The assessed energy bandgap of the bare and doped OMT materials with various transition metals of Fe, Mn, and Ni are 2.59, 2.43, and 2.80 eV, correspondingly. The energy bandgap is witnessed to reduction with a TM-doping. It is worthy to note that the materials' bandgap is definitely tuned to an exact level to choose different TM-ions into host OMT materials; thus, red-shifting of optical absorption edge from UV to the visible region is witnessed. Obviously, the bare OMT materials exhibit a sharp absorbance at 400 nm, which can be assigned to the electronic transition from the valence band (VB) to the CB, corresponding to the bandgap (∼3.09 eV) of bare OMT. However, the transition metal doping over OMT materials makes absorption above a wider range of the visible-light area with the broad absorption maxima at about 500 nm (Fe-OMT), 550 nm (Mn-OMT), and 480 nm (Ni-OMT). The enhancement in the absorption owing to the positive shift in the CB edge validates the TM-doped OMT's reduced bandgap and, thereby, a red-shift in light absorption (Liu et al., 2018).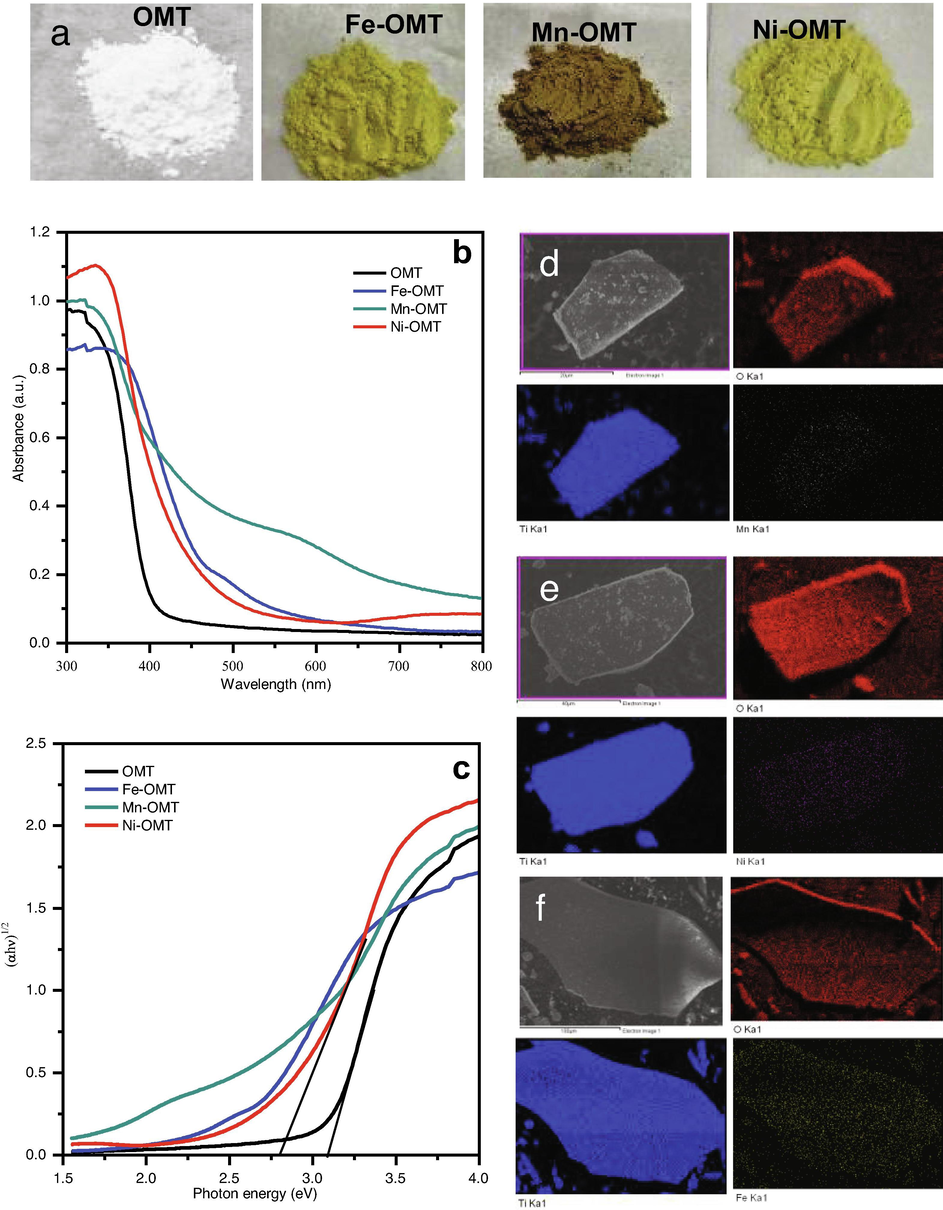
Photographs are showing the color changes of OMT upon 4% molar ratio (M/Ti) Mn, Fe, and Ni doping (a) UV–visible absorbance spectra of TM-doped OMT. (b) and (c) its corresponding tauc plots, elemental EDAX mapping images of Mn/OMT (d), Ni-OMT (e), and Fe-OMT (f) materials prepared by self-assembly template route.
Samples
Crystal phase
XRD Crystallite (grain) Size
SBET (m2/g)
Pore volume (cm3/g)
Pore size (nm)
Band gap eV
OMT
Anatase/TiO2
6.97
218
0.33
2.65
3.09
Fe-OMT
Anatase/TiO2
6.59
260
0.35
2.44
2.59
Mn-OMT
Anatase/TiO2
7.16
249
0.25
2.12
2.43
Ni-OMT
Anatase/TiO2
6.80
259
0.30
2.67
2.80
Moreover, energy-dispersive X-ray spectroscopy (EDX) mapping was applied to examine the controllability and elemental dispersion of the TM dopant profile, as presented in Fig. 2(d,e,f). The TM-doping percentages in the fabricated materials were evaluated by EDX spectroscopy measurements (Fig. S1). The EDX mapping of TM-doped OMT samples shows the even distribution of the elements of Ti, Fe, Mn, and Ni on the OMT materials, exhibiting the uniform deposition of the TM dopants on the OMT framework.
The surface morphologies, lattice spacing, and porous structure of TM-doped OMT samples were directly observed by TEM and HRTEM analysis, and the results are presented in Fig. 3. The low magnification TEM images show that all the TM-doped OMT samples tend to have a highly-ordered mesoporous structure by possessing a pore size of (2–2.7 nm) and an identical shape to the undoped OMT (Fig. 3 a,b). All the TM-doped OMT materials display the uniform arrangement of nanostructured particles with its fine crystalline areas. Further, the uniform fringes can be evidently witnessed in the photographs of Fig. 3(a-e) agreeing to the bare and TM-doped OMT materials, which look like that the OMT materials exhibited highly crystalline in nature. The degree of crystallinity of the obtained anatase phase matches the premier intensity peak noticed in the diffraction pattern. The lattice fringes spacings were assessed to be (0.29, 0.32, and 0.35) agreeing to (0 0 1),(1 0 0), and (1 0 1) planes of anatase TiO2, respectively (Nguyen et al., 2011). The HRTEM images show that all the TM-doped TiO2 (Fig. 3c–e) are crystalline with the same lattice fringe spacing as the pure anatase OMT, suggesting that the lattice of OMT doesn’t change after TM doping. The crystallinity of the Fe-OMT materials was examined by pattern and depicted in Fig. 3f. The ring pattern observed in HR-TEM approves polycrystalline anatase features of TM-doped materials with brighter spots demonstrating the greater crystallinity of the Fe-doped-OMT with (1 0 1) plane. The SAED pattern recorded on the shell displays well-definite diffraction rings and spots (Fig. 3f inset), further confirming a crystalline anatase shell.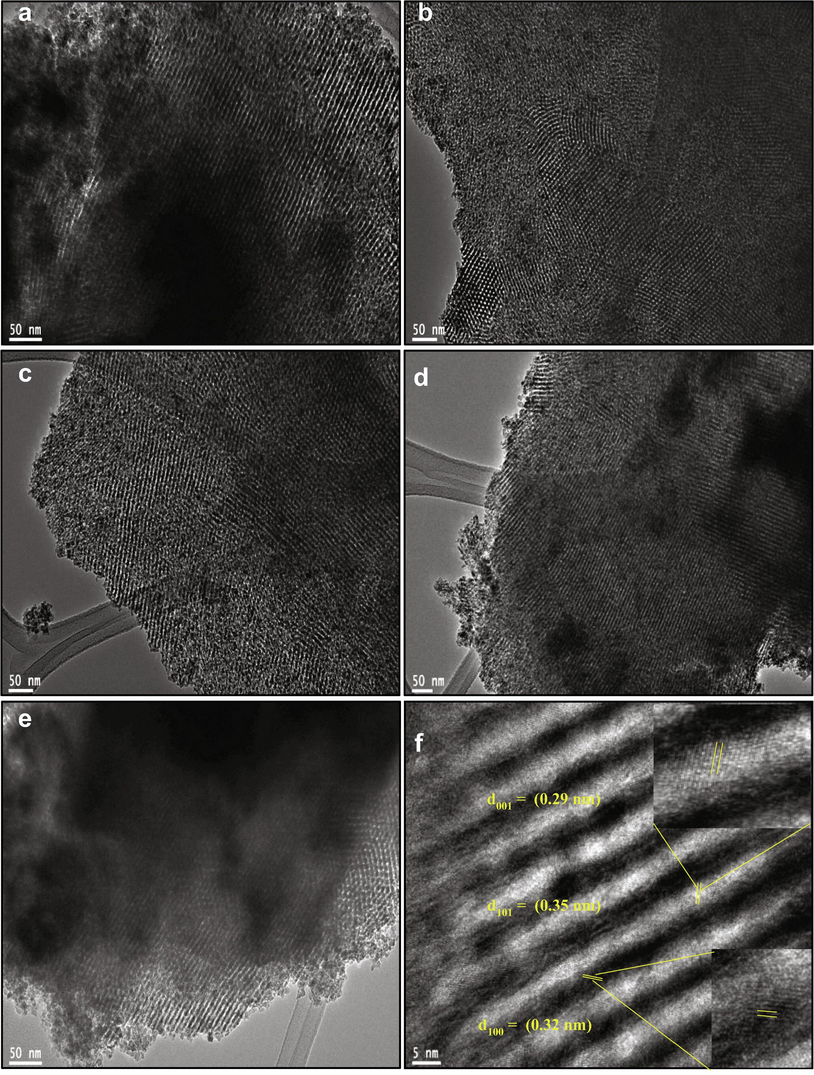
TEM images of (a, b) OMT, (c) Fe-OMT, (d) Ni-OMT, (e) Mn-OMT, (f) High-resolution TEM image of Fe-OMT.
In order to further confirm the influence of the doped amount of TM on the crystalline features of TiO2 was studied by XRD and N2 sorption isotherms. The XRD spectra of TM-doped OMT samples are displayed in Fig. 4. The obtained diffraction patterns evidenced that the TM (Fe, Mn and Ni) doped OMT samples have a greatly crystalline anatase TiO2 phase (JCPDS card No. 01–086 –1157; Space group I41/amd) and have peaks that are identical to those of pure anatase OMT materials and have no distinct metal or metal oxide on the OMT surface. The assessed crystallite sizes of the OMT, Fe-OMT, Mn-OMT, and Ni-OMTsamples are 6.97, 6.59, 7.16, and 6.80 nm corresponding to the XRD patterns shown in Fig. 4a, respectively. These results suggest that a homogeneous dispersion of metal dopants without varying the crystalline features of TiO2. The enlarged (1 0 1) diffraction peak of the pure TiO2, TM doped/OMT materials indicates no shift in 2θ values, and the (1 0 1) diffraction feature in all the OMT materials is evidenced to be strongly specifying the major crystal growth beside the same plane of anatase TiO2 (Roy et al., 2013) (Fig. 4b). Also, there are no additional specific peaks of phases observed in the TM-doped samples, evidencing no existence of doping-related elements like metal oxides. Thus, the nonexistence of any additional peaks in XRD patterns reveals the occupancy of TMs to the major sites of the TiO2 lattice.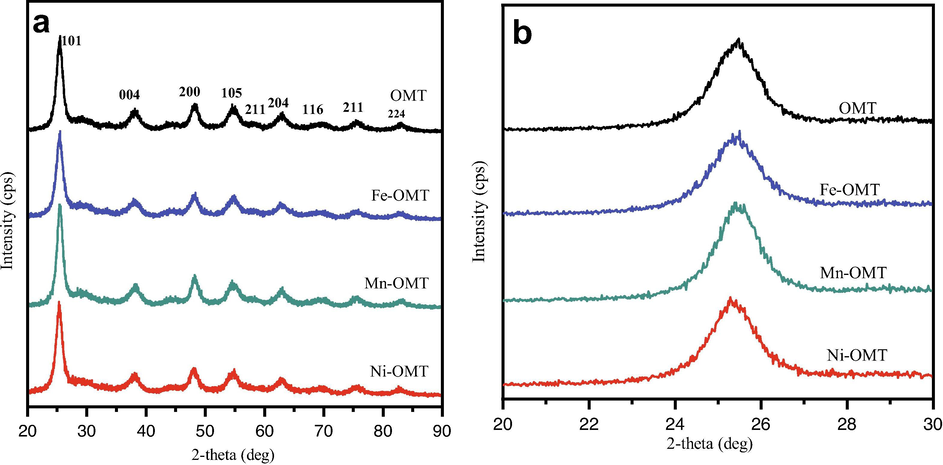
(a) Wide-angle XRD pattern of the pure OMT and TM-OMT samples, (b) The enlarged (1 0 1) diffraction peak of the pure OMT, and TM–OMT samples.
The textural features of obtained OMT and TM-OMT materials were examined by the N2-physisorption method (Fig. 5a, b). The N2 sorption isotherm of all the OMT samples reveals distinguishing type-IV curves and H1 hysteresis loops with capillary condensation steps, typical of the mesoporous materials agreeing with IUPAC classification (Kruk and Jaroniec, 2001). According to the TEManalysis shown in Fig. 5a, the discrete capillary condensation at P/P0 = 0.4–0.8 signifies the existence of cylindrical mesoporous pores. The OMT and TM-OMT samples' PSDwas estimated from the adsorption data using the BJH method (Fig. 5b). Furthermore, the textural parameters, namely specific surface area, mesopore volume, and size of OMT, TM-OMT, and Co-OMT materials gotten from the N2 sorption isotherms and BJH method (Fig. 5b) are shown in Table 1. All the TM/OMT samples had exceptional textural features with a specific surface area (218–260 m2 g1 for TM-OMT), big pore volume (0.2–0.35 cm3 g−1), and uniform pore size (2–2.7 nm). The introduction of transition metals into the OMT leads to a reduction in the surface area. In contrast, compared with pure OMT, the surface area of TM-OMT materials was improved with the introduction of transition metals.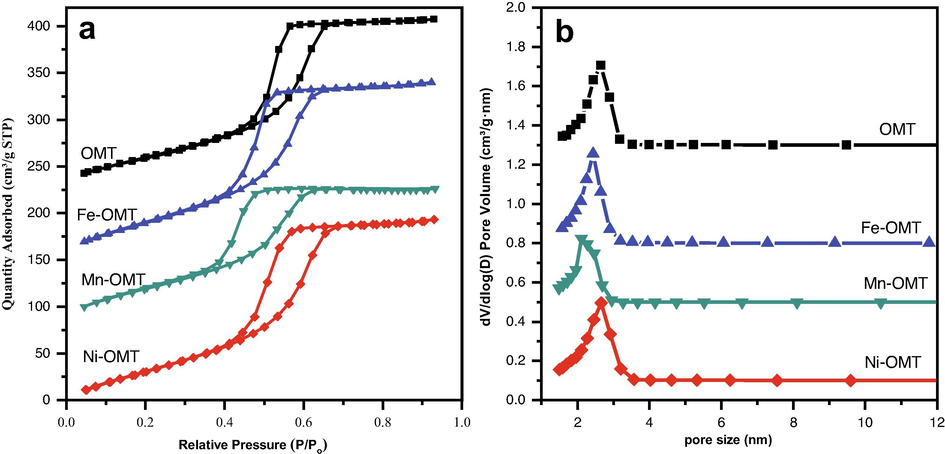
(a) N2 adsorption–desorption isotherms (b) BJH pore size distributions of pure OMT, and different transition metal-doped OMT samples prepared from self-assembly template route.
The amounts of TMs in the fabricated TM-OMT materials are evaluated through ICP. As listed in Table S2, the contents of metal oxides ranging from 0.0413 to 0.058. The XPS spectra analysis of TM-OMT samples was also performed to further ascertain TM's doping into OMT and their oxidation states (Li and Zeng, 2007) XPS survey spectrum of the fabricated TM-doped samples are presented in Fig. S2. The XPD fine Ti 2p of pure OMT, TM-OMT samples are shown in Fig. 6a. The Ti 2p3/2 and 2p1/2 photoelectron peaks of pure OMT are symmetric and their binding energy (BE) situations are at 459.53 eV and 465.28 eV, correspondingly suggesting the Ti4+ state (Tian et al., 2012). The spin–orbit splitting energy of the observed two peaks estimated to be 5.74 eV, identical to the literature value (Tian et al., 2012). As the metal is doped into OMT, the Ti 2P3/2 peaks of Ti in TM-OMT samples are also similar with the pure except a slight red-shifted in the BE by 0.15 eV, for Fe, 0.1 eV, and 0, for Mn and Ni, respectively, signifying the substitution of Ti (iV) by TMs dopants (Duan et al., 2012). The shifting in BE values indicates that the Mn and Ni dopants' electron donation occurs significantly from Fe, probably due to the electron cloud's movement toward electronegativity TMs. Fig. 6(b-e) displays XPS spectra of O1s region of pure OMT and TM-OMT samples. Fig. 6c displays that the O 1s of pure OMT show two dissimilar features of oxygen. Further, two fitted Gaussians peaks allotted as (1) and (2) were applied to fit the experimental data. The first peak (1) is observed at the lower BE of 530.7 eV, and is allotted to lattice oxygen of oxide of the TiO2. The other peak (2) centered at 532.2 eV is correlated to the OH group adsorbed onto the OMT materials' surface. However, The O 1s region of TM-OMT samples can be fitted into three Gaussian peaks labeled as (1), (2), and (3), (Fig. 6(b-e)). The peak situated at 530.7 eV fits to the Ti–O bonding in TiO2. Peaks observed at 532.3 and 535.8 eV can be credited to the Ti–OH bonding and to the existence of adsorbed water. These examinations illustrated that TM dopants' loading in OMT materials generates an improved amount of surface oxygen vacancies in the nature of metal–OH bonds.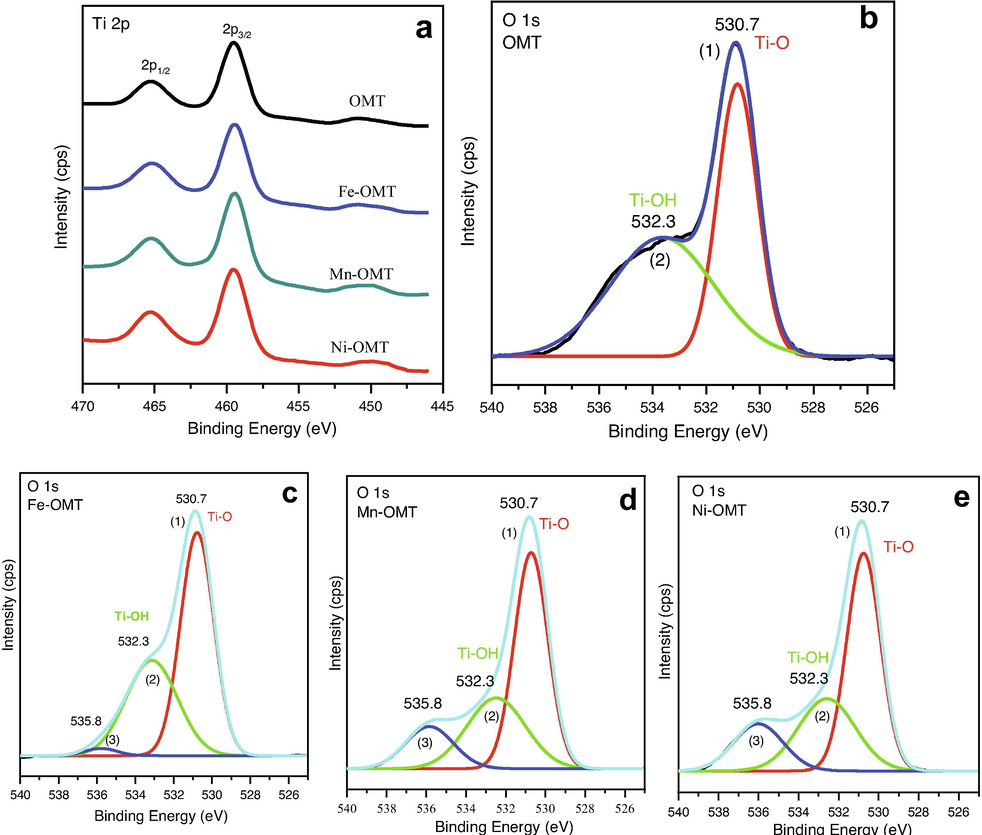
Surface features of OMT and TM doped OMT samples. (a) Ti 2p XPS region spectra of pure OMT and different TM–doped OMT. O 1s region spectra of pure OMT (b), Fe–OMT (c), Mn–OMT (d), and Ni–OMT (e) prepared by self-assemble template route.
4 Conclusions
The soft-templating method was employed to fabricate different TM-doped ordered mesoporous TiO2 materials and its physicochemical properties were discussed in detail. The diffraction peak at 2θ = 25.4° in the XRD spectrum specifies that TM/OMT reveals a pure anatase crystalline phase. The influence of TM doping level in OMT materials in the structural and morphological features was examined. The TM-doped OMT materials show that OMT substrates' formation is successfully modified with transition metal oxide particles. The optical examination of TM/doped OMT materials validates that the bandgap can be definitely modified with the types of transitional metal-ions into mesoporous TiO2. Particularly, the present in-situ EISA dopant approaches permit or homogeneous transition metals dopant dispersion with greater surface-active sites. This work opens a way to develop different transition metals loaded over highly ordered mesoporous TiO2 substrate for various renewable energy production applications.
Funding
No funding was received for this work.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sustain. Energy Rev.. 2017;77:89-108.
- [Google Scholar]
- Synthesis of hexagonally packed mesoporous TiO2 by a modified sol–gel method. Angewandte Chemie International Edition in English. 1995;34:2014-2017.
- [Google Scholar]
- Study on the synthesis of high-surface-area mesoporous TiO2 in the presence of nonionic surfactants. Ind. Eng. Chem. Res.. 2004;43:2485-2492.
- [Google Scholar]
- Well-organized mesoporous TiO2 photoanode by using amphiphilic graft copolymer for efficient perovskite solar cells. J. Phys. Chem. C. 2016;120:9619-9627.
- [Google Scholar]
- Sn-doped TiO2 photoanode for dye-sensitized solar cells. J. Phys. Chem. C. 2012;116:8888-8893.
- [Google Scholar]
- Importance of spin–orbit coupling in hybrid organic/inorganic perovskites for photovoltaic applications. J. Phys. Chem. Lett.. 2013;4:2999-3005.
- [Google Scholar]
- Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37-38.
- [Google Scholar]
- Block-copolymer-assisted one-pot synthesis of ordered mesoporous WO3− x/carbon nanocomposites as high-rate-performance electrodes for pseudocapacitors. Adv. Funct. Mater.. 2013;23:3747-3754.
- [Google Scholar]
- Synthesis, characterization and photocatalytic properties of nickel-doped TiO2 and nickel titanate nanoparticles. J. Mater. Sci.: Mater. Electron.. 2016;27:3599-3607.
- [Google Scholar]
- Gas adsorption characterization of ordered organic− inorganic nanocomposite materials. Chem. Mater.. 2001;13:3169-3183.
- [Google Scholar]
- Hollowing Sn-doped TiO2 nanospheres via Ostwald ripening. J. Am. Chem. Soc.. 2007;129:15839-15847.
- [Google Scholar]
- Mesoporous titania (A) nanoparticles prepared by a solvothermally treated organic small molecule system: formation mechanism and behavior in photocatalysis and dye-sensitized solar cells. J. Phys. Chem. C. 2010;114:1444-1450.
- [Google Scholar]
- The effect of Co-doping on the humidity sensing properties of ordered mesoporous TiO2. Appl. Surf. Sci.. 2017;412:638-647.
- [Google Scholar]
- Influence of VB group doped TiO2 on photovoltaic performance of dye-sensitized solar cells. Appl. Surf. Sci.. 2013;277:231-236.
- [Google Scholar]
- Black NiO-TiO2 nanorods for solar photocatalysis: Recognition of electronic structure and reaction mechanism. Appl. Catal. B Environ.. 2018;224:705-714.
- [Google Scholar]
- Axis-oriented, anatase TiO2 single crystals with dominant 001 and 100 facets. Cryst. Growth Des.. 2011;11:3947-3953.
- [Google Scholar]
- Quantum dot solar cells. Physica E: Low-dimensional Systems and Nanostructures. 2002;14:115-120.
- [Google Scholar]
- Photocatalytic activity of nitrogen and copper doped TiO2 nanoparticles prepared by microwave-assisted sol-gel process. Arabian J. Chem.. 2020;13:86-95.
- [Google Scholar]
- Synergy of low-energy 101 and high-energy 001 TiO2 crystal facets for enhanced photocatalysis. ACS Nano. 2013;7:2532-2540.
- [Google Scholar]
- Raman spectroscopy: a new approach to measure the percentage of anatase TiO2 exposed (001) facets. J. Phys. Chem. C. 2012;116:7515-7519.
- [Google Scholar]
- Mesoporous spherical aggregates of anatase nanocrystals with wormhole-like framework structures: their chemical fabrication, characterization, and photocatalytic performance. Langmuir. 2004;20:11738-11747.
- [Google Scholar]
- In situ synthesis of ordered mesoporous Co-doped TiO 2 and its enhanced photocatalytic activity and selectivity for the reduction of CO 2. J. Mater. Chem. A. 2015;3:9491-9501.
- [Google Scholar]
- Improved performance of dye-sensitized solar cells by trace amount Cr-doped TiO2 photoelectrodes. J. Power Sources. 2013;224:168-173.
- [Google Scholar]
- Hydrothermal preparation and photocatalytic activity of hierarchically sponge-like macro-/mesoporous titania. J. Phys. Chem. C. 2007;111:10582-10589.
- [Google Scholar]
- Organized mesoporous TiO2 films exhibiting greatly enhanced performance in dye-sensitized solar cells. Nano Lett.. 2005;5:1789-1792.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101469.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







