Translate this page into:
Structural and functional analysis of Japanese encephalitis virus drug targets in focus on immune evasion mechanisms
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Japanese encephalitis (JEV) is a well-known arthropod-borne pathogen that is considered an emerging pathogen that causes brain infections. It has a high mortality rate, and morbidity is responsible for 10,000 to 15,000 deaths each year. Nerve cell entry and central nervous system infection are where the virus begins its neuronal invasion phase. It weakens the body's natural defences and sets in motion the neurotrophic effects. There is no effective treatment or drug to combat the inhibition; however, there are some vaccine candidates on the market. JEV drug targets and their functional analysis determine which drugs are most effective in inhibiting the invasion and hijacking of host immune cells. Viral entry-level drug targets have been shown to be the starting point for the immunological vaccine, and their inhibitory profiles are discussed in this review for the entry-level drug targets. Replication of a virus begins with viral entry, and the process continues with infection, maintenance and dissemination. Among the proteins, non-structural and structural proteins are known for their function, and blocking those will result in inhibition, and thus the drug targets of JEV are discussed. This review discusses the JEV overview of drug targets, infection, and immunity to help with the future of JEV drug development.
Keywords
Viral Infection
Immunological drug targets
Japanese encephalitis
NS1 protein
E protein
Fusion inhibitors
1 Introduction
The Japanese encephalitis virus (JEV), which causes Japanese fever, is well known for its lethality, and its phylogeny is assigned to the family Flaviviridae and the genus flavivirus. JEV, ZIKA virus (ZIKV), West Nile virus (WNV), and 70 other species are well-known flaviviruses (Holbrook, 2017). These viruses have recently gained popularity due to their re-emergence, and they are notably ssRNA (single-stranded RNA) viruses that can mutate quickly and spread through arthropod-based vectors such as mosquitoes and ticks. Even though arthropod-based vectors spread flavivirus, it causes serious harm to global health and can lead to severe epidemics and geographic spread(Holbrook, 2017). DENV has recently spread in India, as has the ZIKV outbreak in South America, the yellow fever virus (YFV) outbreak in Africa, and the WNV outbreak in North America. JEV is a major flavivirus that causes viral encephalitis, particularly in Asia-Pacific and Australia (Mackenzie et al., 2004). According to the WHO (World Health Organization), more than 68,000 clinical case reports exposing 3 billion people in 24 countries pose a risk of infection. The intriguing aspect of JEV is that it has the characteristics of double, endemic, and epidemic epidemiological models. Most Asian and Pacific countries are highly vulnerable to this endemic pattern, which occurs sporadically throughout the year and spreads as seasonal diseases in some areas (Grant-Klein et al., 2010).
As of now, there is no valid cure or solution for JEV infections, and it poses a high risk to regional travellers as a significant health problem. To that end, current JEV treatment aims to alleviate severe clinical symptoms while also assisting patients in overcoming viral infections (Kemenesi and Bányai, 2019). In this extension, the WHO requires an active vaccination program that is integrated into the vaccination schedule for JEV-infected areas. Several JEV awareness programs were launched in 2012 by countries like Japan, China, and Thailand (Carro and Cherry, 2020). These immunization programs have been effective in demonstrating a decrease in infection rates. Because vaccination is the only solution, the infections are re-emerging, and there is currently a lack of antiviral inhibitors treating these flavivirus infections, with researchers worldwide struggling to find potent inhibitors (Cumberworth et al., 2017).
1.1 Genotypes of JEV
JEV has five genotypes, which differ by 10% at the nucleotide level depending on geographic location (Mehta et al., 2021). Most JEV isolates from the Asia Pacific and Australia are genetically characterized by genome sequence and gene codes E glycoprotein, spanning region C/PrM and NS5/3′ UTR (Hameed et al., 2021). The phylogenetic representation of various geographical locations for the above three genes represents a similar genotype is found in specific countries, and the other differs for each country, determining JEV is sequentially varied from country to country. In other words, the JEV is widely distributed among ethnic populations; for example, genotype IV is prevalent in Indonesia, while genotype III is prevalent in India, the world's second-most populous country. Other countries, including China, Taiwan, and Japan, have different JEV genotypes (Karna and Bowen, 2019). As of now, five genotypes have been reported, ranging from G-I to G-V, with G-I and GII being more prevalent in temperate regions and G-III, G-IV, and G-V being more prevalent in tropical regions (Zhang et al., 2017).
1.2 Structural aspects of JEV
The JEV and WNV infection mechanisms are neuro-invasive, causing severe damage to the central nervous system (CNS) by spreading infections through the olfactory bulb. JEV causes both symptomatic and asymptomatic infections in this case, resulting in acute flaccid paralysis (AFP) and meningoencephalitis (Chávez et al., 2010). The JEV can infect a variety of neural cells, including microglia, astrocytes, and pericytes. Infecting these cells disrupts the blood–brain barrier (BBB), resulting in widespread inflammation in the human brain (Mishra et al., 2008). The evidence for JEV's neurotropic nature is clear, but the molecular mechanism or neuro-invading mechanism is still unknown. There is currently a lack of information about the factors influencing CNS cell entry, but TLRs (C-type lectin receptors) and TIMs (T-cell immunoglobulin and mucin domains) have high expression profiles in CNS cells. TAM family receptors express themselves in neuronal cells simultaneously (Ghoshal et al., 2007).
Cell surface proteins play a functional role in flavivirus entry into the host cell variety. When the virus infects a human host, it is deposited in the epidermal cells of human skin by vectors, most notably the mosquito. The host cell is encountered here, and viral proteins divert human immunity by infecting keratinocytes and dendritic cells from the skin(Zhang et al., 2019). This disease affects dendritic cells, which enter lymphoid organs and initiate the functional replication mechanism, after which the virus spreads to other internal organs(Bidet and Garcia-Blanco, 2014). DENV, JEV, and ZIKV all exhibit a similar pattern with vector and dendritic cells. JEV's genome architecture includes an open reading frame (11 kb in size) as well as an untranslated region encoding three structural proteins(Leyssen et al., 2000). C (Capsid), E (Envelope), and M (Membrane) proteins are the three structural proteins. Fig. 1 depicts the architecture of flavivirus, which includes structural proteins and 7 non-structural (NS) proteins. This structural feature is found not only in JEV but also in the majority of flavivirus species. The mature virion is completely encased in the envelope (E), membrane (M), and core capsid (C) proteins. Among these proteins, E-Glycoprotein initiates interactions with host cellular receptors, resulting in clathrin-mediated endocytosis and the formation of endosomes(Garcia et al., 2017). In addition, after endosome acidification, vesicular membranes fuse with viral, resulting in the release of viral RNA into the host cytoplasm (Paula et al., 2009). The newly synthesized untranslated RNA acts as mRNA, translating into a single Open Reading Frame (ORF) in the endoplasmic reticulum (ER) (Aktepe and Mackenzie, 2018). As shown in Fig. 1, this polyprotein is post-translationally modified into three structural proteins (C, E, and M) and seven non-structural proteins (NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5).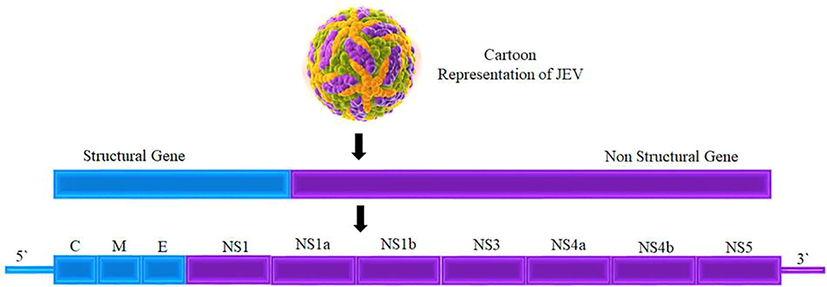
A JEV structural and non-structural protein representation.
1.3 Biochemistry of flavivirus
The JEV, like all other flaviviruses, has the smallest particle, 50 nm in size, with an ssRNA viral genome that is capped at the 5′ terminus and is approximately 11 kb in length. A poly(A) tract is missing from the 3′ end of this RNA genome (Barrows et al., 2018). The translated viral RNA produces single polyproteins with approximately 3400 amino acids, cleaved into three structural and seven non-structural proteins by both viral protease enzymes. Capsid (C) protein has positively charged residues at one end that aid in interactions with viral RNA and membrane protein (Diosa-Toro et al., 2020). The intriguing fact is that multiple capsid proteins form the strong nucleocapsid in complex with viral genomic RNA, and this nucleocapsid is embedded by ER-derived lipid bilayers that are integrated with E and M proteins (Ke, 2018). This function is responsible for the invasion of the host cell and the release of nucleocapsid protein into the cytoplasmic layers. In translation, another M protein (prM) is synthesized alongside the E protein through the ER membrane. The E protein (500 AA) has the largest structure of the structural proteins and functions to mediate and fusion of virions in the early stages of viral entry [39]. The structural morphology of E protein is complicated by the fact that it is formed with ninety homodimers in mature virions that contain ectodomains separated into three prominent domains (Central domain I (DI), extended domain II (DII), and globular domain III (DIII)) (Huber et al., 2021). Two loops in DII are highly flexible and serve as a fusion loop at its tip, assisting in binding/fusion with the host membrane. DIII mimics human immunoglobulin and initiates functional interactions with human cellular receptors(Campos et al., 2018).
Binding with receptor domain binding causes clathrin-mediated endocytosis and endosomal formations, which mediate the structural change of trimeric spike formation from the dimer structure, resulting in a slight pH change. Endosomal penetration, which moves alongside host and viral membranes, exposes the loops to fuse and releases the nucleocapsid towards the cytosol. The GAG binding region of E protein contains positively charged residues that influence communication with various host cells(Neufeldt et al., 2019). Aside from structural proteins, the non-structural proteins (NS1) mechanism is intriguing, and the NS1 exists as a membrane-associated dimer and a secreted hexamer found on the ER surface and in the plasma membrane. NS1 plays a dominant role in RNA replication, immune modulation, and evasion, allowing viruses to hijack host cell mechanisms(Vonderstein et al., 2018). Another NS protein, NS2A, is a membrane-associated hydrophobic protein that functions for RNA replication and cleaves the NS1-NS2A junction after translation. It functions as a component of the viral replicase complex, virus assembly, and secretion. Furthermore, it modifies the host's viral response mechanism by inhibiting the interferon signalling pathway. Along with NS3, NS2B remains as a heterodimer that initiates the heterodimeric complex's anchoring into the endoplasmic reticulum membrane(Esposito et al., 2015). It is not a simple protein because it functions as a co-factor for NS2-NS3 serine protease and as a cause for viral polyprotein at the NS2A/NS2B, NS2B/NS3, NS3/NS4A, and NS4B/NS5 junctions(Zhang et al., 2018).
For the RNA helicase and NTPase activity, NS3 enzymes are involved in viral replication and viral assembly(Edeling et al., 2014). Another hydrophobic protein, NS4A is a small non-structural protein that acts as an interferon antagonist, while NS5 is the largest and contains MTase activity in the N-terminus and RNA-dependent RNA polymerase (RDRP) activity in the C-terminus(Arenas, 2020). NS5 is a multifunctional protein that includes terminal RNA transferase, RNA helicase, and RDRP. NS5, like NS3, is a multi-enzymatic protein with guanylyl-transferase/methyltransferase domains that are required for 5′ capping. It has an RNA-dependent RNA polymerase domain at the C-terminus. It also collaborates with other viral replication factors such as viral RNA, the host, and viral co-factors to amplify the viral genome(Martins and Santos, 2020). We can deduce that NS proteins, which are essential for viral replication, play a dominant role and that inhibiting these proteins may result in disruption of viral replication. As a result, these proteins are highly concentrated for the detection of viral inhibitors.
1.4 Host-pathogen interaction
JEV initiates host-pathogen interactions by entering mammalian host cells via caveolin-mediated endocytosis and neuronal cells' dopaminergic signal transduction pathway. Later on, it improves the surface's focal adhesion and the binding phenomenon, as well as the entry of JEV(Singh et al., 2020). The major component for this is the v3 integrin, which uses vimentin to the surface and significantly influences JEV interaction and infection of BHK-21 cells(Yun and Lee, 2018). The literature reports the role of SM (sphingomyelin) in JEV entry using a mouse model, and the stride database provides details of Host-Pathogen interactions based on available data and clinical samples(Grifoni et al., 2020). JEV hijacks several host proteins that are required for the formation of replication complexes. Following viral entry into the host cell, the JEV releases its positive-sense genome and is transcribed into negative-sense RNA replication intermediates, which serve as a template for replicating large numbers of copies of the positive-sense RNA genome mediate the RNA-RNA interaction (Niu et al., 2018). During the initial stage of infection, the positive-sense RNA acts as mRNA and hijacks the essential factors of the host organism that play a key role in the translation and cleavage of polypeptides in the lumen of the endoplasmic reticulum. Later in the replication process, the viral proteins are cleaved and interact with the cellular proteins of the host cell(Ye et al., 2013). Fig. 2 depicts a protein–protein interaction between JEV and human cells, indicating that the JEV hijacks human cells and initiates infection by rupturing immune cells. Furthermore, we discovered that the JEV actively participates in the invasion of host cells by interacting with more human cancer drug target proteins.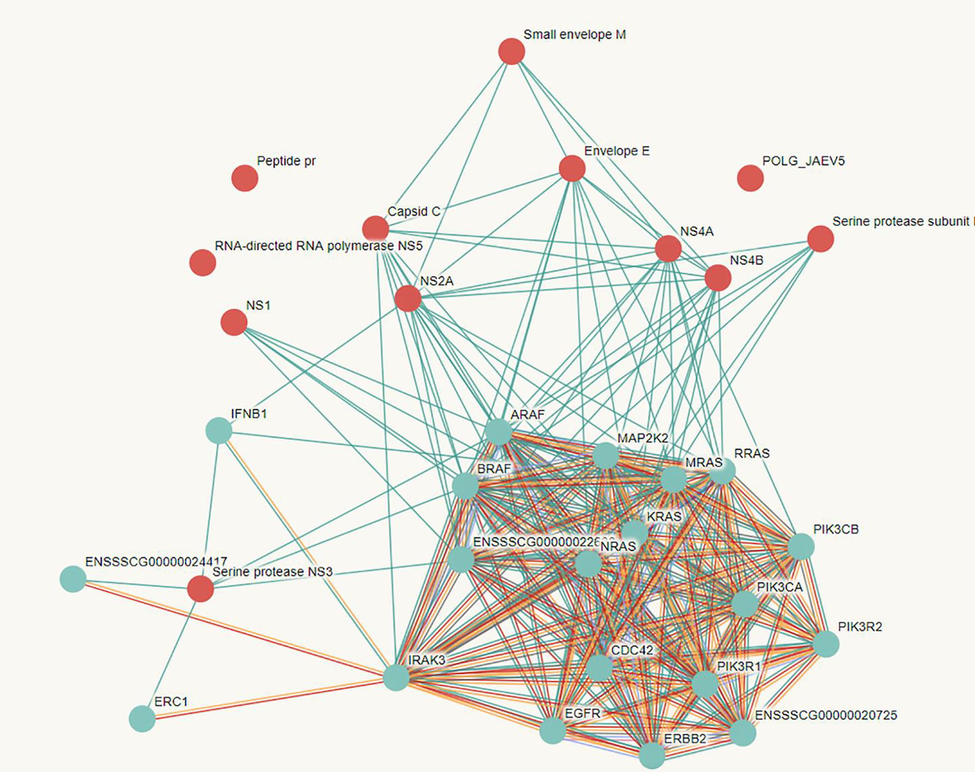
The string database predicted host-pathogen interactions between JEV and Homosapiens.
1.5 Neuro-invasion
The neuro-invasiveness property is critical in all mosquito-borne flavivirus mechanisms(Banerjee and Tripathi, 2019). Understanding the protein–protein interactions, drug targets, and neuroinvasion mechanism of the primary host (Culex mosquitoes) is critical to understanding the protein–protein interactions, drug targets, and neuroinvasion mechanism of the second host (Homosapiens). Recently published research revealed that host cell receptor proteins such as glycoproteins, HSP70, and glucose-regulated protein 78 (GRP78) serve as reservoirs for JEV. While JEV binds to the host cell, neurological symptoms begin after the incubation period, altering Parkinsonian movement and dystonias(Sethi et al., 2019). As a result, five to twenty percent of infected people have poliomyelitis-like disorders characterized by paralysis and permanent (neuro)psychiatric sequelae. As the infection progresses, the neuronal cells exhibit edema, vascular congestion, pre-vascular inflammation, and the formation of microglial nodules. The literature strongly supports the direct infection mechanism of infected endothelial cells, also known as the “Trojan-horse” mechanism. Infection in the CNS region is controlled by specific receptors such as heat shock protein. Through this process, the JEV causes necrosis and apoptosis by hyper activating microglia and releasing reactive oxygen species, which causes damage to neuronal cells. Several studies have found that the envelope (E) protein plays a critical role in neuro-invasion and neurovirulence mechanisms. To deal with viral host cell interactions, the architecture of E-protein domain III and II is adjusted explicitly for receptor binding(Seitz et al., 2018). The viral capsid (C) protein and membrane (M) or pre-membrane (prM) also contribute to the neuro-invasion and neurovirulence mechanism during this process. The non-structural (ns) protein's role in the immune system is initiated by the ns1 function for ribosomal shifting, and mast cells significantly activate pericytes, microglia, and neurons.
1.6 Targeting the neuro-invasion drug targets
Interactions between viral proteins and host cell surface proteins have paved the way for a neuro-invasion mechanism initiated by viral entry. As a result, the E protein, C protein, prM protein, and ns1 proteins function in the neuro-invasion mechanism, and these proteins are discussed as potential drug targets for neuro-invasion(Lv et al., 2018). Furthermore, two novel neuronal membrane receptors, PLVAP and GKN3, and HSP70 and GRP78, have been identified as playing a critical role in the JEV entry mechanism. Protein-protein interactions in the mouse brain suggest 42 protein interactions with E-protein, of which PLVAP, GKNE, SRC8, LRAT, and EXOC8 have high mRNA levels. Similarly, the other protein discussed in the neuroinvasion mechanism has multiple functions, and because of their multiple interactions with host cells, these proteins can be successive drug targets for JEV inhibition(Hsieh et al., 2019).
1.7 E protein inhibitors
The JEV E-protein has three domains: I lateral ridge, fusion loop, domain III lateral ridge, and domain I-II hinge region, and studies show that the dimer form influences virion assembly, receptor interaction, and uncoating. The E-protein has a heterolytic pocket, and compounds such as NITD-448 and other quinazoline chemical forms that functionally bind to the heterolytic pocket have been reported(Zheng et al., 2018). PO2 compounds, as well as D02, D04, and D05, are some compounds that inhibit JEV, YFV, and WNV growth by disrupting viral entry and maturation. Similarly, Castano spermine and Celgosivir are specialized for viral inhibitors in dengue by blocking the E-protein, prM, and Ns1 folding mechanisms. The research evidence of DN59 peptide mimic shows enormous activity for the inhibition of DENV E-protein and may also inhibit the inhibition of other flaviviruses(Banerjee et al., 2017). Furthermore, non-steroid anti-inflammatory drugs such as aspirin and sodium salicylate significantly inhibit JEV proliferation in neuroblastoma cells by inducing cell death and effectively reducing viral protein expression. JEV infection, on the other hand, was suppressed by reducing PGE2 production. Other agents, such as curcumin and minocycline, also effectively inhibit JEV virus replication. Several studies have shown that tumour necrosis factor- (TNF- α) plays a vital role in JEV proliferation, so TNF- α inhibitors are also used as effective inhibiting agents in treating JEV infection. Wang et al. discovered that FDA-approved drugs, specifically calcium inhibitors like pimecrolimus and nelfinavir mesylate, effectively block viral replication. Cilndipine has been shown to effectively block both L and N type calcium channels in smooth muscle and has been used to inhibit JEV infection. Recently, potential effective inhibitors such as lonafarnib, nitroxoline, and hexachlorophene have been identified as effective JEV inhibiting agents to treat the viral infection using a high throughput screening approach. Several bioactive compounds, including ajwain oil, act as potent antiviral agents in addition to synthetic compounds. In vitro antiviral studies have shown that the purified form of ajwain oil has significant inhibitory activity in both in vitro and in vivo evaluation(Yamaguchi et al., 2013).
1.8 Capsid inhibitors
E-protein inhibitors have received extensive research, but capsid protein inhibitors have received little attention. ST-148, with an EC50 value of 0.016 (M), is a drug molecule with a proclivity for inhibiting DENV replication and causing cytopathic effects. Researchers believe that selecting capsid inhibitors may act as potent inhibitors that can be a common inhibitor for nearly all flavivirus family members. Several studies have found evidence of strong potential activity for capsid inhibition, which reduces viremia and viral replication.
1.9 Non-Structural protein 1: The drug target
Among non-structural proteins, the protein NS1 plays an essential role in the host immune evasion and invasion mechanism by directly entering the host cell machinery, resulting in effective virus propagation. In the human cytoplasm, viral internalization and nucleocapsid uncoating are initiated, followed by viral RNA genome replication. The RNA serves as mRNA, and the signal peptide from it guides into the ER lumen, where the mRNA-based polyproteins are cleaved into structural and non-structural proteins with the help of membrane-bound ribosomes. In this case, the viral polypeptide is cleaved by both human and viral proteases; for humans, signalases and furin are involved, while for the virus, the NS3 serine protease is involved. The C-terminal-based signal peptide from E protein is in charge of cleaving the NS1 and translocating it from the previously mentioned polypeptide into the ER lumen. Because NS1 is the main protein, the C-terminal region contains the L/M−V−X−S−X−V−X−A octapeptide sequence, which is functionally essential for cleaving. Interestingly, this octapeptide sequence is highly conserved across the flavivirus family. The NS1 structure, along with other NS proteins and RNA, forms dimer chains that target the human ER, resulting in replication complexes from vesicle packets. This replication complex is critical for viral replication, and NS1 plays a vital role in its formation.
Furthermore, research suggests that a GPI-anchored form of NS1 can modulate flavivirus pathogenesis by affecting the signal transduction mechanism. The structural biology of NS1 reveals that it can form a wide-open barrel structure and is associated with multiple lipid molecules and that its hexameric form has -ladder and wing domain features. This specialized feature allows for interaction with host proteins, which is why NS1 plays such an essential role in host immune evasion. Mutations in NS1 cause the virus to re-emerge and cause lethal infections, according to research in DENV and ZIKV. NS1 interacts with several host proteins in the human intracellular environment that aid in viral replication, translation, and virion production. As illustrated in Fig. 3, the 60 s ribosomal proteins RPL18, RPL18a, and RPL7 are critical for initiation.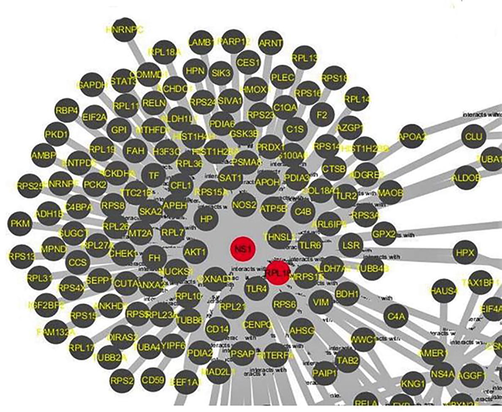
Protein-protein interactions between human host proteins invaded by JEV NS1, with a focus on NS1-RPL18.
1.10 Protein-Protein interactions of NS1 with RPL18
While NS1 (Fig. 4) invades the host cell, it is critical to understand the protein–protein interactions between NS1 and human proteins. Protein-protein interactions, which are physical contacts between two or more proteins that occur in biochemical events or mechanistic actions, result in various interactions via hydrogen bonding interactions, hydrophobic effect, electrostatic interactions, and other physical forces. There is a significant reduction in viral translation and replication when RPL18 is silenced, suggesting RPL18 is required during the DENV replication cycle. The interactions between NS1 and RP18 are examined in this study from the complex protein–protein interactions, as shown in Fig. 5.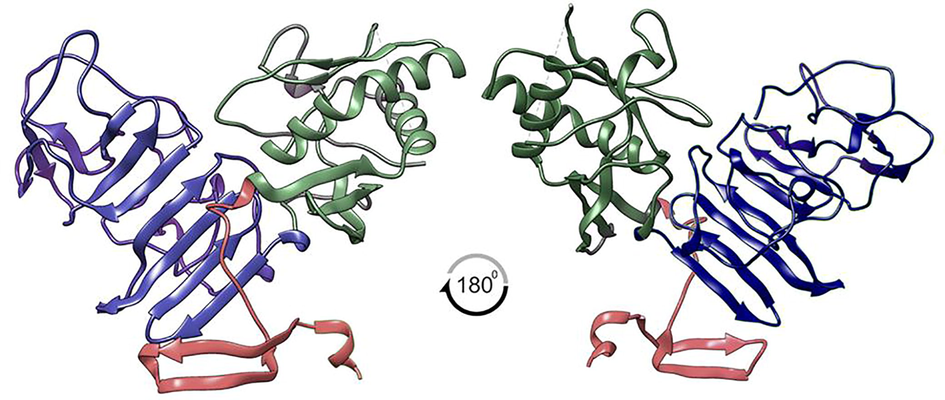
JEV-NS1 has a three-domain structure that plays an essential role in the viral host hijacking mechanism.
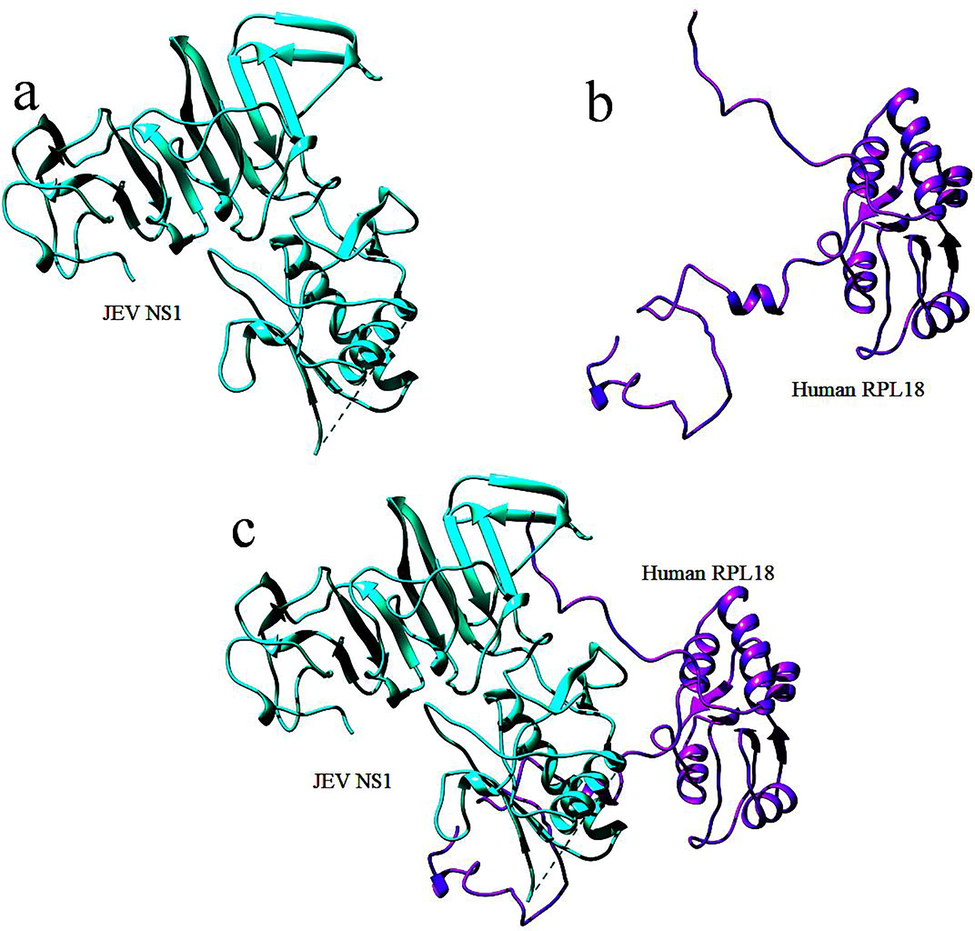
Protein-protein interactions between the JEV NS1 and human RPL18.
1.11 Important biomarkers of JEV
Clinical symptoms and a serological examination of the infected individual are used to diagnose JEV infection. The diagnosis is made based on the availability of a biomarker that distinguishes the severity of the infection. The number of cellular components in cerebrospinal fluid serves as a biomarker. The number of interacting molecules released by infected individuals is counted in order to identify the disease. This frees cells from the interacting molecules that are used to determine the severity of the infection in infected cells. Host cells have produced several immune systems viral particles, and CSF antigens are tested to determine the presence and rate of infection. Host cells have produced a number of immune system viral particles for diagnosing the presence and severity of infection, and CSF antigens have been tested. Conventional diagnostic techniques such as immunofluorescent staining, viral neutralization, and complex fixation are frequently used; however, they are expensive and are not sensitive to the CSF antibodies found. Serum proteins can also be used to determine whether or not a person is infected. SELDI-TOF mass spectrometry is a widely used technology for detecting the presence of viral infection proteins. Proteomic analysis of serum proteins could be used to identify biomarkers for a variety of diseases and infections.
As a result, proteomic analysis of serum proteins using a combination of SELDI-TOF-MS and ProteoMiner or Chip arrays revealed protein interaction in biological samples. Protein binding could be discovered using such arrays due to the hydrophobic and hydrophilic properties of the protein being selectively bound with specific molecular weights. Various antigens are expressed on the membranes of infected cells. For example, an appropriate antibody, CTL is available with additional support for the destruction of antibody-dependent cytotoxic viral replication. However, in response to a viral infection, it promotes cell absorption by causing the synthesis of pro-inflammatory peptides and proteolytic fragments (such as CSb, C3bi, C3d, and C3dg). Plasma fluids contain immune globulins, enzymes, and major proteins responsible for virus and toxic substance destruction. The mast cell also produces conjugated polysaccharide heparin, which aids in blood coagulation during circulation. Osma regulation and other ions are interconnected with the mediator production system by receptors coupled to the G protein and release mediators, including metabolites, in plasma.
2 Future perspective
JEV infection spread beyond its traditional geographical boundaries as a result of primary host mosquito travel and dispersal. The primary causes of the virus's spread into immunologically naive populations are urbanization and climate change. These criteria may have an impact on JEV infection prevention. JEV chronic infection has been reported in 171 districts and 19 states in India. The identification of infections is the primary source of information for the system designed to develop potent antiviral drug molecules. The discovery of potent novel and safe JE vaccines is critical for accelerating and detecting an effective modality with a higher level of safety and efficacy that will meet the needs of changing genotypes. To reduce the prevalence of JEV in India, the recently inactivated cell-culture-based vaccine JENVAC was developed from an Indian strain. The vast majority of research into Japanese encephalitis has gone unnoticed. Interferon, for example, has been shown to be effective in vitro and in vivo models for more than 15 years but is only now being tested in human diseases. As a result, research into new and effective antiviral drugs to combat Japanese encephalitis should be prioritized. Because effective vaccines are in short supply, the most pressing concern is JEV diagnosis and prevention, significantly reducing disease prevalence. More research is required to determine the potential impact of protective immunity and whether co-administration of available vaccines disrupts the immune response. Furthermore, the restoration of diagnostic tools for people with disabilities must take place alongside the capacity building. As a result, the ultimate goal is to replenish the vaccine deficit while also lowering mortality.
3 Conclusion
Flavivirus infection is spread widely by insect vectors and has a significant illness impact on global health, particularly in developing countries. The Japanese encephalitis virus (JEV) is a well-known flavivirus that can be found throughout Asia and the Pacific. They control the neurologic, cognitive manifestations that lead to death. Viral entry and fusion have been linked to the NS1 domain and the E glycoprotein, respectively. All flaviviruses contain NS1, which is involved in genome replication as well as immune evasion. Molecular understanding of viral infection lifecycles is still need to study in detail for developing effective drugs against flavivirus infection. Through this review, secreted and cell surface-associated proteins, as well as essential biomarkers for early diagnosis along with high focus on NS1 protein, and its immunological role is detailly studied. Overall, this review provides, the deep understanding of molecular mechanism of host-pathogen interaction proteins allows to understand their potential role in viral infection better.
4 Consent for publication
None
Acknowledgement
The author would like to thank the Deanship of Scientific Research at Majmaah University for supporting this work under project number R-2021-254.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Shaping the flavivirus replication complex: It is curvaceous! Cell. Microbiol.. 2018;20(8)
- [CrossRef] [Google Scholar]
- Banerjee, A., Tripathi, A., 2019. Recent advances in understanding Japanese encephalitis. F1000Res 8
- Insight into SNPs and epitopes of E protein of newly emerged genotype-I isolates of JEV from Midnapur, West Bengal, India. BMC Immunol. 2017;18:13.
- [Google Scholar]
- Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev.. 2018;118(8):4448-4482.
- [Google Scholar]
- Flaviviral RNAs: weapons and targets in the war between virus and host. Biochem. J.. 2014;462:215-230.
- [Google Scholar]
- Roles of Pro-viral Host Factors in Mosquito-Borne Flavivirus Infections. Curr. Top. Microbiol. Immunol.. 2018;419:43-67.
- [Google Scholar]
- Beyond the Surface: Endocytosis of Mosquito-Borne Flaviviruses. Viruses. 2020;13(1):13.
- [CrossRef] [Google Scholar]
- Domain III peptides from flavivirus envelope protein are useful antigens for serologic diagnosis and targets for immunization. Biologicals. 2010;38(6):613-618.
- [Google Scholar]
- Inhibition of type I interferon induction and signalling by mosquito-borne flaviviruses. Cell. Microbiol.. 2017;19(5):e12737.
- [CrossRef] [Google Scholar]
- Role of RNA-binding proteins during the late stages of Flavivirus replication cycle. Virol. J.. 2020;17:60.
- [Google Scholar]
- Structural basis of Flavivirus NS1 assembly and antibody recognition. Proc. Natl. Acad. Sci. U.S.A.. 2014;111(11):4285-4290.
- [Google Scholar]
- Physico-chemical requirements and kinetics of membrane fusion of flavivirus-like particles. J. Gen. Virol.. 2015;96:1702-1711.
- [Google Scholar]
- Pro-inflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia. 2007;55(5):483-496.
- [Google Scholar]
- Rapid identification of vector-borne flaviviruses by mass spectrometry. Mol. Cell. Probes. 2010;24(4):219-228.
- [Google Scholar]
- Transcriptomic immune profiles of human flavivirus-specific T-cell responses. Immunology. 2020;160(1):3-9.
- [Google Scholar]
- Potential Role of Birds in Japanese Encephalitis Virus Zoonotic Transmission and Genotype Shift. Viruses. 2021;13
- [Google Scholar]
- Japanese encephalitis virus neuropenetrance is driven by mast cell chymase. Nat. Commun.. 2019;10:706.
- [Google Scholar]
- Computational modelling of flavivirus dynamics: The ins and outs. Methods. 2021;185:28-38.
- [Google Scholar]
- Experimental Evaluation of the Role of Ecologically-Relevant Hosts and Vectors in Japanese Encephalitis Virus Genotype Displacement. Viruses. 2019;11(1):32.
- [CrossRef] [Google Scholar]
- The Multifaceted Roles of Autophagy in Flavivirus-Host Interactions. Int. J. Mol. Sci.. 2018;19(12):3940.
- [CrossRef] [Google Scholar]
- Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin. Microbiol. Rev.. 2019;32(1)
- [CrossRef] [Google Scholar]
- Leyssen, P., De Clercq, E., Neyts, J., 2000. Perspectives for the treatment of infections with Flaviviridae. Clin Microbiol Rev 13, 67-82, table of contents.
- Drug Repurposing for Japanese Encephalitis Virus Infection by Systems Biology Methods. Molecules. 2018;23
- [Google Scholar]
- Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med.. 2004;10(S12):S98-S109.
- [Google Scholar]
- Intrinsically disordered protein domains in flavivirus infection. Arch. Biochem. Biophys.. 2020;683:108298.
- [CrossRef] [Google Scholar]
- Japanese encephalitis virus differentially modulates the induction of multiple pro-inflammatory mediators in human astrocytoma and astroglioma cell-lines. Cell Biol. Int.. 2008;32:1506-1513.
- [Google Scholar]
- ER-shaping atlastin proteins act as central hubs to promote flavivirus replication and virion assembly. Nat. Microbiol.. 2019;4(12):2416-2429.
- [Google Scholar]
- TIM-1 Promotes Japanese Encephalitis Virus Entry and Infection. Viruses. 2018;10(11):630.
- [CrossRef] [Google Scholar]
- New drug targets for hepatitis C and other Flaviviridae viruses. Infect. Disord. Drug Targets. 2009;9:133-147.
- [Google Scholar]
- Pharmacologic Depletion of Microglia Increases Viral Load in the Brain and Enhances Mortality in Murine Models of Flavivirus-Induced Encephalitis. J. Virol.. 2018;92(16)
- [CrossRef] [Google Scholar]
- Japanese encephalitis virus-induced neuropathology in mouse model infected through the conjunctival route. Indian J. Med. Res.. 2019;150(5):498.
- [CrossRef] [Google Scholar]
- Role of Platelet Cytokines in Dengue Virus Infection. Front. Cell. Infect. Microbiol.. 2020;10:561366
- [Google Scholar]
- Viperin Targets Flavivirus Virulence by Inducing Assembly of Noninfectious Capsid Particles. J. Virol.. 2018;92(1)
- [CrossRef] [Google Scholar]
- Characterization of a serine-to-asparagine substitution at position 123 in the Japanese encephalitis virus E protein. J. Gen. Virol.. 2013;94:90-96.
- [Google Scholar]
- Early Events in Japanese Encephalitis Virus Infection: Viral Entry. Pathogens. 2018;7(3):68.
- [CrossRef] [Google Scholar]
- Evidence of JEV in Culex tritaeniorhynchus and pigs from high altitude regions of Tibet, China. J. Vector Borne Dis.. 2017;54:69-73.
- [Google Scholar]
- Flaviviruses Exploit the Lipid Droplet Protein AUP1 to Trigger Lipophagy and Drive Virus Production. Cell Host Microbe. 2018;23(6):819-831.e5.
- [Google Scholar]
- Flaviviridae Viruses and Oxidative Stress: Implications for Viral Pathogenesis. Oxid. Med. Cell Longev. 2019;2019:1-17.
- [Google Scholar]
- Acidity/Alkalinity of Japanese Encephalitis Virus E Protein Residue 138 Alters Neurovirulence in Mice. J. Virol.. 2018;92(22)
- [CrossRef] [Google Scholar]







