Translate this page into:
Statistical modeling and optimization of cellulase production by Bacillus licheniformis NCIM 5556 isolated from the hot spring, Maharashtra, India
⁎Corresponding author. gselvakumar75@gmail.com (G. Selvakumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Cellulase shows great interest in the field of organic acids, and biotechnology industries. Cellulase-producing thermophilic bacteria was isolated from West Coast hot spring (Rajapur, Ratnagiri District of Maharashtra (Lat. 16°38′42″N; long. 73°31′53″E) and identified as Bacillus licheniformis. Out of seven variables in the cellulase production medium, four variables such as carboxy methyl cellulose (CMC), calcium chloride, Tween-20, and temperature were screened through Plackett–Burman design and further optimized through response surface methodology (RSM) for higher cellulase production. The optimal conditions were found to be CMC, 19.21 g/L, CaCl2·6H2O, 25.06 mg/L, Tween-20, 2.96 mL/L and temperature 43.35 °C. The high cellulase production 42.99 IU/mL was achieved in a 7 L scale bio fermenter using optimal conditions. A 3 fold increase in cellulase production was achieved by RSM model. These methods have proven suitable to optimize cellulase production by a thermophilic Bacillus.
Keywords
Bacillus licheniformis NCIM 5556
Cellulase
Optimization
Response surface methodology
Hot springs
1 Introduction
Cellulose from agriculture and forestry has potential as cheap and renewable feed stock which is abundantly available in nature and produced annually 1.3 billion tons. Cellulase (E.C. 3.2.1.4) is one of the enzymes responsible to break down the lignocellulosic materials into simple sugars. It has remarkable applications in areas such as alternate energy, textile, detergent, cattle feed, pharmaceutical industry, food, nutrition and agriculture industry (Bhat, 2000). Although standard chemical process of breaking down cellulose into simple sugars is easy, the enzymatic process is pollution-free, economical and cost effective (Ding et al., 2008). Because of these above mentioned reasons, most of the industries have increased their demand for less expensive cellulase with value added application. This gives a new horizon to find cost effective cellulase producing microorganisms.
Geothermal sources provide stable hot environment which are the preferred conditions for thermophiles (Brock, 1986). Many hot springs have been identified in the Indian subcontinents by our Geologists, however their microbial diversity has not been explored yet (Majumdar et al., 2000; Gupta et al., 1975). Compositions and properties of hot spring water may vary in terms of organic and inorganic chemical elements, pH and contain sufficient levels of nutrients which may provide favoured condition for microbial biodiversity (Khalil, 2011). It is reported that Unhale hot spring is rich in bicarbonate, heavy metal ions and water temperature is about 42–45 °C and pH is between 8 and 8.5 (Razdan et al., 2008).
Some of the microorganism inhabitants of these hot springs grow and produce enzymes under extreme conditions which can provide high thermal stability. Bacillus species have attracted industrial attention because of different reasons: high biomass development rate that reduces fermentation time, secretes their enzymes extracellularly which is cost effective for enzyme separation (Schallmey et al., 2004). Bacillus cellulases are able to degrade synthetic CMC, but very rarely degrade crystalline cellulose (Balasubramanian and Simoes, 2014). In addition Bacillus licheniformis has been scarcely studied on its potential to produce cellulases. Various parameters like media components (carbon, nitrogen, mineral sources, medium additives and presence of inducers) and physical parameters (pH, aeration, temperature) can be controlled to improve enzyme productivity and yield. These factors are really important and highly influential in the enzyme production cost which are commonly considered as the major bottleneck of the biotechnological processes (Lynd et al., 2002). For screening of physical and chemical parameters, one variable at a time approach (OVAT) is generally used by researchers to optimize the best parameters and the specific effects of variables. However, it will take more time to carry out many experimental trials and hence the interaction between variables becomes difficult to evaluate and often finding the optimum response results unsuccessful (Moorthy and Baskar, 2013; Brijwani et al., 2010). To overcome these problem factorials cum statistics-based investigational methods have been intended and used to produce fast and reliable outputs. Hence two level factorial model is more informative and practically approachable so that the interaction among the factors can be analyzed with ease. Plackett–Burman design (PBD) is a factorial based statistical technique used to evaluate the significance of the critical variables, (Balusu et al., 2005) while response surface methodology (RSM) used to evaluate the interactions of the independent process variables. The aim of this study is to enhance production of an indigenous and inexpensive cellulase from a thermophilic Bacillus species isolated from Unhale hot spring region of Maharashtra, India.
2 Materials and methods
2.1 Chemicals and materials
Media components used were of high purity and purchased from HiMedia Laboratories private limited, Mumbai. Other chemicals and reagents were of analytical grade obtained from SD fine chemicals, Mumbai. All the chemicals, reagents and media were used without any pre-treatment. Sugar cane bagasse was obtained from Sant Tukaram Sugar Factory, Pune, MH, and India. Rice straw and corn cob were obtained from local formers. Sugar cane bagasse, rice straw and corn cob were sized to 1–5 mm. Bagasse, rice straw and corn cob were pre-treated with 1% alkaline (NaOH) solution at 121 °C for one hour and used 1:10 ratio of substrate to alkali. After pre-treatment, the solids were filtered using Whatman Filter paper No. 1, dried and used for further study. All experiments were carried out in 250 mL Erlenmeyer flasks with a working volume of 100 mL medium with of 5% inoculum were used.
2.2 Isolation and identification of cellulase producing microorganism
Bacterial culture was isolated from five different water and soil samples from Unhale Hot Spring, Rajapur (Western Coastal area) Ratnagiri District, Maharashtra, India (Lat. 16°38′42″N; long. 73°31′53″E) using Basic Liquid Media (BLM) (consisted (g/L) of KH2PO4, 1.36; (NH4)2SO4, 1.0; MgSO4·7H2O, 0.2; FeSO4, 0.01; NaCl, 2.0; yeast extract, 1.0. Cellulase producing bacterial culture was screened by the clearance test using Congo red staining method. The colony showing the largest zone of clearance was selected for molecular characterization by 16S rDNA sequencing (Relevant methods have been provided in supplementary information) and biochemical tests have been carried out based on the method described in Bergy’s manual of Determinative Bacteriology (1974). Isolated culture was maintained on BLM with agar, supplemented with 0.5% carboxy methyl cellulose (CMC) slant at 4 °C and grown at 37 °C. The fermentation medium (BLM supplemented with 0.5% CMC) was inoculated with 10% v/v of the culture and incubated in an orbital incubator shaker at 37 °C at 120 rpm for 72 h. The fermented medium was then centrifuged at 12,000 rpm for 10 min at 4 °C to remove the cell and debris. The clear supernatant thus obtained after centrifugation served as crude enzyme source.
2.3 Total cellulase assay and protein quantification
Total cellulase activity was measured by the DNS method (Miller, 1959). Briefly, 0.5 mL of cell free culture supernatant was incubated with 20 mg of Whatman No. 1 filter paper (Whatman Inc., Florham Park, NJ, USA) and 1 mL of 0.05 M Sodium citrate buffer (pH 4.8) at 50 °C for 60 min. After incubation 3 mL of DNS was added and kept in a boiling water bath for 5 min. The reducing sugar released was measured at 540 nm in an UV–Vis spectrophotometer (Shimadzu) taking glucose as standard. One international unit (IU) of cellulase activity was expressed as the quantity of enzyme, which is required to release 1 μmol of glucose per minute under standard assay conditions (Ghose, 1987). Protein concentration was determined according to the method described by Bradford (1976).
2.4 One-variable-at-a-time approach
According to one-variable-at-a-time (OVAT) approach, BLM medium components were changed in this study to enhance the cellulase activity. Various substrates, namely, pre-treated bagasse, corn cob, rice straw, filter paper and CMC at 0.5% (w/v), were used separately as supplements in the media to enhance the growth of bacteria and the production of cellulase (Table S1 in supplementary information). Bagasse, corn cob and rice straw were sized to 5–10 mm size, before using them as supplements for the fermentation medium. Different substrates along with the media (BLM) were prepared, inoculated with the strain (10%v/v) and incubated on shaker at 150 rpm at 37 °C. The cell free supernatant was used for the analysis of cellulase activity. The substrate produced higher cellulase enzyme activity was considered as suitable substrate for further studies. In order to study the enhancement in the production of cellulase, five different nitrogen sources, namely, yeast extract, peptone, soybean meal extract, tryptone and urea at 0.1% (w/v) were used with appropriate substrate. The medium without nitrogen source was used as a control. To check the enhancement or inhibition of cellulase production, seven different metal ions in the form of salts, namely FeSO4·7H2O, NiSO4·7H2O, CaCl2·6H2O, MnCl2·4H2O, and CuSO4·5H2O, 0.001% (w/v) were used with appropriate chosen substrate and nitrogen source. Five surfactants – Polyethylene glycol (PEG), Sodium dodecyl sulphate (SDS), Triton X100, Tween-20, and Tween-80 at 0.2% (v/v) were used with appropriate selected substrate, nitrogen source, and metal ions. Similarly to study the effect of pH, temperature, and inoculum size on cellulase production, the medium was varied with different pH (4.5, 5.5, 6.5, 7.5, 8.5 and 9.5), temperature (32 °C, 37 °C, 42 °C, 47 °C and 52 °C) and inoculum size (1%, 2%, 3%, 4% and 5% v/v).
All the experiments were performed in triplicate and the average values of cellulase activity were used for further studies.
2.5 Plackett–Burman design
Plackett–Burman design (PBD) is a successful tool to examine the major influencing parameters among the large number of variables for the processes. To identify the most important variables that encourage higher cellulase production, a total of seven variables, including four media components (CMC (X1), yeast extract (X2), CaCl2·6H2O (X3) and Tween-20 (X4)) and three cultivation parameters (pH (X5), temperature (X6) and inoculum size (X7)) were studied carefully. The ranges of each variable are shown in Table 1. In this PBD study, 12 experiments were carried out to identify the highly influenced variables for cellulase production, and each experimental run performed in triplicate and the average values were used for further analysis. The experimental data were fitted in the following linear regression equation (1).
Variables
Unit
Symbols
Coded values
−1 Level
+1 Level
CMC
g/L
X1
5
20
Yeast extract
g/L
X2
1
5
CaCl2·62O
mg/L
X3
20
50
Tween-20
mL/L
X4
1
3
pH
–
X5
5
7
Temperature
°C
X6
37
47
Inoculum size
% v/v
X7
2
5
Run no.
Variables
Cellulase activity (IU/mL)
Residual value
X1
X2
X3
X4
X5
X6
X7
Observed
Predicted
1
−1
−1
+1
+1
+1
+1
−1
12.73
12.66
0.07
2
−1
−1
−1
−1
−1
−1
−1
8.07
7.99
0.08
3
+1
+1
+1
−1
+1
+1
−1
24.14
23.90
0.24
4
−1
+1
−1
−1
−1
+2
+1
11.56
11.35
0.22
5
+1
−1
−1
−1
+1
+2
+1
16.32
16.69
−0.37
6
−1
+1
+1
−1
+1
−1
−1
12.18
12.42
−0.24
7
+1
+1
−1
+1
+1
−1
+1
12.89
12.68
0.21
8
+1
−1
+1
+1
−1
+1
−1
25.41
25.36
0.05
9
+1
−1
+1
−1
−1
−1
+1
17.89
17.91
0.07
10
−1
−1
+1
+1
+1
−1
+1
11.19
11.10
0.09
11
−1
+1
+1
+1
−1
+1
+1
17.02
17.24
-0.21
12
+1
+1
−1
+1
−1
−1
−1
15.25
15.46
−0.21
2.6 Response surface methodology
Once the significant variables identified by PBD, Face centred central composite design (FCCCD) was used to evaluate the optimal level of each variable. FCCCD approach was used to analyze the main, quadratic, and interaction effects of the variables. Based on the PBD, four major variables have been selected for further optimization study, and the levels of each variable are given in Table 3. A total number of 30 experimental runs were carried out according to the FCCCD scheme. The following second order non-linear polynomial equation (2) was used to fit the data.
Term
Effect
Coefficient
T
P
Constant
–
15.40
157.35
0.00⁎
X1
6.54
3.27
33.42
0.00⁎
X2
0.22
0.11
1.14
0.32
X3
5.18
2.59
26.49
0.00⁎
X4
0.71
0.35
3.61
0.02⁎
X5
−0.97
−0.49
−4.97
0.01⁎
X6
4.94
2.49
25.23
0.00⁎
X7
−1.80
−0.90
−9.22
0.00⁎
R2 = 98.61%
R2 (adj) = 99.57%
Variables
Levels
−1 Level
0 Level
+1 level
CMC (X1) (g/L)
5
12.5
20
CaCl2·62O (X2) (mg/L)
20
35
50
Tween-20 (X3) (mL/L)
1
2
3
Temperature (X4) (°C)
37
42
47
Run no.
Coded variables
Cellulase activity (IU/mL)
X1
X2
X3
X4
Actual
Predicted
Residual
1
−1
−1
−1
−1
23.25
23.22
0.03
2
1
−1
−1
−1
26.47
26.46
0.01
3
−1
1
−1
−1
18.51
18.53
−0.02
4
1
1
−1
−1
18.05
18.08
−0.03
5
−1
−1
1
−1
12.01
11.98
0.03
6
1
−1
1
−1
34.69
34.71
−0.02
7
−1
1
1
−1
9.78
9.81
−0.03
8
1
1
1
−1
28.86
28.85
0.01
9
−1
−1
−1
1
19.72
19.71
0.01
10
1
−1
−1
1
29.23
29.22
0.01
11
−1
1
−1
1
20.71
20.71
0
12
1
1
−1
1
26.53
26.53
0
13
−1
−1
1
1
10.75
10.74
0.01
14
1
−1
1
1
39.8
39.75
0.05
15
−1
1
1
1
14.27
14.26
0.01
16
1
1
1
1
39.52
39.57
−0.05
17
−1
0
0
0
19.69
19.73
−0.04
18
1
0
0
0
34.04
34.01
0.03
19
0
−1
0
0
26.21
26.34
−0.13
20
0
1
0
0
24.02
23.91
0.11
21
0
0
−1
0
26.41
26.42
−0.01
22
0
0
1
0
27.31
27.32
−0.01
23
0
0
0
−1
10.05
10.04
0.01
24
0
0
0
1
13.62
13.65
−0.03
25
0
0
0
0
22.78
22.48
0.3
26
0
0
0
0
22.52
22.48
0.04
27
0
0
0
0
22.35
22.48
−0.13
28
0
0
0
0
22.46
22.48
−0.02
29
0
0
0
0
22.43
22.48
−0.05
30
0
0
0
0
22.39
22.48
−0.09
2.7 Validation of the predicted model
Batch fermentation studies were performed at the predicted conditions by RSM design in a 7 L fermenter with a working volume of 5 L. The samples were withdrawn at every 6 h up to 84 h for the determination of cellulase activity and cell growth.
3 Results and discussion
3.1 Screening of cellulase producers
Among sixty-eight bacterial strains obtained from Unhale hot spring water and sediment samples, thirty-one morphologically distinct strains were revealed to be positive in zone of clearance test. The higher zone of clearance produced strain was selected and screened for higher cellulase enzyme production (Fig. S1 in supplementary information). Similar screening method was reported by Prasad et al. (2013). Morphology, biochemical and physiological characters were determined for the highest cellulase producing isolate. The isolate was observed under microscope and was found to be rod shape, motile and Gram-Positive bacteria. The bacteria exhibited positive results for catalase, urease, oxidase, spore former and they hydrolyzed citrate, gelatin, starch, and they utilized different substrates such as glucose, fructose, sucrose, arabinose, mannose, glycerol and inulin. The 16S rRNA gene was amplified from genomic DNA (Fig. S2-a1), purified (Fig. S2-b1), sequenced and the relevant details are provided in the supplementary information. The 16S rRNA gene sequences of the isolate was aligned with related species of the genus Bacillus using CLUSTAL W (Roberts et al., 1994) which displayed the existence of high homology ranging of 99% with the closest relatives of with Bacillus licheniformis (Fig. 1). The 16S rDNA sequence was submitted in GenBank (Accession No. KC469614). The strain was submitted in NCIM, Pune (Accession No. NCIM 5556).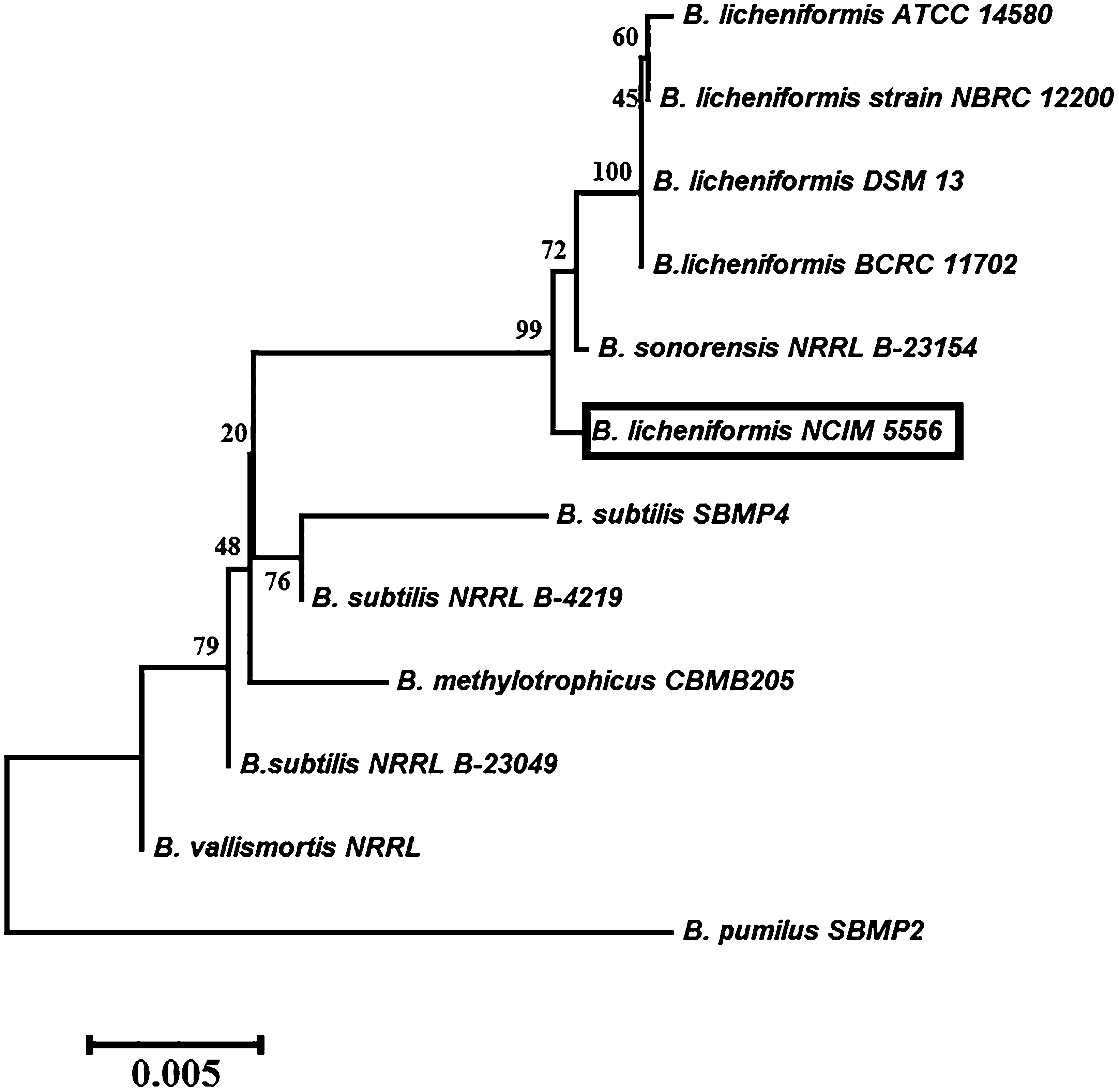
Phylogenetic tree constructed using the neighbor-joining method based on the 16S rDNA sequence of Bacillus licheniformis NCIM 5556 and the sequences of representative strains from GenBank. Bootstrap values (n = 1000 replicates) are reported as percentages. The scale bar indicates the number of changes per nucleotide position.
3.2 One variable at a time approach
Cellulase production has been influenced by several factors like carbon, nitrogen source, inoculum size, temperature, pH value, presence of inducers, and medium additives (Immanuel et al., 2006). In this study, five carbon and five nitrogen sources were evaluated for better cellulase production (Table S1 in supplementary information).
Among five carbon sources, CMC showed significant improvement in cellulase production. In general, the cellulase production was induced by CMC (Thakkar and Saraf, 2014). This is expected and, for instance, Lucas et al. (2001) showed that CMC was the desired substrate for endoglucanase production. It was clearly evident from various researchers reported that yeast extract shows significant effect on cellulase production (Li et al., 2008; Abou-Taleb et al., 2009). In this study yeast extract also exhibited higher cellulase production than with among other nitrogen sources. Abou-Taleb et al. (2009) also stated that organic nitrogen (i.e., yeast extract) was used to enhance cellulase production. CMC (14.27 IU/mL) and yeast extract (12.59 IU/mL) supplemented media resulted in high cellulase activity (Table S1 in supplementary information) and these substrates were selected as optimum medium constituents for cellulase production. The effects of various metal ions in the form of salts on cellulase activity were also evaluated. CaCl2·6H2O used media obtained higher cellulase activity (13.10 IU/mL). Reports also revealed that metal ion in the form of salt such as CaCl2·6H2O, provides protection to some enzymes against thermal denaturation and plays an important role to stabilize the native forms at high temperatures (Aygan and Arikan, 2008). MnCl2.4H2O did not show any effect on cellulase enzyme production. Such a similar trend was also obtained and reported by Shahriarinour et al. (2013). The impact of surfactants on cellulase activity was evaluated. Modified BLM supplemented with Tween-20 media enhanced the higher cellulase activity (13.08 IU/mL) (Matkar et al., 2013) and other surfactants like Tween-80, and Triton X 100 were shown to reduce cellulase activity. Effects of various pH on cellulase activity were evaluated using Modified BLM media. The highest cellulase production was obtained from media at pH 6.5 (13.62 IU/mL) using modified media. Enzyme activity was reduced at lower pH (4.5) and higher pH (9.5). The effect of different temperatures on cellulase activity was also studied. The highest cellulase activity was obtained at temperature 42 °C (13.80 IU/mL). Lower cellulase production was observed at increased temperatures. Effect of different percentage of inoculum on cellulase activity was studied. Highest cellulase activity was achieved at 5% (12.84 IU/mL) of inoculum.
3.3 Plackett–Burman design
In order to screen the major influencing variables for the cellulase production listed in Table 1, a two level fractional factorial Plackett-Burman (PB) design was carried out. The PB design consists of 12 runs and their corresponding cellulase activities are given in Table 1. All the runs were carried out in triplicates and the average values were reported. The effects of each variable are shown in Table 2. Out of seven variables CMC, Yeast extract, CaCl2·6H2O, Tween-20 and temperature showed positive effects, whereas pH and inoculum size showed negative effects. Correspondingly cellulase enzyme production optimization was also reported (Annamalai et al., 2013; Thakkar and Saraf, 2014). From the half normal plot and pareto chart (Figs. S3-a and S3-b (see supplementary information)), it was clearly evident that the most significant variables were CMC followed by CaCl2·6H2O, temperature, inoculum size, pH and Tween-20. All the variables were significant (probability p-value <0.05) (Table 2) except yeast extract because its p-value was higher than 0.05 (p = 0.317). The following linear regression model (Eq. (3)) was developed based on the experimental data in terms of actual values of the tested variables, and validated by correlation coefficient (R2). The R2 value was found to be 0.998, which indicates that there was only 0.2% variation in the data that could not be explained by the model (Table 2)
Further optimization studies were carried out, considering the most significant variables such as CMC, CaCl2·62O, temperature and Tween-20. Inoculum size and pH were kept at a lower level (−1) in the production medium since they exhibited a negative effect. However, yeast extract showed very little positive result, and hence it also was kept at a lower level in the medium for further studies.
3.4 Response surface methodology
The FCCCD was used to find the optimum conditions and to study the interaction effects of CMC (X1), CaCl2·6H2O (X2), Temperature (X3), and Tween-20 (X4) on the cellulase production. The variables and their levels are given in Table 3. Based on the FCCCD, 30 experiments were performed with four independent variables at three levels (−1, 0 and +1) (Table 3). A quadratic non-linear polynomial equation (4) was developed based on the experimental results and the independent variables in terms of actual values.
Model fitness and its adequacy were examined by ANOVA and Fisher’s F-test. The results are tabulated in Table 4. The higher F-value for the model (11870.59) and lower probability value (p < 0.05 at 95% confidence level) indicates that the model was significant. Further, the higher F value revealed that the maximum variation in the response (cellulase activity) could be explained by the model equation. From Table 4, the F-values are high as well as their probability p-values are less than 0.05 for all the individual, interaction and square terms of the variables, which implies that all the individual, interaction and square effects of each variables were highly significant. Besides, an insignificant lack of fit value (p > 0.05) indicated that the model was a good fit. However, the goodness of fit can be further ensured by the determination coefficient (R2), predicted R2 and adjusted R2 (Haaland, 1989). The determination coefficient was found to be 0.9991 indicating that model 99.9% of experimental data were compatible. Also, the high values of adjusted (0.99983) and predicted R2 (0.9998) indicated the high correlation between predicted and experimental values. Signal to noise ratio can be measured by adequate precision. The desirable value of adequate precision was considered to be greater than 4. In this study, the adequate precision was found to be 406.503 which indicate a satisfactory signal to noise ratio. Hence, the predicted model can be used to navigate the design space. The coefficient of variation was found to be 0.454%, which revealed the reliability and precision of the conducted experiments (Weisberg, 1985).
Source
SS
df
MS
F value
Prob > F
Model
1803.53
14
128.82
11870.59
<0.0001⁎
X1
917.35
1
917.35
84529.95
<0.0001⁎
X2
26.60
1
26.60
2450.75
<0.0001⁎
X3
3.65
1
3.65
336.70
<0.0001⁎
X4
58.61
1
58.61
5400.53
<0.0001⁎
X1X2
13.63
1
13.63
1256.37
<0.0001⁎
X1X3
379.96
1
379.96
35011.60
<0.0001⁎
X1X4
39.41
1
39.41
3631.20
<0.0001⁎
X2X3
6.31
1
6.31
581.69
<0.0001⁎
X2X4
32.35
1
32.35
2980.71
<0.0001⁎
X3X4
5.16
1
5.16
475.87
<0.0001⁎
X12
49.94
1
49.94
4602.16
<0.0001⁎
X22
18.06
1
18.06
1664.60
<0.0001⁎
X32
49.83
1
49.83
4591.69
<0.0001⁎
X42
293.29
1
293.29
27025.22
<0.0001⁎
Residual
0.16
15
0.01
Lack of Fit
0.04
10
0.00
0.183
0.9888⁎⁎
Pure error
0.12
5
0.02
Cor total
1803.69
29
For better understanding the interaction between the variables and their effects on the responses, three dimensional response surface graphs were constructed (Fig. 2a–f). In these 3D graphs, cellulase activity was plotted on z-axis against any two parameters, while other variables were kept at a constant level, particularly at its centre level. The graphs are clearly demonstrating a strong interaction between the independent variables.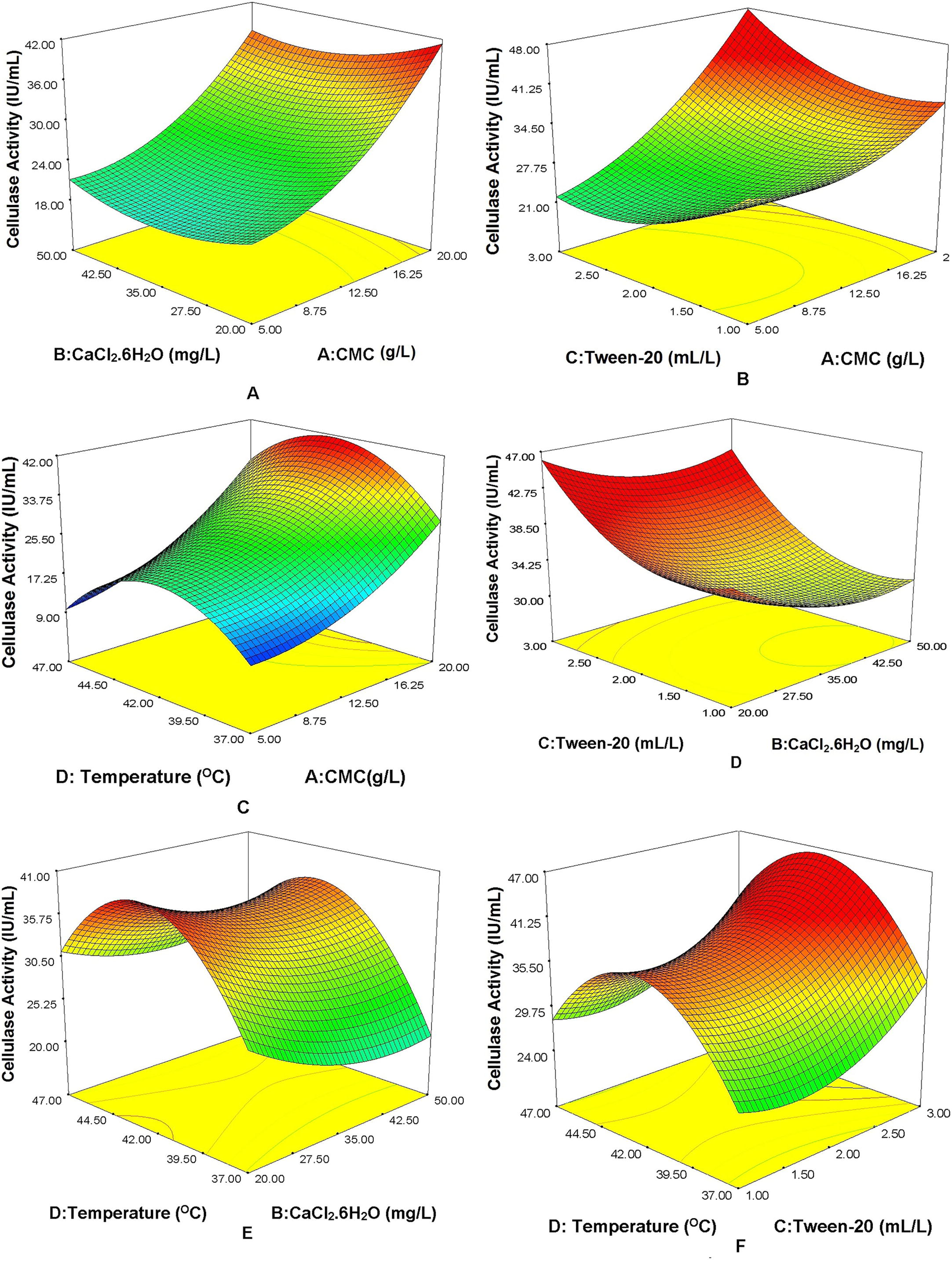
Three dimensional response surface plots showing effects of variables and its interaction on cellulase activity. (a) CMC vs CaCl2·62O; (b) CMC vs tween-20; (c) CMC vs temperature; (d) CaCl2·62O vs Tween-20; (e) CaCl2·62O vs temperature; (f) Tween-20 vs temperature.
CMC is a crucial factor for the cellulase production. The cellulase activity increases with CMC (Fig. 2a–c) at lower concentrations of CaCl2·6H2O, Tween-20 and at moderate temperature. Incubation temperature is an important parameter and exhibits a significant effect on the cellulase production. The effect of temperature on cellulase production was studied in between 37 °C and 47 °C. Fig. 2c, e and f shows the effect of temperature on cellulase activity. It is clearly observed that the higher cellulase activity was obtained at 42 °C. The cellulase activity decreased with the higher concentration of calcium chloride (Fig. 2d and e). The effect of Tween-20 concentration was studied along with other parameters to check the activity of cellulase as represented in Fig. 2b, d and f. The higher concentration of surfactant Tween-20, enhanced the oxygen transfer rate of microorganism and resulted in increased cellulase activity (Fig. 2b, d and f). Maximum cellulase activity was obtained at the highest concentration of Tween-20 (3 mL/L).
Fig. S4a–b in supplementary information shows perturbation plot and predicted vs actual cellulase activity plot respectively. The curvature of each variable line in the perturbation plot indicates the strong interaction between the independent variables. As specified above, the higher values of R2 and predicted R2 showed a high correlation between predicted and experimental values. This is also evident from the predicted vs actual cellulase activity plot (Fig. S4b). Fig. S4b shows that the experimental values were quite similar to that of the predicted values. From the analysis of response surface curves the optimal conditions were found to be CMC, 19.21 g/L, CaCl2·62O, 25.06 mg/L, Tween-20, 2.96 mL/L, and temperature 43.35 °C. The cellulase activity was observed to be 40.88 IU/mL at the optimum conditions predicted by RSM.
3.5 Verification of the predicted model
Submerged fermentation was carried out to validate the optimized conditions predicted by RSM. Fermentation was carried out for 84 h and the sample was withdrawn at every 6 h interval for the determination of cellulase activity and growth of the strain. The cellulase activity was increased along with biomass growth up to 72 h. The microorganism entered into the stationary phase after 72 h and hence resulted in a decrease in cellulase activity (Fig. 3). The maximum cellulase activity was found to be 42.99 IU/mL, which substantiated cellulase activity (40.88 IU/mL) predicted by RSM. RSM optimized media produced the highest cellulase activity so far reported (Rashid et al., 2009; Saravanan et al., 2012; Thakkar and Saraf, 2014; Dave et al., 2015). Hence, Bacillus licheniformis NCIM 5556 is a promising producer of cellulase compared to previous studies (Acharya and Chaudhary, 2011, 2012) and it can be utilized for enzyme production at an industrial scale.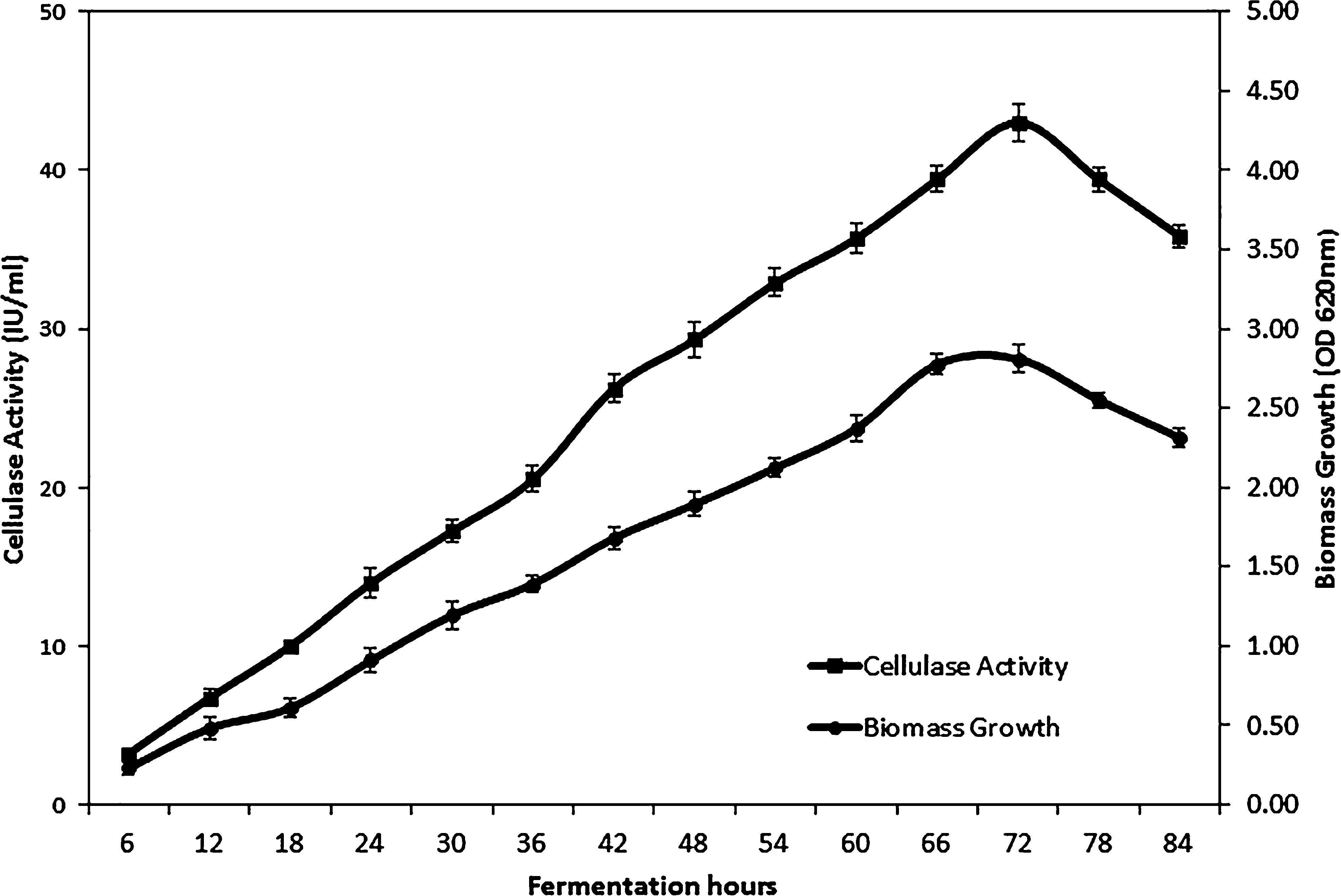
Growth of Bacillus licheniformis NCIM 5556 and cellulase production in bioreactor using optimized conditions CMC, 19.21 g/L, CaCl2·62O, 25.06 mg/L, Tween-20, 2.96 mL/L, and temperature 43.35 °C. (■) Cellulase activity (IU/mL); (●) Biomass estimation (OD at 600 nm).
4 Conclusion
Cellulase producer Bacillus licheniformis was isolated from samples of Unhale Hot spring water and soil sample. Culture and its gene sequence were deposited in NCIM and GenBank respectively. OVAT approach was used to select the variables for cellulase production. Four out of seven significant variables were (CMC, CaCl2·6H2O, Tween-20, temperature) screened through PB design. These four significant variables were further optimized through RSM based face centred central composite design. The optimal conditions achieved by RSM-FCCCD were experimentally validated. The cellulase production was found to be increased by 3-fold with optimized media as compared to unoptimized basal media. A comparative table for the production of cellulase B. licheniformis is shown in Table S2 (see supplementary information). Thus the media and stain could be utilized for cellulase production at large scale operations. However, further studies on enzyme purification and characterization would pave the way for an efficient cellulase for industrial applications.
Acknowledgements
We would like to thank TATA Chemicals Ltd, Innovation Centre, Pune 412111, Maharashtra, India for utilizing the infrastructure and support to carry out this work. We would like to thank Manonmaniam Sundaranar University, Abisekapatti, Tirunelveli 627012, Tamil Nadu, India, for recognizing our work. We thank our colleagues for their moral support.
References
- Nutritional and environmental factors affecting cellulase production by two strains of cellulolytic Bacilli. Aust. J. Basic Appl. Sci.. 2009;3(3):2429-2436.
- [Google Scholar]
- Effect of nutritional and environmental factors on cellulase activity by thermophilic bacteria isolated from hot spring. J. Sci. Ind. Res.. 2011;70:142-148.
- [Google Scholar]
- Optimization of fermentation conditions for cellulases production by Bacillus licheniformis MVS1 and Bacillus sp. MVS3 isolated from Indian hot spring. Braz. Arch. Biol Technol.. 2012;55(4):497-503.
- [Google Scholar]
- Thermostable, haloalkaline cellulase from Bacillus halodurans CAS 1 by conversion of lignocellulosic wastes. Carbohydr. Polym.. 2013;94(1):409-415.
- [Google Scholar]
- A new halo-alkaliphilic, thermostatble endoglucanase from moderately Bacillus sp. C14 isolated from van Soda Lake. Int. J. A. Biol.. 2008;10(4):369-374.
- [Google Scholar]
- Bacillus pumilus S124A carboxymethylcellulase; a thermo stable enzyme with a wide substrate spectrum utility. Int. J. Boil. Macromol.. 2014;67:132-139.
- [Google Scholar]
- Optimization of critical medium components using response surface methodology for ethanol production from cellulosic biomass by for ethanol production from cellulosic biomass by Clostridium thermocellum SS19. Process Biochem.. 2005;40(9):3025-3030.
- [Google Scholar]
- Cellulases and related enzymes in biotechnology. Biotechnol. Adv.. 2000;18:355-383.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-754.
- [Google Scholar]
- Production of cellulolytic enzyme system in mixed culture solid state fermentation of soya bean hulls supplemented with wheat bran. Process Biochem.. 2010;45(1):120-128.
- [Google Scholar]
- Brock T.D., ed. Thermophiles: General: Molecular and Applied Microbiology. New York: John Wiley & Sons; 1986. p. :1-16.
- Optimization of process parameters for cellulase production by Bacillus licheniformis MTCC 429 using RSM and molecular characterization of cellulase gene. J. Bioprocess Biotechnol.. 2015;2015(5):212.
- [Google Scholar]
- A biophysical perspective on the cellulosome: new opportunities for biomass conversion. Curr. Opin. Biotechnol.. 2008;19:218-227.
- [Google Scholar]
- Geochemistry of thermal waters from various geothermal provinces of India. In: Proceedings of the Grenoble Symposium of the International Association of Hydrological Sciences. Wallingford, Oxfordshire, United Kingdom: IAHS Press, Institute of Hydrology; 1975. p. :47-58.
- [Google Scholar]
- Statistical problem solving. In: Haaland P.D., ed. Experimental Design in Biotechnology. New York and Basel: Marcel Dekker Inc; 1989. p. :1-18.
- [Google Scholar]
- Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int. J. Environ. Sci. Technol.. 2006;3(1):25-34.
- [Google Scholar]
- Screening and characterization of thermophilic bacteria (lipase, cellulase and amylase producers) from hot springs in Saudi Arabia. J. Food Agric. Environ.. 2011;9:672-675.
- [Google Scholar]
- Cloning of the thermostable cellulase gene from newly isolated Bacillus subtilis and its expression in Escherichia coli. Mol. Biotechnol.. 2008;40(2):195-201.
- [Google Scholar]
- Production, purification, and properties of an endoglucanase produced by the hyphomycete Chalara (Syn. Thielaviopsis) paradoxa CH32. J. Agric. Food Chem.. 2001;49(1):79-85.
- [Google Scholar]
- Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev.. 2002;66:506-577.
- [Google Scholar]
- Geoelectric investigations in Bakreswar geothermal area, West Bengal, India. J. Appl. Geophys.. 2000;45(3):187-202.
- [Google Scholar]
- Production of cellulase by a newly isolated strain of Aspergillus sydowii and its optimization under submerged fermentation. Int. Biodeter. Biodegr.. 2013;78:24-33.
- [Google Scholar]
- Use of dinitrosalicylic acid reagent for the determination of reducing sugars. Anal. Chem.. 1959;31(3):426-428.
- [Google Scholar]
- Statistical modeling and optimization of alkaline protease production from a newly isolated alkalophilic Bacillus species BGS using response surface methodology and genetic algorithm. Prep. Biochem. Biotechnol.. 2013;43:293-314.
- [Google Scholar]
- Characterization of the cellulolytic enzyme produced by Streptomyces griseorubens (Accession No. AB184139) isolated from Indian soil. J. King Saud Univ. Sci.. 2013;25:245-250.
- [Google Scholar]
- Optimization of the Nutrient supplients for cellulase production with the basal medium palm oil mill effluent. World Acad. Sci. Eng. Tech.. 2009;60:809-815.
- [Google Scholar]
- Bacillus mojavensis sp.nov., distinguishable from Bacillus subtilis by sexual isolation, divergence in DNA sequence, and difference in fatty acid composition. Int. J. Syst. Bacteriol.. 1994;44(2):256-264.
- [Google Scholar]
- Optimization of cellulase production using Trichoderma reesei by RSM and comparison with genetic algorithm. Front. Chem. Sci. Eng.. 2012;6(4):443-452.
- [Google Scholar]
- Developments in the use of Bacillus species for industrial production. Can. J. Microbial.. 2004;50:1-17.
- [Google Scholar]
- Effect of medium composition and cultural condition on cellulase production by Aspergillus terreus. Afr. J. Biotechnol.. 2013;10(38):7459-7467.
- [Google Scholar]
- Application of statistically based experimental designs to optimize cellulase production and identification of gene. Nat. Prod. Bioprospect.. 2014;4(6):341-351.
- [Google Scholar]
- Applied Linear Regression. NY: John Wiley and Sons; 1985.
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2016.08.001.
Appendix A
Supplementary material







