Translate this page into:
Sensing and antimicrobial activity of polyaniline doped with TiO2 nanocomposite synthesis and characterization
⁎Corresponding author. pragathis46@gmail.com (C. Pragathiswaran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Pure PANI and TiO2/PANI nanocomposite composites with widely different TiO2 weight percentages were synthesized by chemical oxidative method. Polymerization of aniline using ammonium per sulfate (APS) in acidic medium at room temperature for the PANI/TiO2 nanocomposite. UV–visible absorption spectroscopy, X-ray diffraction (XRD), Photo luminescence spectroscopy (PL) and Scanning electron microscopy were used to examine the morphological and structural features of these composites (SEM). Dynamic light Scattering (DLS) investigation is performed to find the size of nanocomposite of PANI/TiO2. The multifunctionality of TiO2 nanocomposite doped with PANI was incorporated. The sensing action of diazepam drugs has been widely used. The antimicrobial activity of PANI/TiO2 nanocomposite inhibition efficiency was investigated.
Keywords
Polyaniline
TiO2
Diazepam
Spectroscopical analysis
Microscopic analysis
Photoluminescence
Biological properties
Antimicrobial activity
1 Introduction
PANI/TiO2 nanocomposite was formed using ammonium persulfate (APS) as the oxidant in the current investigation of aniline with TiO2 (Kang et al., 1998). The polymer should be in its conductive form to generate the PANI/TiO2 for synergistic action (Lin et al., 2012). The PANI conductivity and aggregate sizes, on the other hand, are parameters determined by the synthesis conditions (Sapurina et al., 2012). The goal of this research was to create PANI/TiO2 composite photo catalysts, as well as to investigate the stability, content, and properties of PANI which are all important for the PANI/TiO2 effect. Due to its wide range of applications, the combination of PANI and inorganic materials such as semiconductor nanoparticles is gaining appeal. Using ammonium per sulfate PANI/TiO2 nanocomposites have extremely separate properties than conventional inorganic dopants of mineral acids and they are critical in the production of electronic devices with superior electrical, magnetic, and optical properties (Sudha et al., 2009; Saxena and Malhotra, 2003). TiO2 is an amazing photo material due to its wide band gap range. Photo catalysis is a well-studied method for using light to stimulate semiconductor metals (Manjunath et al., 2008; Singh et al., 2007; Govindan Nadar et al., 2021; Govindan et al., 2019). Nano TiO2 conversion and nanopart dispersion or nanoTiO2 in a suitable medium such as PVA (Gao et al., 2007; Güleryüz et al., 2013), PMMA (Stejskal et al., 1992; Sung et al., 2006), EVA (Khanna et al., 2007; Zhang et al., 1999) is a revolutionary process for preparing another film. Polyvinyl alcohol (PVA) is often utilized as the host matrix in the fabrication of conducting polymer polyaniline nano composite films (Rajivgandhi et al., 2019; Sudha et al., 2009). The microstructure of the conducting polymer polyaniline changes over time when it grows in the presence of inorganic minerals (TiO2) and PVA (González-Benito et al., 2013; Park et al., 2004). The PVA polymer and nano composite are useful application (Hermas et al., 2014; Yu et al., 2011; Rajivgandhi et al., 2019). Several researchers have observed that polyaniline's thermal stability can be increased by mixing it with TiO2, SnO2, polystyrene (Al-Ahmed et al., 2004); xanthenes gum (Ebrahim et al., 2009), and polythiophene (Mo et al., 2008). Antimicrobial activity of PANI/TiO2 nanocomposites against Staphylococcus aureus and Escherichia coli has been observed (Alam et al., 2013). By producing nanostructured forms with the addition of metal oxide, some recent studies have increased (PANI) as a sensitivity and selectivity (Hu et al., 2012; Jiang, 2008). Nanocomposite characteristics are controlled by morphology and interfacial variables in addition to constituent qualities (Dexmer et al., 2008). The majority of these approaches rely on aggregation in the presence of Hg (II) to cause a change in color change in melting transitions and electrochemical measurements (Kaushik et al., 2009; Green and Woodhead, 1912; Govindan Nadar et al., 2020; Muthuchamy et al., 2020; Genies et al., 1990).
2 Material and methods
2.1 Needed chemicals and instruments
Aniline (Sigma-Aldrich), HCl Merck, 37%, ethanol (99.8% Merck), 30%, H2O2 (Sigma-Aldrich), 95%, and H2SO4 (Merck), 98% (Sigma) Ammonium ferrous sulphate, On a Shimadzu FTIR-8400 spectrometer (Tokyo, Japan), FTIR spectra of PANI and its nanocomposites in the wave number range of 400–4000 cm−1 were recorded. On a Hitachi U-2900 spectrophotometer (Tokyo, Japan), In NMP, UV–visible absorption spectra of PANI and its nanocomposites were observed in the range of 250–800 nm. The Expert–PRO X-ray diffract device used for measuring the X-ray diffraction (XRD) of the materials. The Cu as anode (K-Alpha −1.54060), all compounds were examined in the range of 10 to 80. The data were measured with a DLS 5000 laser spectrometer geonimetre (ALV) from Germany (Sung et al., 2006).
2.2 Synthesis of polyaniline
The in-situ chemical oxidation polymerization technique was used to generate the polyaniline composite. To create aniline hydrochloride, 1 M of 9 ml aniline was combined with 1 M of 3 ml hydrochloric acid for 15 min. 0.1 M of ammonium persulphate (APS), an oxidant, was added dropwise with continuous stirring at 50 0C for 4 h to completely polymerize this solution. The precipitate was filtered, rinsed with deionized water and acetone, and then dried in an oven for 24 h to obtain a homogeneous mass.
2.3 Synthesis of polyaniline titanium dioxide (PANI/TiO2) nano composites
An in-situ chemical oxidation polymerization technique was used to create PANI/TiO2 nanocomposites. To create aniline hydrochloride, 1 M of 9 ml aniline was added with 1 M of 3 ml hydrochloric acid (HCl) for 15 min. TiO2 powder is added in the mass fraction to the previous solution with vigorous swirling to keep the TiO2 homogeneously suspended in the solution. 0.1 M of ammonium persulphate (APS), an oxidant, was added drop by drop at 50 °C for 4 h with constant stirring to fully polymerize this solution. The precipitate was filtered, washed with deionized water and acetone, and dried in an oven for 24 h to create a reliable mass of PANI/TiO2 nanocomposite with various TiO2 weight percentages (5 %, 10%, 15%, 20%, and 25%) formed in this manner. The samples were then ground down with an agate mortar (Saxena and Malhotra, 2003). Total synthesis process was available in Scheme 1.
Pictorial PANI/TIO2 nanocomposite synthesis diagram.
2.4 Preparation of antimicrobial assay discs
Bacterial strains of Klebsiella pneumoniae and Proteus mirabilis were developed at the Chandigarh’s Microbial Type Culture Collection Centre (MTCC). The Microbial Type Culture Collection Centre (MTCC) in Chandigarh created the fungus strains Aspergillus Niger and Aspergillus flavus. The Whatmann No. 1 filter paper has been used to make the discs with a diameter of 6 mm. At 121 °C, the discs were autoclaved. The moisture discs were disinfected before being dried in a hot air oven at 50 °C. After that, a quantity of solvent extract and control discs were made.
3 Results and discussion
3.1 Fourier- transforms infrared spectra
Fig. 1 depicts TiO2 and PANI/TiO2 for FT-IR spectra. Polyaniline major distinguishing bands were visible in addition to the TiO2 peaks. The quinoid and benzenoid structures of PANI were represented by the bands at 1513 and 1599 cm−1 respectively. The band at 1285 cm−1 was also assigned to C–N stretching of a secondary aromatic amine. The presence of PANI/TiO2 in the complex is identified by the presence of asymmetric and symmetric C=O stretching peaks at 1206 and 694 cm−1. The peak at 694 shifted to 679 cm−1 due to TiO2 coordinated with PANI. FTIR approach is very important for transition element doped the organic compound below 1000 cm−1 is arrived.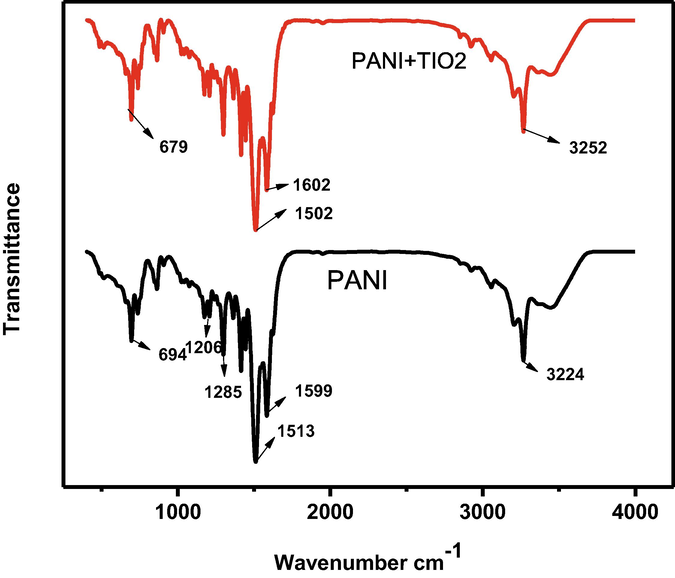
FT-IR spectrum of PANI/TiO2.
3.2 Polyaniline with TiO2 UV–Visible spectrum
Fig. 2(a) and (b) show the UV–Vis spectrum of PANI and PANI/TiO2 nanomaterial. The metals are attributed to the two distinctive bands of doped PANI with TiO2 that emerge at about 384 and over 402 nm. The generated nanocomposite can absorb UV and visible light strongly (Lin et al., 2012) as shown in Fig. 2(b). PANI bands with a wavelength of 295 nm can be seen in the hybrid samples. Furthermore, in the nanocomposite, the peak at over 402 nm in PANI doped with TiO2 is clearly displaced. Encapsulation of TiO2 NPs has an influence on conducting polyaniline doping was determined.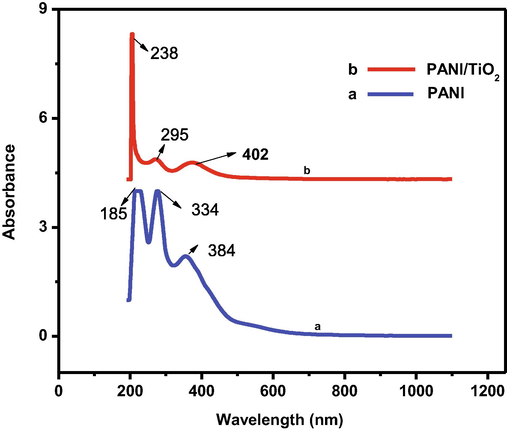
UV–visible of spectrum of (a) PANI (b). PANI/ TiO2.
3.3 PANI/TiO2 X-ray di fraction
Polyaniline and PANI/TiO2 composites were investigated by X-ray diffraction patterns. Polyaniline has an amorphous structure, according to X-ray diffraction studies. Fig. 3 shows that the PANI/TiO2 composites of X-ray diffraction pattern exhibits well-defined broad peaks, indicating that the materials are well-crystalline. The observed 2θ values of 28, 36 and 55 of planes 112, 102 1nd 110 are coordinated in line with the JCPDS no-88-8622 standard. Due to the presence of TiO2, the resultant diffract ion has a perfect crystalline structure. The XRD pattern of the PANI/TiO2 composite reveals that it has an average nano particle size of 1.641 nm due to TiO2 dispersion in polyaniline during the polymerisation.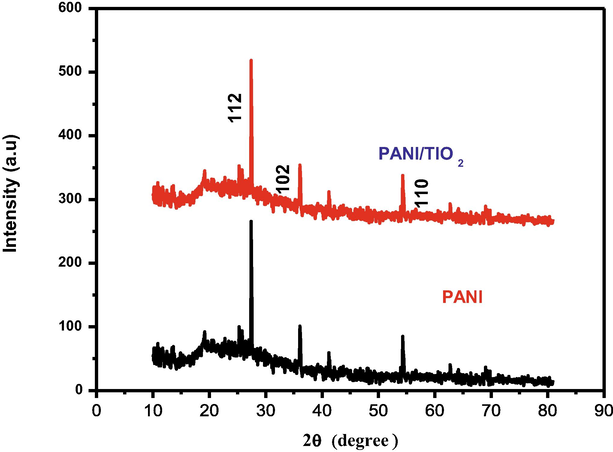
XRD image of nanocomposite PANI/TIO2.
3.4 Photoluminescence studies
Photoluminescence is the light emission and absorption of photons. The emission over a widespread range from the violet to red. PL properties organic polymers emission peak obtains from poly aniline 645 nm and PANI / TiO2 nanocomposite were formed. The emission peak obtained at 645 nm as shown in Fig. 4. It is shown a sharp peak emission of light by conjugated polymer is due to inter chain energy migration and experimental evidence abundance by the presence of two types of excitation (Chen et al., 2005). These excitations are PANI and PANI doped TiO2 nanocomposite through transfer from polaron bond high energy transaction in the presence of photo Luminas spectra (Goncalves et al., 2010). The existence of low energy (1.4 and 2.1 eV) transitions due to the polaron bands and high energy (4.0 eV) show the emission about very high intensity to dopant concentration may be strong the interaction between π bond conjugation of poly aniline doped TiO2 nano particles strong interaction due to the existences polymer multiple electronic state polar and defect to investigated by PLS spectra.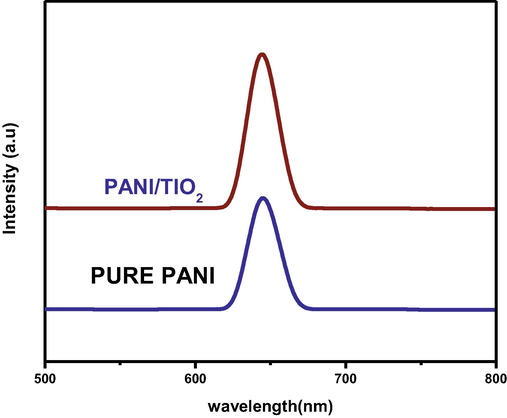
PL spectra of PANI/TIO2 nanocomposite.
3.5 DLS studies
Fig. 5 shows the strong absorption of light by the PANI/TiO2 nanocomposite prevents the DLS approaches from observing light scattering during polymerization. The reaction mixture was diluted with 10 ml of 1 M HCl in 60 s interval. After dilution mixture was the immediate characterized by particles size analyzed using with DLS. The PANI doped TIO2 nanocomposite particles size distribution of curve show intensively two peak at 73.1 nm and 1502 nm as shown in Table 1. They are attributed to diameter and length of PANI/TIO2 nanocomposite (Liu et al., 2006). Moreover DLS results proved that the water content swelling nano fiber.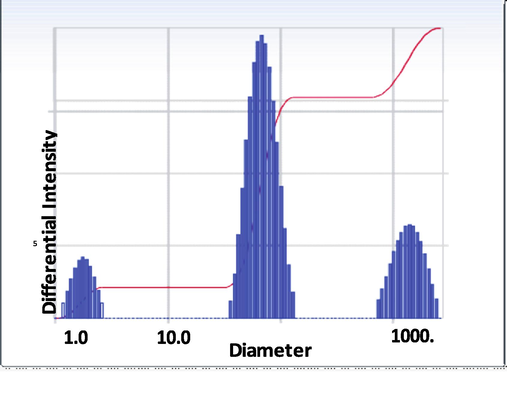
DLS image of PANI/TIO2 nanocomposite.
Distribution Result
Peak
Diameter (nm)
1
1.8
2
73.1
3
1,502
4
0.0
5
0.0
3.6 Scanning electron microscope (SEM)
The SEM image of PANI nanoparticles in 1 mM aniline (pH = 5) solution is shown in Fig. 6. The bulk powder rice grain qualities are fused together rather than distinct particles (Sapurina et al., 2012). The PANI nanospheres were synthesized depending on the outcomes as shown in Fig. 6a. PANI nanospheres have an average width of 20 nm and a thickness of 40 to 100 nm as shown in Fig. 6b. Titanium apparently appeared in EDAX spectrum as shown in Fig. 6c.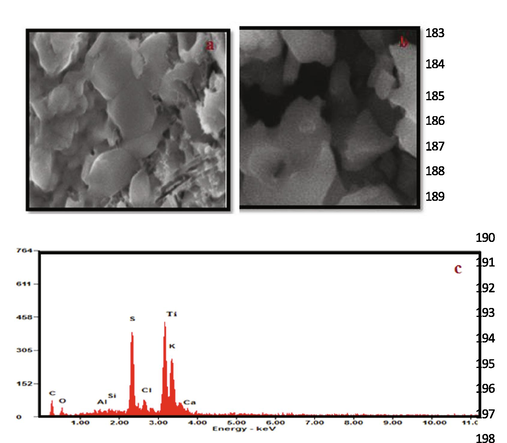
SEM image of (a) PANI and (b) PANI doped with TiO2 (c) EDAX-spectrum.
3.7 Cyclic voltammetry
The electron mediating qualities for a current response are particularly significant for an electrochemical material. Due to the presence of 350 mM diazepam in 0.1 M PBS, the major characteristics of a competent electron mediator include improved current responsiveness and reduced variance in peak potential (DEp). Fig. 7a of PANI/GCE shows that the increased electro catalytic current response of various components of the sensor. The Fig. 7b and Fig. 7c show that the sensing capability increase when PANI doped with Titanium dioxide against Diazepam (Cai et al., 2017) (pH-7). The modest reduction peak current of diazepam was detected at about −1.5 V for the bare electrod whereas the corresponding potential for doped the nanocomposite of GCE electrode was at −2V. In the present study simple and effective sensor set us has been developed by using glass cuvettes. PANI doped TiO2 nanocomposite immerse sensing the diazepam solution was recorded by cyclic voltammeter. The standard value of signals to find out all the immersion time for PANI/TiO2chemical sensor against 100 ppm of diazepam (Govindan et al., 2020). The PANI/TiO2 nanocomposite has recorded an initial increase with time expose to 100 ppm of diazepam (Fig. 7d).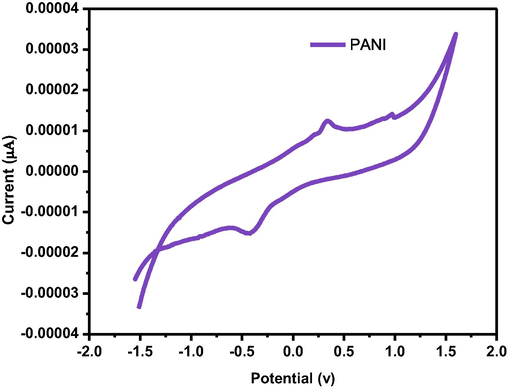
Cyclicvoltammogram of the GCE/PANI.
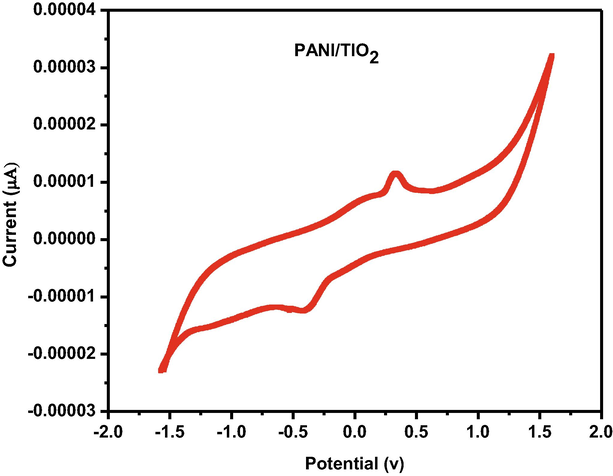
Cyclicvoltammogram of the GCE/PANI/TiO2.
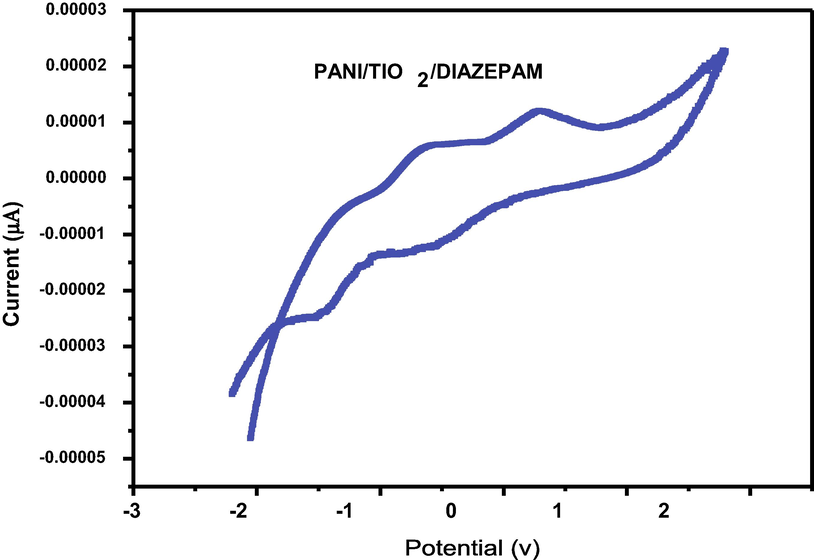
Cyclicvoltammogram of the GCE/PANI/TIO2/DIAZEPAM.
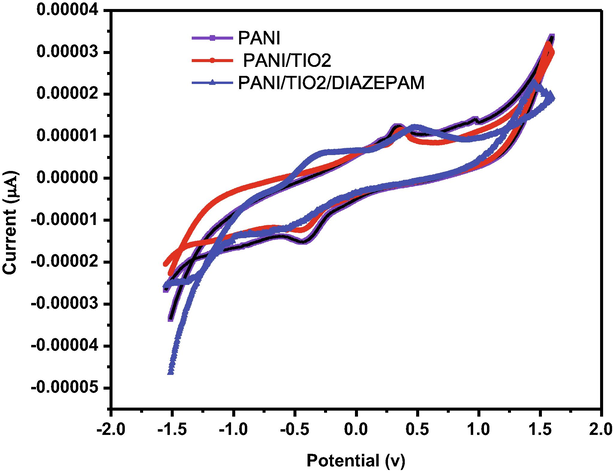
Cyclicvoltammogram of the GCE/PANI/PANI + TIO2/DIAZEPAM.
3.8 Assay of antibacterial activity
A modified Bauer method has been used to investigate antibacterial activity. Before being refrigerated to 45°Celsius, Muller Hinton agar was prepared and autoclaved for 20 min at 15 lb of pressure. The solidified media was transferred to sterile petriplates after cooling. Using a sterile swab, the plates with medium were seeded with the appropriate bacterial isolate. The various solvent extract discs as well as control and standard Nitrofurantoin (300 g) for Bacter discs were placed separately on each petriplate. At 37 °C, the plates were incubated for 24 h. The diameter of the zone formed around the paper disc was measured and expressed in mm after the incubation period (Fig. 8a). The values of the zones were arranged in Table 2.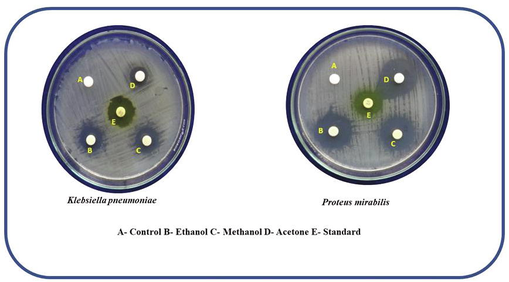
Anti-bacterial activity of the nanomateral against K. pneumoniae and P. mirabilis.
S.No.
Microorganism
Zone of Inhibition (mm in diameter)
Control
Standard*
Acetone
Ethanol
Methanol
2
Klebsiella pneumoniae
–
18
18
21
19
3
Proteus mirabilis
–
20
20
18
16
3.9 Assay of antifungal activity
Antifungal activity was determined using a modified version of the Bauer et al technique (1966). PDA (Potato Dextrose Agar) was prepared, autoclaved for 20 min at 15 lb of pressure and chilled to 45 °C. The cooled fluid was mixed with 10 ml/L tartaric acid (10 percent) which acts as an antibacterial agent and placed on sterile petriplates to solidify. The microorganism vesicles have been seeded onto the medium plates using a sterile cotton swab. The extract discs first from various organic solvents were placed individually on each Petri plate along with control and standard (Iitraconazole (10 g) discs. For 72 h, the plates were kept at 28 °C. The diameter of the zone formed around the paper disc was measured and represented in millimetres after the incubation period was completed. The zone of inhibition against tested fungi was indicated in Fig. 8b and the zone of inhibition values were available in Table 3.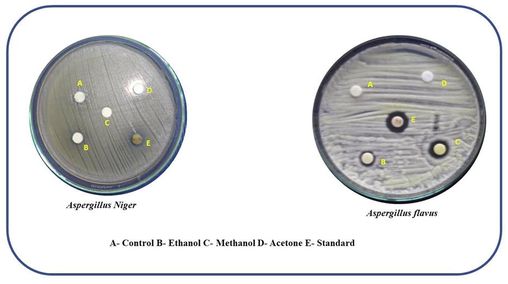
Anti-fungal activity of nanomaterial against Aspergillus niger and Aspergillus flavus.
S.No.
Microorganism
Zone of Inhibition (mm in diameter)
Control
Standard*
Acetone
Ethanol
Methanol
1
Aspergillus Niger
–
20
1
8
8
2
Aspergillus flavus
–
12
1
11
10
4 Conclusion
PANI and TiO2 nanocomposite were successfully encapsulated by in situ chemical oxidative methods PANI on the surface of TiO2 nanoparticles formation of PANI/TiO2 nanocomposite at identification of techniques by FTIR, UV, XRD and SEM. The result showing metal oxide dopped PANI and TiO2 nanocomposites increased the polymerization and more effective sensing capability. When compared to pure PANI and PANI doped TiO2 nanocomposite effective qualities of sensitivity. The nanocomposite interaction between diazepam drug molecules has superior sensitivity was measured by cyclic voltammeter. The antibacterial activity of PANI/TiO2 nanocomposite was examined. Klebsiella pneumoniae and Proteus mirabilis were among the antimicrobial compounds isolated. Disk diffusion method for isolated fungi, such as Aspergillus Niger and Aspergillus flavus were investigated. The disc diffusion method was used to compare the percentage of inhibition of collected PANI/TiO2 nanocomposites and fungus to the positive control Amphotericin-B (200 mg).
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers, Supporting Program number (PNURSP2022R82), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Composites of polyaniline and cellulose acetate: preparation, characterization, thermooxidative degradation and stability in terms of DC electrical conductivity retention. Synth. Met.. 2004;144:29-49.
- [Google Scholar]
- Optical and electrical conducting properties of Polyaniline/Tin oxide nanocomposite. Arab. J. Chem.. 2013;6(3):341-345.
- [Google Scholar]
- Mater. Res. Express. 2017;4:65.
- J. Chem. Phys.. 2005;123:124707
- Vanadium oxide-PANI nanocomposite-based macroscopic fibers: 1D alcohol sensors bearing enhanced toughness. Chem. Mater.. 2008;20(17):5541-5549.
- [Google Scholar]
- Ac and Dc conductivities of polyaniline/poly vinyl formal blend films. Curr. Appl. Phys.. 2009;9(2):448-454.
- [Google Scholar]
- Preparation and photocatalytic activity of PANI/ TiO2 composite film. Rare Metals. 2007;26:1-7.
- [Google Scholar]
- Genies E.M., Boyle A., Laskowski M., Tsintavis C., 1990. Synth. Met. 36, 139; Stejskal J., Gilbert R.G., 2002. Pure Appl. Chem. 74, 857.
- Langmuir. 2010;26:11657.
- Coefficient of thermal expansion of TiO2 filled EVA based nanocomposites, A new insight about the influence of filler particle size in composites. Eur. Polym. J.. 2013;49(7):1747-1752.
- [Google Scholar]
- Graphene/nickel oxide nanocomposites against isolated ESBL producing bacteria and A549 cancer cells. Mater. Sci. Eng. C. 2019;102:829-843.
- [Google Scholar]
- Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumonia. Colloid. Surf., A. 2020;600:124830
- [Google Scholar]
- Photocatalytic reduction and anti-bacterial activity of biosynthesized silver nanoparticles against multi drug resistant Staphylococcus saprophyticus BDUMS 5 (MN310601) Mater. Sci. Eng. C. 2020;114:111024
- [Google Scholar]
- Effect of Ti and Cu doping on the structural, optical, morphological and anti-bacterial properties of nickel ferrite nanoparticles. Results Phys.. 2021;23:104065.
- [Google Scholar]
- Green A.G., Woodhead A.E., 1912. J. Chem. Soc., Trans., 101, 1117; Rao P.S., Satyanarayana D.N., Jeevananda T. In: Advanced Functional Molecules and Polymers
- Novel strategy to prepare polyaniline-modified SiO2/TiO2 composite particles. Synth. Metals. 2013;181:104-109.
- [Google Scholar]
- Electrosynthesis and protection role of polyaniline- polyvinyl alcohol composite on stainless steel. Progr. Org. Coat.. 2014;77:403-411.
- [Google Scholar]
- One-step synthesis and antibacterial properties of polyaniline/TiO2 nanocomposites. Adv. Mater. Res.. 2012;534:78-81.
- [Google Scholar]
- Facile synthesis and characterization of polyaniline/NiFe2O4 nanocomposite in w/o microemulsion. J. Macromol. Phys.. 2008;47(2):242-249.
- [Google Scholar]
- Polyaniline: A polymer with many interesting intrinsic redox states. Rog. Polym. Sci.. 1998;23:277-324.
- [Google Scholar]
- Hybrid cross-linked polyaniline-WO3 nanocomposite thin film for NOx gas sensing. J. Nanosci. Nanotechnol.. 2009;9(3):1792-1796.
- [Google Scholar]
- Synthesis of nano-particles of anatase TiO2 and preparation of its optically transparent film in PVA. Mater. Lett.. 2007;61:4725-4730.
- [Google Scholar]
- Highly efficient, photocatalytic degradation of organic pollutants by PANI-modified TiO2 composite. J. Phys. Chem. C. 2012;116:5764-5772.
- [Google Scholar]
- Electrosynthesis of polyaniline SiO2 composite at high pH in the absence of extra supporting electrolyte. Polym. Bull.. 2006;57(6):825-832.
- [Google Scholar]
- Dielectric spectroscopy of polyaniline/stanic oxide (PANI/SnO2) composites. Ferroelectric. 2008;366(1):22-28.
- [Google Scholar]
- Synthesis and dielectric properties of polyaniline/titanium dioxide nanocomposites. Ceram. Int.. 2008;34(7):1767-1771.
- [Google Scholar]
- Anti-quorum sensing and anti-biofilm activity of nickel oxide nanoparticles against multi-drug resistant Pseudomonas aeruginosa. Environ. Chem. Eng.. 2020;8:104533
- [Google Scholar]
- Synthesis and electrical characteristics of polyaniline nanoparticles and their polymeric composite. Curr. Appl. Phys.. 2004;4(6):581-583.
- [Google Scholar]
- Microb. Pathogen. 2019;127:267-276.
- Biosynthesized silver nanoparticles for inhibition of antibacterial resistance and biofilm formation of methicillin-resistant coagulase negative Staphylococci. Bioorg. Chem.. 2019;89:103008
- [Google Scholar]
- Oxidative polymerization of aniline: molecular synthesis of polyaniline and the formation of supramolecular structures. In: De Souza Gomes A., ed. New Polymers for Special Applications. Brussels, Belgium: Intech; 2012.
- [Google Scholar]
- Prospects of conducting polymers in molecular electronics. Curr. Appl. Phys.. 2003;3(2-3):293-305.
- [Google Scholar]
- CdS/polyaniline nanocomposites: synthesis and characterization. Synth. React. Inorg. Met. Org. Chem.. 2007;37(3):153-159.
- [Google Scholar]
- Polyaniline dispersions: preparation of spherical particles and their light scattering characterization. Polymer. 1992;33:4857-4858.
- [Google Scholar]
- Development of electromagnetic shielding materials from the conductive blends of polyaniline and polyaniline-clay nanocomposite-EVA: Preparation and properties. Compos. Sci. Technol.. 2009;69(3-4):358-364.
- [Google Scholar]
- Novel approach to enhance the dispersion stability of ER fluids based on hollow polyaniline sphere particle. Colloids Surf., A: Physicochem. Eng. Aspects. 2006;274(1-3):37-42.
- [Google Scholar]
- Polyaniline nanowires on TiO2 nano/microfiber hierarchical nano/microstructures: Preparation and their photocatalytic properties. Mater. Chem. Phys.. 2011;129(1-2):666-672.
- [Google Scholar]
- Preparation and optical absorption of dispersions of nano–TiO2/MMA (methyl methacrylate) and nano-TiO2/PMMA (polymethylmethacrylate) Mater. Res. Bull.. 1999;34:701-709.
- [Google Scholar]







