Translate this page into:
Selenium conditioning decreases antioxidant enzyme activity and delays germination potency of Macrotyloma uniflorum and Vigna radiate
⁎Corresponding author. tarasu.bbm@pondiuni.edu.in (Chinnasamy Thirunavukkarasu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
This study focuses on the effect of Selenium (Se) on the germination of Macrotyloma uniflorum and Vigna radiate seeds.
Methods
Both the seeds were soaked in solutions containing different concentrations of Se (0 mg/L, 0.5 mg/L, 2.5 mg/L and 5.0 mg/L), for different time intervals (3 h, 6 h, 9 h and 12 h). After soaking, germination efficacy of the seeds was assayed. Total carbohydrate and starch content of the seeds were monitored along with α-amylase activity. The antioxidant activity was also estimated through measuring Catalase, Superoxide dismutase activity and amount of reduced Glutathione as well.
Results
The result unveiled that at 0.5 mg/L of Se did not affect the germination at 3 h of soaking, however upon increasing the duration of soaking the germination efficacy is statistically decreased. 3 h soaking of seeds with 2.5 mg/L and 5.0 mg/L of Se reduced the germination efficacy of both the seeds. Further it was observed that increasing the duration of soaking from 3 h to 6hrs, 9 h and 12 h, inhibited the germination potency (P < 0.05). Total carbohydrate and starch content were increased (P < 0.05) in both the seeds soaked in 0.5 mg/L of Se for 3 h, on the other hand there is no statistical difference at 2.5 mg/L and 5.0 mg/L of Se. The α-amylase activities in different experimental samples are in line with carbohydrate and starch levels. Furthermore, it was observed that reduced glutathione and antioxidant enzyme activities were increased (P < 0.05) in both the seeds soaked in 0.5 mg/L of Se for 3 h, however these alterations were statistically reduced (P < 0.05) at 2.5 mg/L and 5.0 mg/L of Se.
Conclusion
The present study documents higher concentration of Se could decrease the germination efficacy of M. uniflorum and V. radiate seeds through oxidative stress mediated pathway. On the other hand, lower concentration delays germination. Soaking of M. uniflorum and V. radiate seeds in less concentrated Se solution increases the carbohydrate and starch content compromising germination efficacy.
Keywords
Antioxidant enzymes
Micronutrient
Nutritional value
Selenium
Seed germination
1 Introduction
Germination of seeds can alter the macro and micronutrient content inside them. Nutritional value of the seeds, can be altered through soaking the seeds in essential micronutrients. Among the micronutrients, some trace elements are very crucial for the proper bio-function of certain cellular enzymatic activities and different protein functions. Selenium (Se) is a naturally available micronutrient. Se is found to be present in all-natural materials on earth including rocks, soils, water, air, plant and animal tissues. Se possesses one of the narrowest ranges between dietary deficiency (<40 μg/ day) and toxic levels (greater than400 μg/ day) compared to other micro nutrients in humans (El-Ramady et al., 2015). However, toxic concentration of this vital nutrient is not known for widely consumable plants such as Macrotyloma uniflorum (Horse Gram) (M. uniflorum) and Vigna radiata (Green Gram) (V. radiata). Since Se deficiency and toxic dose falls under narrow range, consumption of the mentioned plants may manipulate human health in positive or negative manner. Se is incorporated into cysteine and methionine and gives rise to selenocysteine and selenomethionine, which get integrated into proteins to form selenoproteins (Stadtman, 1990; Iqbal et al., 2020). Se serves as an essential constituent of biologically important antioxidant enzyme such as glutathione peroxidase (GPx) which prevents oxidative cell damage (Bermingham et al., 2014).
The concentration of Se in plants varies based on its concentration in the soil. Its bioavailability and uptake vary in different plant species (Boon, 1989; Neal, 1995; Fleming, 1980). Rosenfield and Beath (1964) were the first to classify plants in three groups on the basis of Se uptake. Plants that are considered as ‘Se-accumulators’ grow well on high Se containing soils and can accumulate more than 100 mg of Se Kg−1 dry matter (DM) of plant. Plants called ‘Se-indicators’ can tolerate up to almost 1 g of Se Kg−1 DM. The third group includes majority of the plants that cannot tolerate more than 10–100 mg of Se Kg−1 DM, these are called ‘non-accumulator’ plants. A subset of Se-accumulators also exists, the ‘Se-hyperaccumulator’ plants. These plants have leaves containing more than 1 g of Se Kg−1 DM (White, 2018). However, most plants contain<10 mg Se Kg−1 DM. Plants like Haplopappus, Stanleya and Astralagus are characteristic of seleniferous semi-arid environment in Western USA and other part of the world. These plants are often used as an indicator of high Se environments (Boon, 1989; Neal, 1995). During germination and early period of plant growth Se gets incorporated in to different proteins and is utilized later in the period of plant expansion. Seeds of Se hyper-accumulator plants contain the highest Se concentrations than that of any other organs (Fleming, 1980). This infers that Se might have some vital function in seed germination. Germination is a well-regulated process, it is also influenced by the environmental factors such as light and temperature, contamination of soil by metals through natural processes such as volcanic eruptions, weathering of rocks and industrial by-products, which are conceded to be major contributors that influence mineral and metal content in soil (Dame, 2020).
Plants grown in higher amount of Se soil contain more Se than that of other places. For example, brinjal and brown rice cultivated at Tiruvendpuram, Kerala, India- the region known to have excess Se in soil, contain up to 39 mg/kg and 180 mg/kg Se respectively in these plants (Srikumar, 1993). In Se accumulator plants, moister content in soil could influence the Se metabolism, which intern decreases Se-methylselenomethionine (Stadtman, 1990). Se is known to influence seed germination to various extents of plant life, which is further dependent upon plant species. Even though its effects on various plants are being studied; earlier studies by Spencer and Siegel (1978) indicated that Se has the ability to affect seed germination and radical elongation in some plant species. However only in extremely high concentration such as 28 mM of selenate was found to be toxic in germinating seeds (Spencer and Siegel, 1978). The range of concentrations used by Spencer and Siegel (1978) is too wide, so the actual point at which Se became toxic could not be ascertained in germinating seeds. In countries like India, M. uniflorum and V. radiata, also called horse gram and green gram respectively are widely used as a source of food. Among the various parts of the plants, cereals and sprouts are majorly used as food. The current work focuses on the effect of Se on the germination of M. uniflorum and V. radiata seeds and its nutritional and antioxidant status.
2 Materials and methods
2.1 Materials
M. uniflorum and V. radiata seeds were purchased from local seed market of Pondicherry-605005, India. It was authenticated by Prof. B. Kannabiran (late), Department of Biochemistry and Molecular Biology, Pondicherry University, India. All other chemicals used for this study were of analytical grade and purchased from HiMedia, India.
2.2 Germination studies
2.2.1 Germination percent and seed vigour index
M. uniflorum and V. radiate were soaked with distilled water containing different concentrations of Se (0, 5, 25, 50 mg/dL) for the time period of 3 h, 6 h, 9 h and 12 h. At the end of soaking period, seeds were seeded at a density of 30/plate on a petri-plate (100 mm X 15 mm) containing double layer of moisture filter paper. The seeds were allowed to germinate and grow for different periods of time such as 24 h, 48 h and 72 h at 25 °C with 8 h of light and 16 h of darkness. At the end of incubation period the number of germinated seeds were counted manually and the percent germination was calculated. The seed vigour index is calculated by multiplying germination percent (%) and seedling length (mm) (Qijuan et al., 2017).
2.2.2 Imbibition study
Pre-weighed seeds of both M. uniflorum and V. radiata each in replicates were soaked in distilled water without (served as control) and with Se (5 mg/dL, 25 mg/dL and 50 mg/dL). The change in the wet weight was quantified after 10 h, the was repeated with the interval of 2 h. The difference in the respective weights corresponds to the imbibition rate. The imbibition rate was calculated in percent by modifying the procedure described by Clarke and Pauw (1989).
2.3 Biochemical studies
M. uniflorum and V. radiata seeds soaked for 3 h in Se containing solutions of 0 mg/dL, 5 mg/dL, 25 mg/dL and 50 mg/dL were further allowed to germinate for 24 h. This sample was used to study biochemical changes in different experimental conditions.
2.3.1 Total carbohydrate content
The digested samples were filtered and diluted to 100 ml. Total carbohydrate was estimated by Anthrone method (Hedge and Hofreiter, 1962). Starch content was assayed by Anthrone method (Hedge and Hofreiter, 1962).
2.3.2 Protein extraction
The seeds were soaked and germinated as mentioned in section 2.3. The embryos were exercised, snap frozen with liquid nitrogen and subsequently powdered. Total protein was extracted with pre-chilled extraction buffer consist of 0.25 M sucrose, 0.05 M tris (pH 7.4) and 1 mM EDTA which contained the protease inhibitor (PSMF). The homogenates were centrifuged at 10,000 rpm for 20 min in Eppendorf centrifuge (5810R) at 4° C. Supernatants were collected and stored in −80 °C for further analysis. Protein concentration was estimated by Bradford method (Bradford, 1976).
2.3.3 Sds-Page
50 μg of protein was resolved by 10 % lamelli SDS-PAGE and subsequently stained with Coomassie brilliant blue and destained in a mixture of 10 % methanol, 10 % acetic acid and deionized water (Laemmli, 1970). The gel was kept in 7 % acetic acid solution, photographed in Gel Doc (BIO-RAD) and the bands were compared with known molecular weight marker protein.
2.3.4 Glycoprotein staining
Glycoprotein staining was performed according to Schiff’s method.The gel was photographed in Gel Doc (BIO-RAD) and the bands were compared with known molecular weight marker protein. SDS-PAGE preparation and quantification procedure is followed as explained in Subastri et al., (2015).
2.3.5 α-Amylase activity
α-Amylase activity was assayed by employing 3, 5-dinitrosalicylic acid (DNS) method described by Cui et al. (2002). The enzyme activity is expressed as IU (specific activity).
2.4 Antioxidant content
The non-enzymatic anti-oxidant such as reduced glutathione (GSH) was assessed by using 5, 5′-dithio-bis (2-nitrobenzoic acid) reagent (Moron et al., 1959). Catalase (CAT) activity was determined by the method of Caliborne (1985). The enzyme activity is represented as IU (specific activity). Superoxide dismutase (SOD) was assayed by Marklund and Marklund (1974) using pyrogallol. The enzyme activity is represented as IU (specific activity). One IU consists of the amount of SOD required to inhibit the auto oxidation of pyrogallol by 50 %.
2.5 Statistical analysis
The experimental data were represented as Mean ± SEM and analyzed using GaphPad Prism 6 software. The significance was analyzed by Tukey’s One Way ANOVA.
3 Results
3.1 Germination studies
Figs. 1 and 2 show the germination profile of seeds soaked with increasing concentration of Se for different duration and germinated for 24 h. Radicle length of more than 1 mm was considered to be germinated. The number of seeds germinated in M. uniflorum (Fig. 1) and V. radiata (Fig. 2) soaked in distilled water (control) is high when compared with different concentrations (5 mg/dL, 25 mg/dL and 50 mg/dL) of Se-soaked seeds. Further the radicle emergence is less in Se-soaked seeds when compared to control. In both plants, a significant reduction in germination was observed in all the different period of incubation with different concentration of Se. Increasing Se concentration or duration of soaking time, negatively influenced the M. uniflorum and V. radiata seed germination. Further increase in germination time (48 h and 72 h) confirmed the above findings (Fig. S1, S2, S3 and S4) in M. uniflorum and V. radiata seeds. It is observed that, higher concentration of Se (25 and 50 mg/dL) has reduced the germination in M. uniflorum and V. radiata seeds. At higher concentrations of Se seedlings fail to emerge. Also, Se treatment for long periods showed reduced growth and curvature of roots.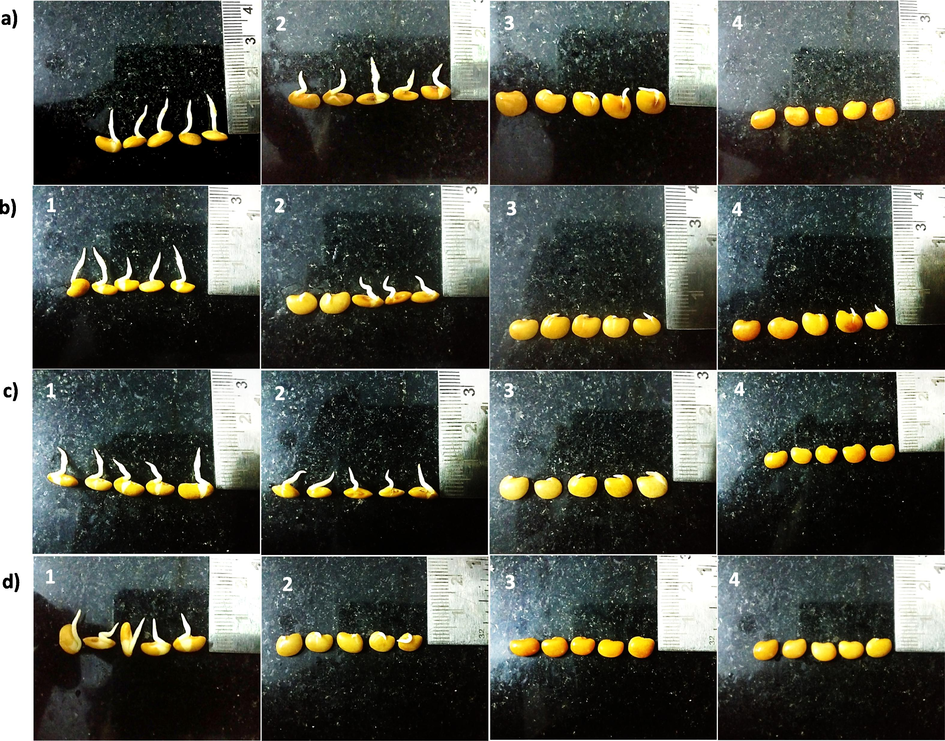
M. uniflorum seeds soaked in solutions without and with (different concentrations) Se for different periods of time and germinated for 24 h. Right side of the photograph shows scale par. a) Soaked 3 h, b) Soaked for 6 h, c) Soaked for 9 h, d) Soaked for 12 h. 1) 0 mg Se /dL (control); 2) 5 mg Se/dL; 3) 25 mg Se/dL and 4) 50 mg Se/dL.
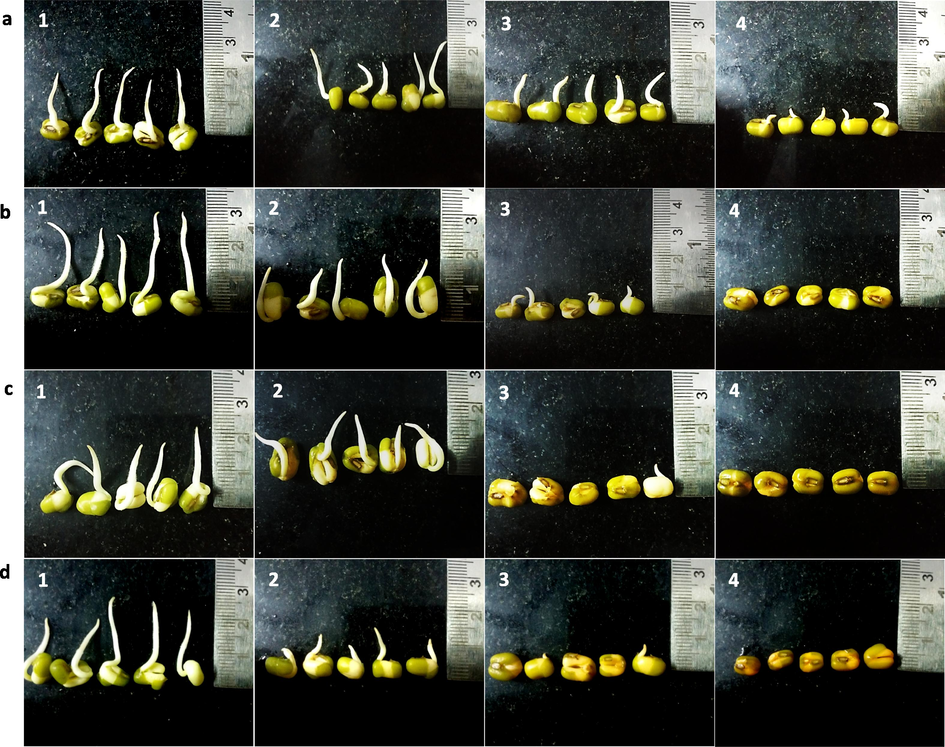
V. radiata seeds soaked in solutions without and with (different concentrations) Se for different periods of time and germinated for 24 h. Right side of the photograph shows scale par. a) Soaked 3 h, b) Soaked for 6 h, c) Soaked for 9 h, d) Soaked for 12 h. 1) 0 mg Se /dL (control); 2) 5 mg Se/dL; 3) 25 mg Se/dL and 4) 50 mg Se/dL.
The germination percent in M. uniflorum (Fig. 3A) and V. radiata (Fig. 3B) seeds are shown in Fig. 3. Different concentrations of Se were soaked for 3 h and allowed to germinate for 24 h, 48 h and 72 h. Increasing the concentration of Se, decreases percent germination of both the seeds. Further we observed that, when duration of Se soaking is increased to 6 h, 9 h and 12 h, (Fig. S5, S6 and S7) the statistically significant decrease in germination percent is highly visible. Altogether, it is revealed that increase in the Se level reduces the germination percentage. Tables 1 and 2 depicts the seed vigour index of M. uniflorum and V. radiata without or with Se. Seed vigour is an important quality parameter which needs to be assessed to supplement germination and viability tests to gain insight into the performance of a seed lot in the field or in storage. Here in case of seeds soaked in Se for 3 h, 6 h, 9 h and 12 h, with increase in the concentration of Se, the seed vigour decreases for 24 h, 48 h and 72 h of germination.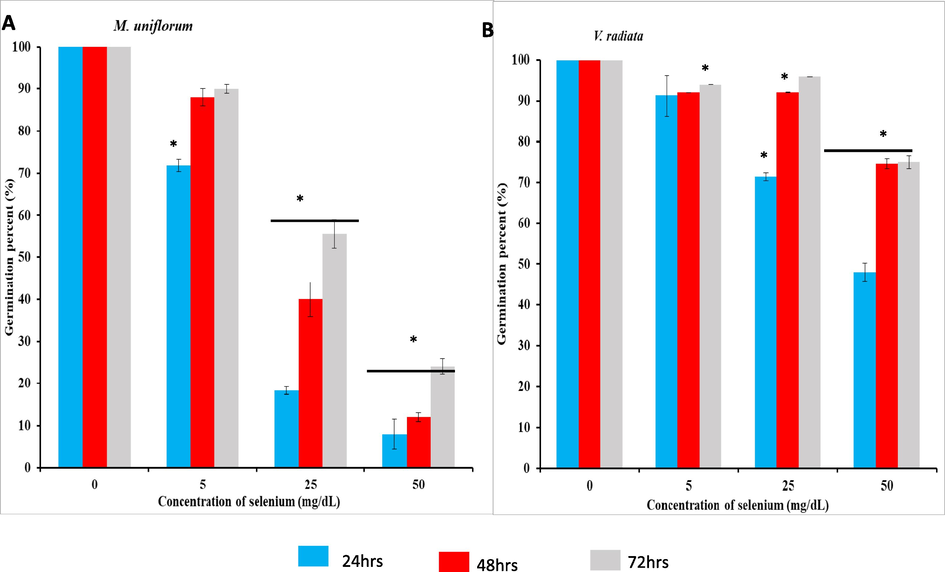
Effect of Se on germination. A) Percent germination of M. uniflorum seeds soaked without or with different concentrations of Se for 3 h. B) Percent germination of V. radiata seeds soaked without or with different concentrations of Se for 3 h. Data were expressed as mean ± SEM. *represents the level of significance at P < 0.05 compared to control seeds.
M. uniflorum germinated for 24 h
Se concentration (mg/dL)
Soaking time
3 h
6 h
9 h
12 h
Se (Control) (0 mg/dL)
1450 ± 50
1500 ± 20
1050 ± 50
850 ± 50
Se (5 mg/dL)
933.2 ± 72.32*
605.7 ± 35.63*
760 ± 76*
108.17 ± 7.21*
Se (25 mg/dL)
44.86 ± 10.07*
49.8 ± 2.67*
48 ± 4.2*
0
Se (50 mg/dL)
15.72 ± 4.21*
14.3 ± 3.62*
0
0
M. uniflorum germinated for 48 h
Se (Control) (0 mg/dL)
2500 ± 119
2200 ± 100
3200 ± 100
3150 ± 89
Se (5 mg/dL)
1862 ± 98*
1585 ± 75.49*
2697 ± 123*
968.7 ± 27.67*
Se (25 mg/dL)
320 ± 18*
560.2 ± 50.92*
379.6 ± 87.6*
96.42 ± 16.07*
Se (50 mg/dL)
24 ± 8.4*
67.67 ± 36*
75.78 ± 25*
42.85 ± 14.28*
M. uniflorum germinated for 72 h
Se (Control) (0 mg/dL)
4600 ± 100
6400 ± 100
4750 ± 250
4750 ± 200
Se (5 mg/dL)
3500 ± 400*
2735 ± 210*
3187 ± 245*
2785 ± 214*
Se (25 mg/dL)
777.8 ± 55.55*
2189 ± 123.9*
2008 ± 223*
718.7 ± 102.7*
Se (50 mg/dL)
264.8 ± 24.07*
232.3 ± 21.12*
478.6 ± 73.62*
117.8 ± 10.71*
V. radiata germinated for 24 h
Se concentration (mg/dL)
Soaking time
3 h
6 h
9 h
12 h
Se (Control) (0 mg/dL)
2100 ± 100
2600 ± 100
1650 ± 150
2250 ± 250
Se (5 mg/dL)
1870 ± 45.62*
1571 ± 98.21*
1119 ± 223.83*
760.3 ± 84.48*
Se (25 mg/dL)
713.9 ± 80*
307.1 ± 76.48*
48.89 ± 48.89*
124.1 ± 41.37*
Se (50 mg/dL)
215.8 ± 23.97*
0
0
0
V. radiata germinated for 48 h
Se (Control) (0 mg/dL)
4900 ± 100
4100 ± 100
3150 ± 50
3900 ± 50
Se (5 mg/dL)
4250 ± 250*
2750 ± 50*
3050 ± 30*
2612 ± 220.8*
Se (25 mg/dL)
2656 ± 241.5*
1558 ± 183.33*
1260 ± 60*
230 ± 38.33*
Se (50 mg/dL)
484.9 ± 111.9*
70.83 ± 14.16*
36.66 ± 21.65*
0
V. radiata germinated for 72 h
Se (Control) (0 mg/dL)
6000 ± 500
4650 ± 150
6500 ± 500
5600 ± 600
Se (5 mg/dL)
5750 ± 250
4400 ± 150
4900 ± 400*
5225 ± 118
Se (25 mg/dL)
1900 ± 100*
2658 ± 345*
1250 ± 227*
632.5 ± 57.5*
Se (50 mg/dL)
0
0
0
0
Imbibition marks the commencement of germination. Imbibition facilitates the uptake of water and solutes in the seed. Thus, with time the imbibition rate increases and gradually it reaches an equilibrium. As represented in Fig. 4, M. uniflorum (Fig. 4A) and V. radiata (Fig. 4B) seeds soaked in water and different concentrations of Se solution (5 mg/dL, 25 mg/dL, 50 mg/dL) did not show any statistically significant change in the seed imbibition rates when compared with corresponding controls.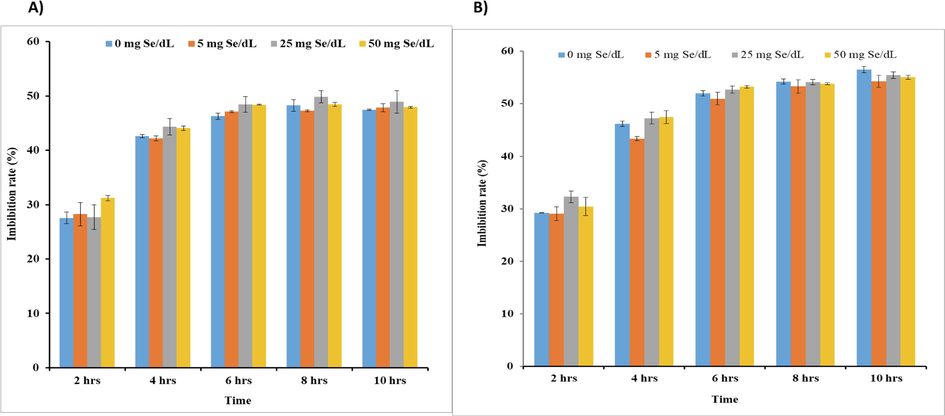
Imbibition rate of M. uniflorum (A) and V. radiate (B) seeds soaked with (different concentrations) or without Se for different time period. All values were expressed as mean ± SEM. *represents the level of significance at P < 0.05 compared to control seeds.
3.2 Biochemical studies
Fig. 5A denotes the total carbohydrate content in M. uniflorum and V. radiata seeds. Total carbohydrate content in the seeds treated with 5 mg/dL of Se showed statistical (P < 0.05) increase when compared with corresponding control seeds. However, further increase Se (25 mg/dL and 50 mg/dL) concentration gradually decreased (P < 0.05) total carbohydrate content than that of control.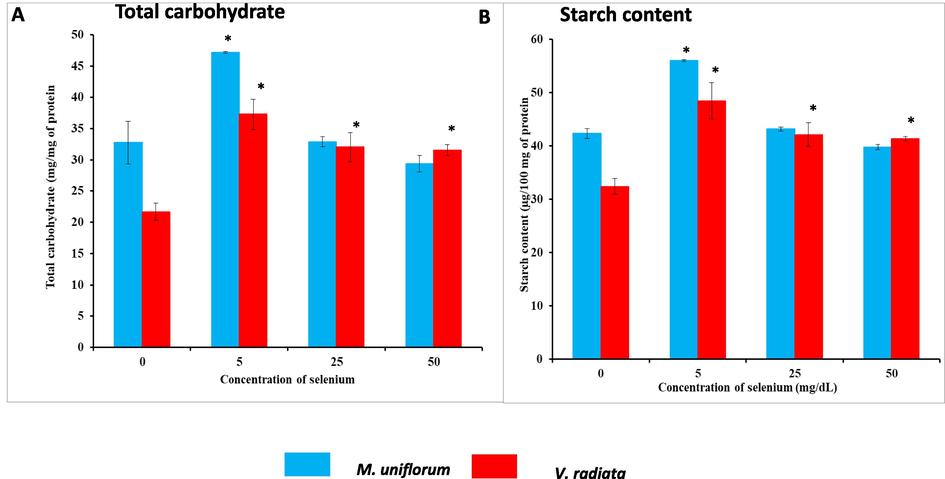
A) Total carbohydrate content of M. uniflorum and V. radiata seeds in different experimental groups. B) Starch content of M. uniflorum and V. radiata seeds in different experimental groups. Seeds were soaked with (different concentrations) or without Se for 3 h and germinated for 24 h. All values were expressed as mean ± SEM. *represents the level of significance at P < 0.05 compared to control seeds.
The starch content is shown in Fig. 5B in M. uniflorum and V. radiata seeds. The starch level was found to be statistically increased (P < 0.05) in 5 mg/dL of Se-soaked M. uniflorum seeds than that of control seeds. However, upon increasing the concentration of Se to 25 mg/dL and 50 mg/dL, there was no statistically significant difference in starch content when compared to controls. On the other hand, V. radiata seeds soaked in different concentrations of Se showed statistically significant (P < 0.05) increase in starch content than that of control seeds.
Fig. 6A shows the α-amylase activity in control and different experimental groups. M. uniflorum and V. radiata seeds were soaked in solutions with or without Se (5 mg/dL, 25 mg/dL and 50 mg/dL) for 3 h and the α-amylase activity was estimated after 24 h germinated seed extracts. The results indicate that seeds soaked in 5 mg/dL of Se solution showed increased (P < 0.05) α-amylase activity than control. On the other hand, higher concentration of Se (25 mg/dL and 50 mg/dL) were found to inhibit α-amylase activity (P < 0.05) than that of control.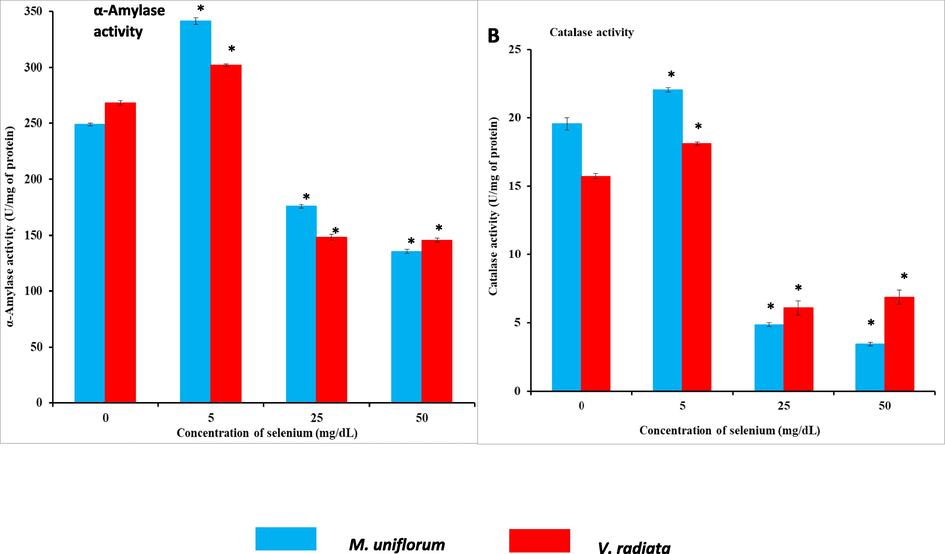
A) Activity of α-amylase in M. uniflorum and V. radiata seeds in different experimental groups. B) Activities of CAT in M. uniflorum and V. radiata seeds in different experimental groups. Seeds were soaked with (different concentrations) or without Se for 3 h and germinated for 24 h. All values were expressed as mean ± SEM. *represents the level of significance at P < 0.05 compared to control seeds.
The enzymatic antioxidants such as CAT (Fig. 6B) and SOD (Fig. 7A) showed decreased activity when Se level increases. The activity of CAT and SOD indicates that seeds soaked in 5 mg/dL Se solution showed increased (P < 0.05) CAT and SOD activity than control. Whereas, seeds soaked in 25 mg/dL and 50 mg/dL of Se concentrations showed reduced enzyme activity (P < 0.05). All the Se-soaked seeds show gradual decrease in anti-oxidant enzyme level. Detectable level of glutathione peroxidase activity was not observed in different experimental samples.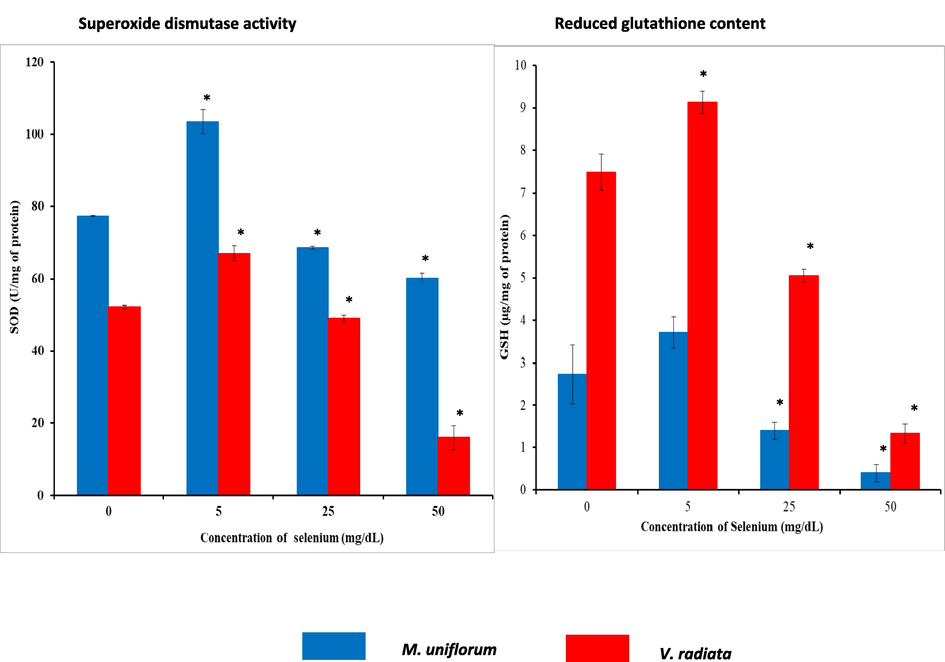
A) SOD activity in M. uniflorum and V. radiata seeds in different experimental groups. B) Reduced glutathione content in M. uniflorum and V. radiata seeds in different experimental groups. Seeds were soaked with (different concentrations) or without Se for 3 h and germinated for 24 h. All values were expressed as mean ± SEM. *represents the level of significance at P < 0.05 compared to control seeds.
The level of non-enzymatic anti-oxidant such as GSH is shown in Fig. 7B. The results indicate that soaking of seeds in 5 mg/dL of Se showed higher (P < 0.05) (except M. uniflorum seeds) level of reduced glutathione than that of control. While, Seeds soaked in 25 mg/dL and 50 mg/dL concentrations of Se showed decreased GSH content than that of control.
The resolved protein pattern on gel showed that reduction in the protein content of both seeds soaked with different concentration of Se. Treatment with Se has reduced the protein as well as glycoprotein content in both seeds. Fig. 8 shows the changes in protein (Fig. 8A) and glycoprotein (Fig. 8B) content of both seeds soaked with Se.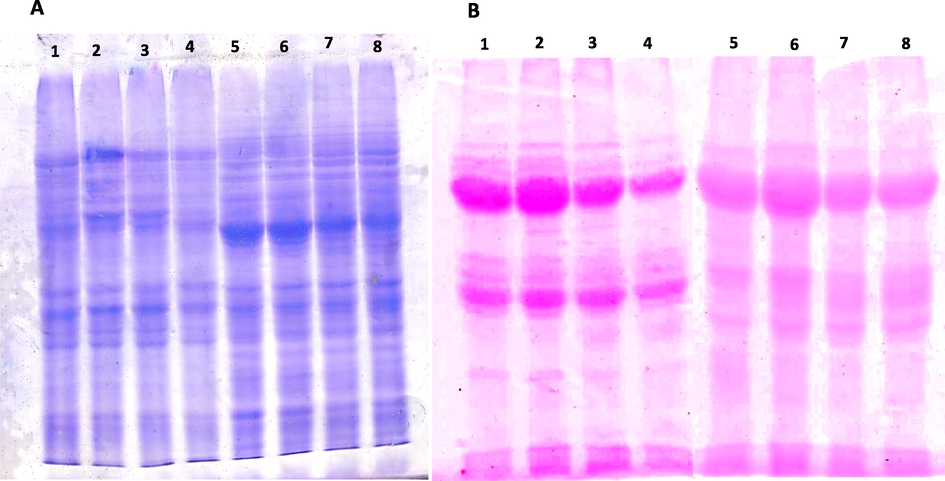
A) SDS PAGE of M. uniflorum and V. radiata seeds soaked with Se and germinated for 24 h. B) Glycoprotein content of M. uniflorum and V. radiata seeds soaked with Se and germinated for 24 h. Lane 1- control (0 mg Se/dL); Lane 2–5 mg Se/dL; Lane 3–25 mg Se/dL; Lane 4–50 mg Se/dL soaked M. uniflorum seeds; Lane 5- control (0 mg Se/dL); Lane 6–5 mg Se/dL; Lane 7–25 mg Se/dL; Lane 8–50 mg Se/dL soaked V. radiate seeds.
4 Discussion
Seed germination is a physiological phenomenon that marks the commencement of plant growth. Harbored inside the seed coat, the embryo remains dormant unless it detects cues facilitating it towards dormancy breakdown. Both in natural as well as artificial conditions seeds can germinate and there are wide variety of factors that influence seed physiology and thus can affect germination. It is widely accepted that germinated seeds contain more nutrients than that of non-germinated (Huang et al., 2014). There is a believe that soaking a seed with essential micronutrients may further enhance the nutrient value of seeds. Role of heavy metals on seed germination has long been an extensive area of research. It has been shown that Seed morphology and physiology are strongly affected by lead (Pb) exposure. Pb hinders seed germination, seedling development, root elongation, plant growth, chlorophyll production, transpiration and affects the activity of peroxidases and polyphenol oxidases, oxidizing ability of roots and overall lowering of carbohydrate-metabolizing enzymes such as β-amylases, α-amylases, acid phosphatases and acid invertases (Pourrut et al., 2011).
Among all the methods, ‘germination percent’ is often followed to assess the potential of seeds to germinate. It is an estimate of the viability of a population of seeds and basically indicates the percentage of seeds germinated from the experimental lot. Thus, by altering germination conditions, effect of those factors on the corresponding germination percentage can be easily obtained.
Even though at lower concentrations Se has shown to be breaking dormancy of certain seeds, in this study the concentrations were too high to promote germination (Shao et al., 2005). As the seeds germinate certain metabolic changes start to occur. Control seeds, soaked in water in all cases showed greater germination percentage than any of the Se-soaked seeds. Thus, Se mediated phytotoxicity may have resulted in the reduction of germination percentage by interfering with the mechanisms that promote dormancy breakage & seed germination and a gradual decrease is observed with increase in Se concentration. Another vital study that exactly represents germination is 'seed vigour'. The seed vigour has been used to indicate the performance of a seed lot in the field or in seed storage. As represented in Tables 1 and 2, the seed vigour index is getting reduced with the increasing concentration of Se and the time of exposure. This states that Se at high level induces phytotoxicity.
Commencement of germination takes place by uptake of water by the dry seeds and is completed when the radical emerges out by penetrating its surrounding structures. Germination and seedling growth require energy and molecular building blocks (substrates) for the synthesis of new tissues. Energy and substrates are obtained by enzyme-catalyzed metabolic processes in the tissues of germinating seeds (Bewley and Black, 1978). Water is essential for cellular metabolism for at least three reasons: (a) for enzymatic activity, (b) solubilization and transport of reactants, and (c) as a reactant, especially in the hydrolytic digestion of stored reserves of protein, carbohydrate and fat. In this study Seed Imbibition rate was estimated with respect to time. As shown in Fig. 4 M. uniflorum and V. radiata seeds soaked in water and 3 different concentrations of Se solutions (5 mg/dL, 25 mg/dL, 50 mg/dL) did not show any significant changes in the seed imbibition. Thus, it can be stated that Se addition did not result in any significant alteration in the water uptake by the seeds when compared with control seeds.
Carbohydrates along with the proteins acts as one of the major nutrient reserves in the seed and have significant effect on seed germination and seedling growth. Not only carbohydrates act as a food reserve which get digested to supply nutrition to the emerging seedling but also relative quantities of various carbohydrates have shown to confer tolerance to stress conditions (Garg et al., 2002). In case of plants treated with different cadmium concentrations (10 mM, 20 mM, 30 mM) carbohydrate contents were found to be decreased (Gubrelay and Rajneesh, 2013). In this study carbohydrate content showed significant decrease with increase in Se concentrations in both M. uniflorum and V. radiate which is similar to the toxicity raised by the Pb mentioned previously. In case of both the samples, seeds soaked in 5 mg/dL and 25 mg/dL Se solutions, total carbohydrate contents were less than that of controls. However, in case of M. uniflorum seeds soaked in 50 mg/dL Se solution the carbohydrate content was less than control and in case of V. radiata it is more.
Analysis of carbohydrate breakdown in germinating seeds show a rapid breakdown of starch reserves in endosperm, as the seeds germinate. Although the major soluble carbohydrate in the dry seed is sucrose, a marked increase in the production of glucose and malto-oligosaccharides accompanies the breakdown of starch (Stitt and Zeeman, 2012). The starch content of the M. uniflorum and V. radiata seeds soaked in Se showed significant decrease with increase in Se concentration in the soaking solution. In case of both the samples, seeds soaked in 5 mg/dL and 25 mg/dL Se solutions, total carbohydrate contents were more than that of controls. However, in case of M. uniflorum seeds soaked in 50 mg/dL Se solution the carbohydrate content was less than control and in case of V. radiata it is more.
α-Amylase is the key marker enzyme for the germination. The activity of the enzyme showed a pattern of gradual decrease with increase in Se, but seeds soaked in 5 mg/dL Se solution showed more activity than control. Both starch content and α-amylase activity showed a significant increase in case of M. uniflorum and V. radiata seeds soaked in 5 mg/dL of Se solution for 3 h. This implies that, Se at a concentration of 5 mg/dL, though inhibits radicle emergence, results in the elevation of carbohydrate and starch content, as well as the activity of α-amylase. This could be as a result of the facilitative effect of Se in seed germination especially in lower concentrations. As in the experimental sample the set amount of time for which seeds get exposed to Se is comparatively less, Se in 5 mg/dL concentration results in the least amount of intake of Se among all which may result in the data obtained. The early report by Qijuan et al. (2017) showed that reduction in the α-amylase activity while inhibiting the seed germination by eugenol similarly higher concentrations of Se reduced the α-amylase activity.
After imbibition, high energy cost of germination of seeds are fulfilled through rapid enhanced oxygen uptake and oxidative phosphorylation (Tommasi et al., 2001). Mobilization of food storage generate reactive oxygen species (ROS) through oxidative phosphorylation, this causes the structural and functional damage in cells. The mechanisms that scavenge the ROS play vital role in the successful accomplishment of seed germination. Also, it has been reported that percent seed germination might be associated to the proficiency of free radical scavenging in dry seeds because this scavenging can merely affect seed storage and vigor (Priestley, 1986). Researchers have reported that generation of ROS during seed germination may be a useful biological mechanism. Further this has been connected with germination capacity, seedling development, and defense against parasitic organisms during germination (Schopfer et al., 2001). The reported results show increasing interest in the efficient role of ROS and corresponding scavenging enzymic systems in seed germination.
Antioxidant enzymes such as SOD, peroxidase and CAT are considered to be the main protective enzymes engaged in the removal of free radicals and ROS (Blokhina et al., 2003). Among antioxidant enzymes CAT and SOD are the most efficient antioxidative enzymes that play major roles in scavenging oxygen free radicals (Scandalios, 1993). In both M. uniflorum and V. radiata, seeds soaked in 5 mg/dL of Se solution show a significantly higher SOD and CAT activity than the control. Seeds soaked in 25 mg/dL and 50 mg/dL Se solutions show significantly less activity of both of these enzymes which might have resulted in more oxidative stress and free radical mediated damage in those seeds. The balance between ROS generation and ROS scavenging determines ROS deposition in the cells. Further, the increase in ROS also depends on the environment such as presence of heavy metal, light intensity, temperature, etc (García-Caparrós et al., 2021). It is known that the photosynthetic electron transport chain serves as a major site of ROS generation. The existence of surplus heavy metals results into a limitation of CO2 fixation in the chloroplasts, which connected with an over production of ROS (Mittler et al., 2004). More reduction of electron transport chains in the mitochondria is also a main place of ROS production (Davidson and Schiestl, 2001). As reported by Moller et al. (2007) isolated mitochondria convert 1–5 % of O2 consumed into ROS. Hydrogen peroxide (H2O2), a strong ROS generating substance is produced in the peroxisomes after glycolate is oxidized to glyoxylic acid during photorespiration. Therefore, peroxisomes are the additional sites where more ROS such as superoxide anion, H2O2, singlet oxygen (O2) and hydroxyl radicals (∙OH) are generated. This ROS is more feasible in these organelles because of spin inversion and one-two and three-electron transfer reactions to O2, reactions are taking place during electron transport chain action. The redox active heavy metals can induce ROS generation directly through Haber–Weiss and Fenton reactions or indirectly through inhibiting the activities of enzyme in the cellular antioxidant defense mechanisms (Schutzendubel and Polle, 2002; Halliwell, 2006).
In case of both M. uniflorum and V. radiata, seeds soaked in 5 mg/dL of Se solution the seeds show higher GSH content than control. On the other hand, Seeds soaked in 25 mg/dL and 50 mg/dL Se solutions show lesser GSH content. This indicates greater amount of free radical mediated damage in the seeds treated with higher concentration of Se.
In SDS PAGE, by visually observing the banding pattern, band density of protein and glycoproteins seems to be higher than control in case of seeds soaked in 5 mg/dL of Se solution. 25 mg/dL and 50 mg/dL Se gradually reduced both protein content. All the above findings states that treatment of Se in germinating seeds reduces their potential to germinate, thereby it reduced the nutrient composition of germinated seeds. When higher organisms feed on such food, it may result in malnutrition and the risk factors are associated with diseases and high metal accumulation in animals.
Heavy metals are an essential part of life as they act as important trace elements necessary to sustain living systems. But in higher concentrations they can be lethal. Se, like many other heavy metals is essential in living organisms in trace amounts. Certain plants can grow on soil with very high Se content. Se accumulator plants can even accumulate much higher levels of Se than others due to their metabolism. In germinating seeds, Se incorporation in lower concentrations may promote seed germination but in higher concentration like other heavy metals, Se shows its phytotoxic effects and thus inhibits germination rate.
5 Conclusion
The present findings show that Se-soaked M. uniflorum and V. radiata seeds (2.5 mg/L and 5.0 mg/L concentrations) have lower percentage of germination, which is through oxidative stress mediated mechanisms. However, lower concentration of Se (0.5 mg/L) did not affect the percent germination at 3 h of soaking. On the other hand, long time soaking (>3 h) is not advisable. A motive of increasing the nutritional value of germinated seeds by soaking in micronutrient needs extensive research. Even though low concentration of Se (0.5 mg/L) at 3 h of soaking time increases the carbohydrate and starch content of the seeds, with increased duration of soaking time (>3 h) it delays germination. The Se induced phytotoxicity may affect the nutritional value of germinated seeds.
Acknowledgement
The authors acknowledge the Department of Science and Technology, Govt. of India, New Delhi for their support through DST-FIST program.
Disclosure of Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Thirunavukkarasu C reports administrative support was provided by Pondicherry University. Thirunavukkarasu C reports a relationship with Pondicherry University that includes: employment. Thirunavukkarasu C has patent NIL pending to NIL. NIL].
References
- Selenium-enriched foods are more effective at increasing glutathione peroxidase activity compared with selenomethionine: a meta-analysis. nutrients. Nutrients.. 2014;6:4002-4031.
- [CrossRef] [Google Scholar]
- Bewley J.D., Black M., eds. Physiology and Biochemistry of Seeds in Relation to Germination. Berlin, Heidelberg: Springer Berlin Heidelberg; 1978.
- Antioxidants, oxidative damage and oxygen deprivation stress. Ann. Bot.. 2003;91:179-194.
- [CrossRef] [Google Scholar]
- Potential selenium problems in great plains soils. In: Jacobs L.W., ed. Selenium in Agriculture and the Environment. New Orleans, LA: SSSA special publication; 1989. p. :107-131.
- [Google Scholar]
- A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [CrossRef] [Google Scholar]
- Water imbibition rate of wheat kernels as affected by kernel color, weather damage, and method of threshing. Can. J. Plant. Sci.. 1989;69:1-7.
- [CrossRef] [Google Scholar]
- Molecular dissection of seedling-vigor and associated physiological traits in rice. Theor. Appl. Genet.. 2002;105(5):745-753.
- [Google Scholar]
- Analysis of major and trace elements in teff (Eragrostis tef) J. King. Saud. Univ. Sci.. 2020;32:145-148.
- [CrossRef] [Google Scholar]
- Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae. Mol. Cell. Biol.. 2001;21:8483-8489.
- [CrossRef] [Google Scholar]
- Selenium in soils under climate change, implication for human health. Environ. Chem. Lett.. 2015;13:1-19.
- [CrossRef] [Google Scholar]
- Essential micronutrients II: Iodine and selenium. In: Davis B.E., ed. Applied Soil Trace Elements. New York: John Wiley and Sons; 1980. p. :199-234.
- [Google Scholar]
- Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot. Rev.. 2021;87:421-466.
- [CrossRef] [Google Scholar]
- Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Appl. Biol. Sci.. 2002;99:15898-15903.
- [CrossRef] [Google Scholar]
- Effect of heavy metal Cd on some physiological and biochemical parameters of barley (Hordeum vulgare L.) Int. J. Agric. Corp. Sci.. 2013;5:2743-2751.
- [Google Scholar]
- Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant. Physiol.. 2006;141:312-322.
- [CrossRef] [Google Scholar]
- Carbohydrate chemistry 17. In: Whistler R.L., Be Miller J.N., eds. New York. Academic Press; 1962.
- [Google Scholar]
- Kinetic changes of nutrients and antioxidant capacities of germinated soybean (Glycine max L.) and mung bean (Vigna radiata L.) with germination time. Food Chem.. 2014;143:268-276.
- [CrossRef] [Google Scholar]
- Effect of supplemental selenium in fish feed boosts growth and gut enzyme activity in juvenile tilapia (Oreochromis niloticus) J. King. Saud. Univ. Sci.. 2020;32:2610-2616.
- [CrossRef] [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature.. 1970;227:680-685.
- [CrossRef] [Google Scholar]
- Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.. 1974;47:469-474.
- [CrossRef] [Google Scholar]
- Reactive oxygen gene network of plants. Trends. Plant. Sci.. 2004;9:490-498.
- [CrossRef] [Google Scholar]
- Oxidative modifications to cellular components in plants. Annu. Rev. Plant. Biol.. 2007;58:459-481.
- [CrossRef] [Google Scholar]
- Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Arch. Biochem. Biophys.. 1959;82:70-77.
- [CrossRef] [Google Scholar]
- Selenium. In: Alloway B.J., ed. Heavy Metals in Soils. London. Blackie Academic & Professional; 1995. p. :260-283.
- [Google Scholar]
- Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol.. 2011;213:113-136.
- [CrossRef] [Google Scholar]
- Seed aging. Implications of seed storage and persistence in the soil. New York. Ithaca: Cornell University Press; 1986.
- Inhibitory effect of eugenol on seed germination and pre-harvest sprouting of hybrid rice (Oryza sativa L.) Sci. Rep.. 2017;7:1-9.
- [CrossRef] [Google Scholar]
- Selenium, geobotany, biochemistry, toxicity and nutrition. New York: Academic Press; 1964.
- Oxygen stress and superoxide dismutases. Plant. Physiol.. 1993;101:7-12.
- [CrossRef] [Google Scholar]
- Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant. Physiol.. 2001;125:1591-1602.
- [CrossRef] [Google Scholar]
- Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot.. 2002;53:1351-1365.
- [Google Scholar]
- Comparison of ecotoxic effect of selenium on seed germination of wheat and paddy rice. Chin. J. Ecol.. 2005;12:1440-1443.
- [Google Scholar]
- Effects of sulfur and selenium oxyanions on Hg-toxicity in turnip seed germination. Water. Air. soil. pollut.. 1978;9:423-427.
- [CrossRef] [Google Scholar]
- The mineral and trace element composition of vegetables, pulses and cereals of southern India. Food. Chem.. 1993;46:163-167.
- [CrossRef] [Google Scholar]
- Starch turnover: Pathways, regulation and role in growth. Curr Opin Plant Biol.. 2012;15:282-292.
- [CrossRef] [Google Scholar]
- Nutrient profile of porridge made from Eleusine coracana (L.) grains: effect of germination and fermentation. J. Food. Sci. Technol.. 2015;52:6024-6030.
- [CrossRef] [Google Scholar]
- Comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J. Exp. Bot.. 2001;52:1647-1654.
- [CrossRef] [Google Scholar]
- Selenium metabolism in plants. Biochim. Biophys. Acta. Gen. Subj.. 2018;1862:2333-2342.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102501.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







