Translate this page into:
Seed inoculation with Rhizobium japonium bacteria improved fatty acid composition of different soybean (Glycine max L.) genotypes
⁎Corresponding author. aynurbilmez@siirt.edu.tr (Aynur Bilmez Özçınar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Soybean is an important crop for food security as it fulfills global oil requirements. Seed inoculation with bacteria is frequently used to increase its production; however, it could change the seed composition. Nevertheless, the genotypes respond differently to the bacteria. Therefore, it is necessary to assess the impact of Rhizobium bacteria on the seed composition.
Methods
This two-year (2018 and 2019) study investigated the effects of seed inoculation with Rhizobium japonicum on fatty acid composition of different soybean genotypes. Three frequently cultivated soybean genotypes, i.e., ‘Gapsoy16′, ‘Traksoy’, and ‘İlksoy’ were included in the study. The seeds were either inoculated with R. japonicum or sown without inoculation. The fatty acid profile, i.e., saturated fatty acids (palmitic and stearic acid) and unsaturated fatty acids (oleic, linoleic, linolenic, and arachidic acids) was determined, and the collected data were analyzed by single and multivariate analysis.
Results
Seed inoculation with R. japonicum significantly altered the fatty acid composition of different genotypes; however, varied effects were recorded for the genotype. Linoleic acid, oleic acid, and palmitic acid made up ∼ 33 % of total fatty acids in seeds. Linoleic acid contents varied between 30.78–34.02 %, whereas oleic acid contents ranged between 27.85–31.04 %. Similarly, palmitic acid contents differed between 15.53–16.93 %. The ‘İlksoy’ and ‘Gapsoy’ had the highest contents of palmitic and oleic acids, respectively. Overall, inoculation of bacteria increased the composition of unsaturated fatty acids and lowered saturated fatty acids.
Conclusion
Seed inoculation with R. japonicum increased the essential fatty acid composition in ‘Traksoy’ genotype. However, ‘İlksoy’ genotype recorded a decrease in unsaturated fatty acids. Therefore, ‘Traksoy’ can be inoculated with R. japonicum to improve fatty acid profile.
Keywords
Soybean
Rhizobium
Fatty acid composition
1 Introduction
Soybean [Glycine max (L.) Merr.] is a major source for food, feed, and biofuel production (Anderson et al., 2018; Toomer et al., 2023). Soybeans are globally cultivated to fulfill the oil need, animal and human nourishment, and many industrial uses. The improved soybean productivity may be attributed to significant research relating to novel agricultural practices, and resistant genotypes over the past several decades. More than 50 % of edible oil consumed globally is derived from soybean (Kim et al., 2017; Toomer et al., 2023). Soybean oil possesses abundant polyunsaturated and monounsaturated fats and less saturated fat. Therefore, the oil has the potential to reduce cholesterol levels and lower the risk of heart disease. Soybean oil is a nutritious choice for baking and cooking due to its essential fatty acids, beneficial fats for heart health, and vitamins. Soybean oil might potentially enhance bone strength, promote skin and hair health, and improve cardiovascular function, making it a valuable for a well-balanced diet (Swallah et al., 2023).
Soybean oil is the second most prevalent plant oil globally following palm oil. The global annual consumption of soybean oil between 2016 and 2021 was 56.3 million metric tons. After industrial and biofuel uses, 45.4 million metric tons (29.0 % of the overall consumption of plant-based food oils) were added to the food supply in the form of soybean oil (Bukowski and Goslee, 2024; Meijaard et al., 2022). Fatty acid composition is an important quality parameter in soybean which may influence consumer choice (Lee et al., 2013) and its final use (Rowntree et al., 2014). Soybean seeds contain 18–22 % oil, of which 85 % are unsaturated fatty acids (Anwar et al., 2016). The fatty acid composition of soybean oils is essential for its functional qualities and nutritional benefits. Enhancing the fatty acid profile of soybean oil may increase its benefits for cooking and overall health (Abdelghany et al., 2020).
Soybean oil contains saturated fatty acid [palmitic acid (16 %) and stearic acid (18 %)], unsaturated fatty acids, i.e., linoleic acid, and polyunsaturated fatty acid like linolenic acid (Azam et al., 2021). Higher concentrations of polyunsaturated fatty acid, such as linolenic acid, are needed in human nutrition and are effective for consumption (Anwar et al., 2016). Higher concentration of oleic acid is preferred due to the long shelf life and oil stability for industrial applications (Bellaloui et al., 2009). Higher oleic acid concentration is also preferred for biofuel production, higher oxidative stability and for lubricating characteristics (Graef et al., 2009). Oleic acid lowers cholesterol levels, cardiovascular disease, and has anti-diabetic and anti-inflammatory properties (Oliveira et al., 2010). A decrease in essential fatty acid content has a major nutritional impact, and accurate soybean oil composition data is essential for determining its dietary status.
Soybean has a marginal demand for nitrogen (Alam et al., 2015). The symbiotic relationship between legume plants and Rhizobia in root nodules is the most effective and prolific method of nitrogen fixation, making it critical in agriculture (Yuan et al., 2016). Due to the mounting expenses of chemical fertilizers and the rising environmental concerns, there is a growing attraction in the contribution of soil microorganisms in crop nutrition (Tarekegn and Kibret, 2017). The symbiotic relationship between soybeans and Bradyrhizobium bacteria leads to biological nitrogen fixation, which has both economic and ecological advantages by reducing the need on synthetic nitrogen fertilizers (Chang et al., 2015).
Soybean seed composition are a function of genetics, environment and management practices (Abdelghany et al., 2020; Assefa et al., 2019; Attia et al., 2021). The stability of soybean oil is determined mainly by its fatty acid composition. Abdelghany et al. (2020) analyzed the lipid profile of 1025 Chinese soybean accessions obtained from various ecological areas. Substantial variations were noted in the levels of palmitic acid, stearic acid, oleic acid, linoleic acid, and linolenic acid across different accessions.
Rhizobium is a Gram-negative soil bacterium. It has a broad geographical range and can induce nodulation in legumes; hence, facilitates the process of nitrogen fixation via symbiotic associations (Han et al., 2020). Rhizobium not only facilitates the assimilation of iron and phosphorus, but also stimulates the synthesis of plant hormones that improve crop development. Consequently, there is an increase in crop productivity and uptake of nutrients (Tarekegn and Kibret, 2017; Wei et al., 2023; Yuan et al., 2016). Furthermore, they can improve agricultural productivity, reduce the prevalence of diseases and pests, and increase the number of beneficial microorganisms in the soil. Inoculating soybean seeds with Rhizobium japonicum enhances the fatty acid composition of the seeds, resulting in improved oil profiles and higher-quality seeds (Israilov et al., 2023; Silva et al., 2013; Szpunar-Krok et al., 2021a). This approach has the potential to enhance the nutritional composition of soybeans and make them more beneficial components of diets when combined with careful nitrogen management. The effect of Rhizobium inoculation on soybean seed quality characteristics, i.e., chemical composition is very limited in the literature (Rahim et al., 2015). Therefore, the objective of the present study was to infer the effects of seed inoculation with R. japonicum on the fatty acid composition of soybean seeds.
2 Materials and methods
2.1 Experimental site, soil, and climate
Field experiments were carried out at Siirt University, Faculty of Agriculture, during 2018 and 2019. The soil was clayey in texture, salt-free, low in organic matter with low phosphorus and potassium content, and slightly alkaline (Table 1). The average temperature during the 2018 study period was higher than long-term values. Total rainfall during the study period was 100.6 mm in 2018 which was almost double the long-term averages (50.3 mm). In 2019, total rainfall was 31.6 mm which was lower than the long-term averages. Monthly and long-term monthly average temperatures and rainfall data are presented in Table 2.
0–30 cm depth soil
30–60 cm depth soil
Properties
Value
Remark
Value
Remark
pH
7.12
Neutral
7.72
Slightly basic
Total salt (dS/m)
0.11
Salt-free
0.10
Salt-free
Calcium carbonate (%)
2.73
Slightly calcareous
3.20
Slightly calcareous
Soil texture (%)
Clay: 47.24
Clay:47.24
Clayey
Silt: 18.26
Clayey
Silt:17.28
Sand: 34.40
Sand:35.48
Organic matter (%)
1.48
Low
1.16
Low
Available Phosphorus (kg/ha)
47.4
Low
0.96
Very low
Available Potassium (kg/ha)
826.7
Sufficient
46.3
Sufficient
Available Calcium (kg/ha)
6980.5
11294.4
Available Magnesium (kg/ha)
1216.8
121.4
Zinc (ppm)
0.54
0.36
Manganese (ppm)
35.04
27.5
Iron (ppm)
10.88
9.33
Copper (ppm)
1.73
1.38
Boron (ppm)
0.03
0.02
Months
Monthly Average temperature (°C)
Monthly average relative humidity (%)
Monthly average rainfall (mm)
2018
2019
Long-term (Average)
2018
2019
Long-term (Average)
2018
2019
Long-term Years (Average)
June
27.40
29.10
25.90
31.70
26.50
28.70
10.00
1.20
9.30
July
32.30
30.20
30.60
20.10
23.00
18.10
0.60
0.00
2.70
August
32.10
30.50
30.30
21.40
19.50
17.20
1.60
2.60
1.70
September
27.90
25.60
25.40
22.50
30.00
24.00
0.00
12.50
6.90
October
20.40
18.10
18.20
46.30
47.00
45.30
100.60
31.60
50.30
2.2 Treatments and design
Three frequently cultivated soybean genotypes in the study area (‘Gapsoy16′, ‘Traksoy’, and ‘İlksoy’) with and without Rhizobium seed inoculation were included in the study. The experiment was arranged according to factorial design where soybean genotypes were main plots and seed inoculation was taken as sub plots.
2.3 Crop management
Soybean seeds were sown using a seeder machine on 5 June and 29 May during 2018 and 2019, respectively after harvesting of wheat crop. Seeds were inoculated with Rhizobium japonicum bacteria before sowing. The bacteria were collected from “Soil Fertilizer and Water Resources Central Research Institute” (Ankara, Turkey). The 4 % sugar solution was mixed with bacteria culture to ensure better adhesion of the bacteria to the seeds. The inoculated seeds were kept in a shaded place for 8 h prior to sowing. Urea (46 % N) was applied during the sowing time as a basal fertilizer.
Earthing up of soil was performed approximately one month after sowing. Irrigation was provided using a drip irrigation system and a total of 10 irrigations were applied. No pesticides were applied. The crop was harvested on 10 and 25 October in 2018 and 2019, respectively. The middle two rows were harvested by hand and 50 cm from both ends of the rows were not considered for the yield data measurement. The threshing was done with a thresher machine.
2.4 Fatty acid composition
For the esterification process, 0.8 mL of oil was transferred into tubes. Subsequently, 1.6 mL of 1 M KOH (dissolved in methanol) and 2.5 mL of hexane were added to the oil and mixed vigorously using a vortex. The upper phase that was generated in the tube was transferred to the vial using a syringe and a 0.22 PVDF filter, and then supplied to the GC–MS apparatus. The GC–MS analysis was conducted using a Trace 1310 gas chromatograph that was equipped with an ISQ single quadrupole mass spectrometer manufactured by Thermo Fisher Scientific in Austin, TX, USA.
The protocol started with an initial temperature of 140 °C for a duration of 5 min. Subsequently, the temperature increased gradually at a rate of 2 °C per minute until 190 °C. It was then maintained at 190 °C for 1 min, after which the temperature was further increased at a rate of 3 °C per minute until 240 °C. Finally, the temperature was held at 240 °C for 35 min. The ion source and detector were both maintained at 250 °C. The material was diluted in 5 mL of 100 % CHCl3 and then filtered using a 0.22 µm disposable syringe filter. A 1 μL volume was injected using the spitless model. The material was separated using a Thermo TG-WAXMS GC column with dimensions of 60 m × 0.25 mm ID × 0.25 µm. Helium was used as the carrier gas at a flow rate of 1.2 ml/min. The mass spectral scan range was configured to span from 55 to 550 atomic mass units (amu). The proportion of each component was determined by comparing its average peak area to the overall area. The Xcalibur program was used to analyze the mass spectra and chromatograms. The retention indices (RI) were adjusted by consulting the relevant literature (Adams, 2007). The identification of peaks was performed by comparing the known components recorded in the NIST, Wiley7, Wiley9, redlip, mainlip, and WinRI databases.
2.5 Statistical analysis
The data of the fatty acid profile were analyzed by two-way analysis of variance (ANOVA) (Steel et al., 1997). The year effect proved significant; hence, the data of each year was analyzed and presented separately. The means were compared by least significant difference test where ANOVA indicated significant differences. Furthermore, principal component analysis was executed to make the data interpretation easier. All analyses were performed on SPSS statistical software version 20.0 (IBM SPSS Inc., 2012).
3 Results
The individual and interactive effects of genotypes and seed inoculation significantly altered the fatty acid composition of soybean genotypes. The individual effects of seed inoculation with bacteria remained non-significant for some of the fatty acids; however, their interaction with the genotypes always remained significant during both years of the study (Table 3). DF = degree of freedom, SS = sum of squares, MS = mean squares, the bold values in P value column are representative of significant differences among the relevant individual and interactive effect.
Source of Variation
2018
2019
DF
SS
MS
F value
P value
SS
MS
F value
P value
Palmitic acid
Varieties (V)
2
1.79
0.899
359.78
< 0.0001
3.27
1.63
15.24
0.001
Bacteria inoculation (I)
1
0.08
0.084
33.62
< 0.0001
0.01
0.011
0.10
0.757
V × I
2
0.12
0.062
24.74
< 0.0001
0.16
0.082
0.76
0.488
Stearic acid
Varieties (V)
2
8.623
4.312
1724.66
< 0.0001
10.30
5.15
343.33
< 0.0001
Bacteria inoculation (I)
1
0.006
0.006
2.42
0.146
1.62
1.62
108.00
< 0.0001
V × I
2
1.797
0.899
359.42
< 0.0001
3.42
1.71
114.19
< 0.0001
Oleic acid
Varieties (V)
2
2.95
1.47
589.04
< 0.0001
14.09
7.04
48.23
< 0.0001
Bacteria inoculation (I)
1
0.27
0.27
106.58
< 0.0001
0.38
0.38
2.61
0.132
V × I
2
1.05
0.52
209.84
< 0.0001
1.57
0.78
5.37
0.021
Linoleic acid
Varieties (V)
2
18.24
9.12
3647.58
< 0.0001
22.12
11.06
198.86
< 0.0001
Bacteria inoculation (I)
1
0.04
0.04
16.82
0.001
0.20
0.20
3.60
0.082
V × I
2
1.97
0.99
394.46
< 0.0001
0.72
0.36
6.48
0.012
Linolenic acid
Varieties (V)
2
0.04
0.02
7.22
0.009
0.36
0.180
69.39
< 0.0001
Bacteria inoculation (I)
1
0.09
0.09
35.28
< 0.0001
0.001
0.001
0.36
0.559
V × I
2
0.08
0.04
16.38
0.000
0.007
0.004
1.36
0.293
Arachidic acid
Varieties (V)
2
0.067
0.034
13.50
0.001
0.09
0.048
16.00
0.000
Bacteria inoculation (I)
1
0.001
0.001
0.32
0.582
0.000
0.000
0.09
0.769
V × I
2
0.004
0.002
0.86
0.448
0.01
0.005
1.60
0.242
The concentration of palmitic acid was greatest in the ‘İlksoy’, with an average of 16.74 %, followed by ‘Traksoy’ at 16.13 %, and ‘Gapsoy’ at 16.03 %. This suggests that the ‘İlksoy’ genotype has greater concentration of palmitic acid during 2018. Similarly, ‘İlksoy’ had the greatest palmitic acid concentration, with an average of 16.91 % during 2019. It was followed by ‘Gapsoy’ with 16.01 % and ‘Traksoy’ with 16.00 %. This implies that the ‘İlksoy’ contains a little larger amount of saturated fat in comparison to the other genotypes (Fig. 1). The ‘Gapsoy’ genotype had the greatest stearic acid concentration (13.76 %) followed by ‘İlksoy’ (12.76 %) and ‘Traksoy’ (12.08 %), respectively during 2018. Similarly, ‘Gapsoy’ and ‘Traksoy’ had the greatest (13.82 %) and the lowest (11.97 %) stearic acid level, respectively.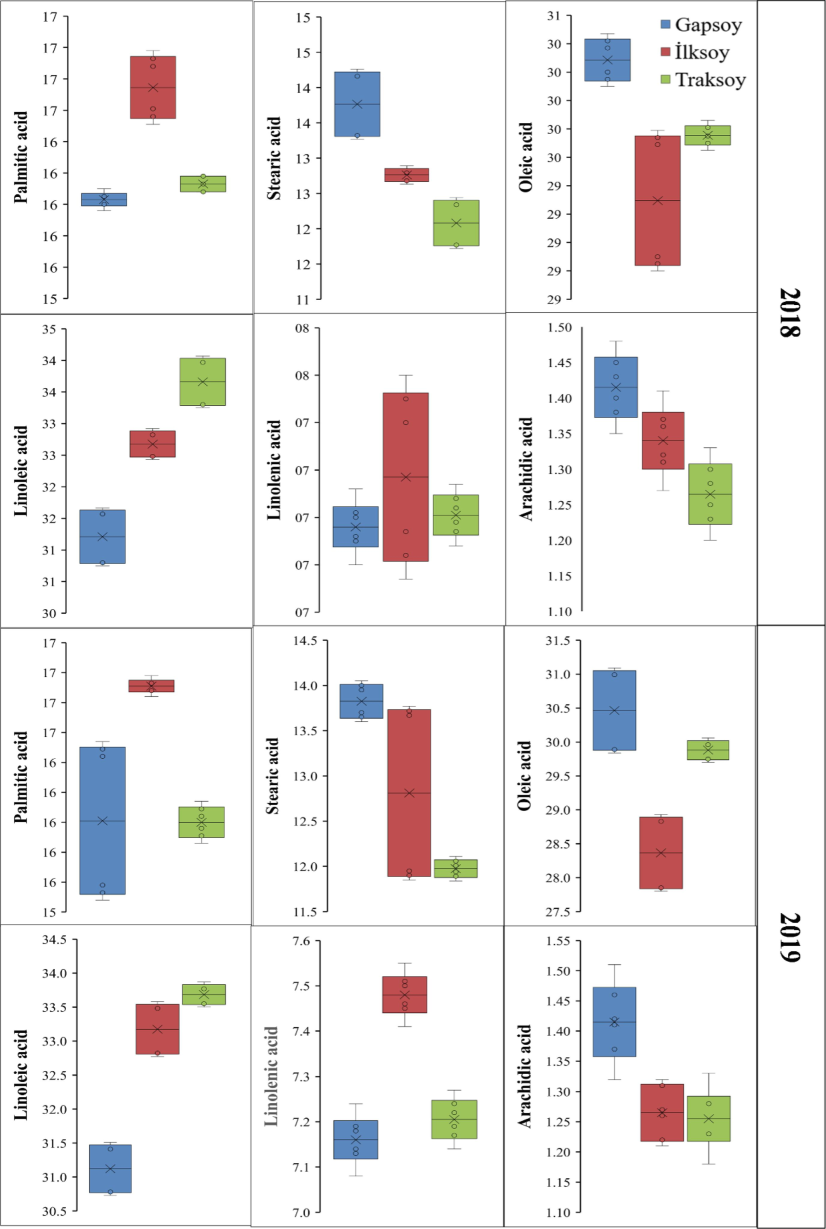
The influence of different soybean genotypes on fatty acid profile.
The ‘Gapsoy’ and ‘İlksoy’ genotypes recorded the most (30.18 %) and the least (29.19 %) oleic acid, respectively during 2018. Similarly, ‘Gapsoy’ (30.46 %) and ‘İlksoy’ (28.36 %) had the highest and the lowest oleic acid contents, respectively during 2019. The greater oleic acid concentration in ‘Gapsoy’ may help heart health and cooking stability. The enhanced oleic acid concentration in ‘Gapsoy’ may improve its health and stability. The highest (33.66 %) and the lowest (31.210 %) linoleic acid was recorded for ‘Traksoy’ and ‘Gapsoy’ genotypes, respectively during 2018. Likewise, ‘Traksoy’ had the highest linoleic acid (33.68 %) during 2019, whereas ‘Gapsoy’ had the lowest (31.12 %) linoleic acid. These results suggest that ‘Traksoy’ and ‘İlksoy’ are better polyunsaturated fatty acids. The linolenic acid level was greatest in the ‘İlksoy’ (7.28 %) followed by ‘Traksoy’ and the lowest was noted for ‘Gapsoy’ during 2018. Similarly, ‘İlksoy’ (7.48 %) had higher linolenic acid than ‘Traksoy’ (7.20 % higher) and ‘Gapsoy’ (7.16 % higher) during 2019 (Fig. 1).
Palmitic acid concentration was marginally higher in inoculated seeds (16.37 %) than non-inoculated ones (16.23 %) during 2018. This variation suggests that seed inoculation may affect palmitic acid concentration. Stearic acid concentration did not change between inoculated (12.88 %) and non-inoculated (12.85 %) seeds. Oleic acid concentration was marginally lower in inoculated seeds (29.55 %) than non-inoculated seeds (29.80 %). Inoculation reduced oleic acid. Linoleic acid concentration was slightly higher in inoculated seeds (32.56 %) than in non-inoculated seeds (32.46 %). Seed inoculation with R. japonicum may increase linoleic acid production. The inoculated seeds had more linolenic acid (7.29 %) than non-inoculated seeds (7.15 %). This rise in linolenic acid shows that bacteria inoculation promotes its accumulation. Inoculated seeds had 1.33 % arachidic acid, compared to 1.34 % in non-inoculated ones. This variation suggests that bacteria inoculation may not substantially alter arachidic acid production (Fig. 2). These results suggest that bacteria may affect soybean fatty acid composition, increasing linoleic and linolenic acids in the plants growing from inoculated seeds. Small changes in oleic acid content may affect these soybeans’ nutritional and industrial usage.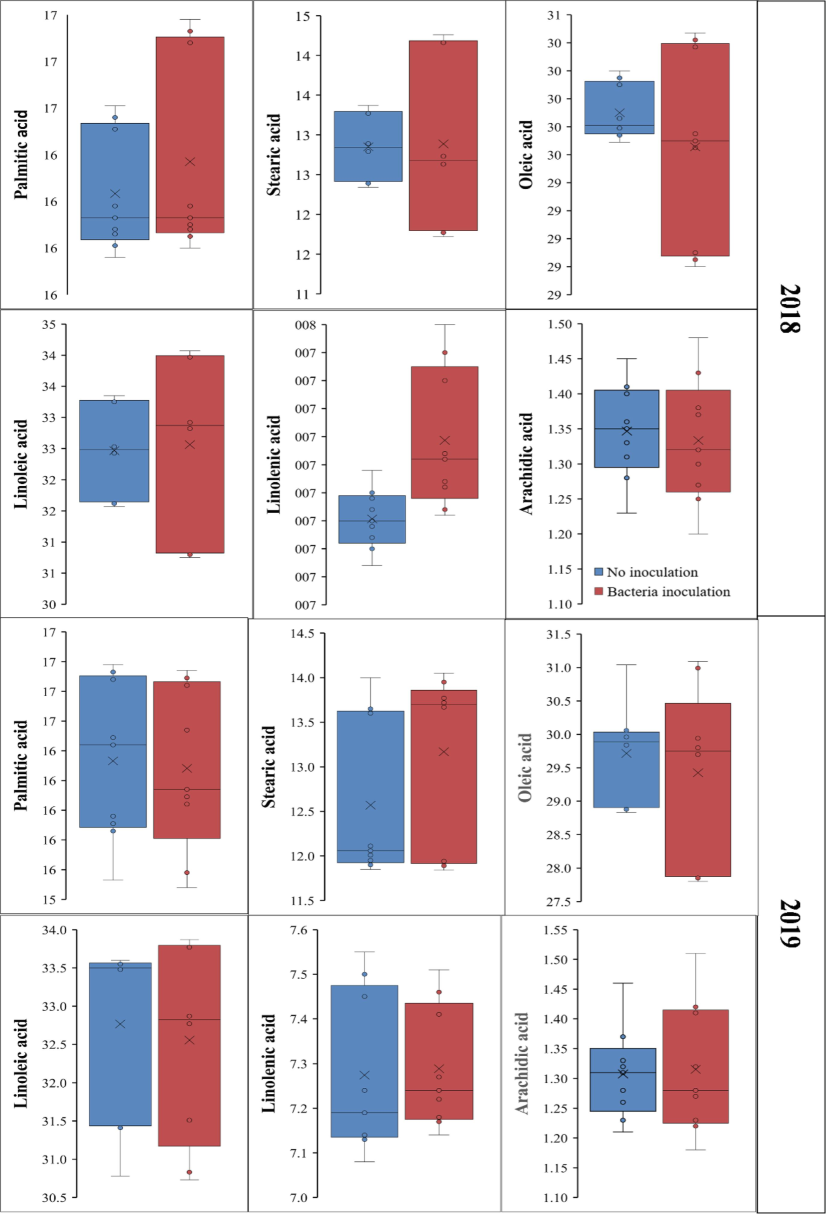
The influence of seed inoculation with bacteria on the fatty acid profile of soybean genotypes.
The palmitic acid exhibited non-significant difference between the plants growing from inoculated (16.28 %) and non-inoculated (16.33 %) seeds during 2019. The plants grown from bacteria inoculated seeds had a greater quantity of stearic acid (13.17 %) than non-inoculated seeds (12.57 %). The observed difference, although statistically significant, indicates a little increase in the stearic acid concentration. The concentration of oleic acid was similar in both inoculated (29.42 %) and non-inoculated (29.71 %) seeds, indicating that seed inoculation had no significant impact on oleic acid. The concentration of linoleic acid in non-inoculated seeds was higher (32.76 %) than in inoculated seeds (32.55 %) (Fig. 2).
Fatty acid content showed notable variations for interactive effects of genotypes and seed inoculation during 2018. The bacteria-inoculated seeds of ‘İlksoy’ had the greatest palmitic acid content (16.93 %), whilst non-inoculated seeds of ‘Gapsoy’ had the lowest concentration (16.01 %). Similarly, inoculated seeds of ‘Gapsoy’ had the greatest stearic acid concentration (14.20 %), while the ‘Traksoy’ had the lowest stearic acid level (11.77 %) when inoculated. The ‘Gapsoy’ genotype exhibited the highest (30.32 %) oleic acid under inoculation conditions, whilst ‘İlksoy’ had the lowest (28.75 %) under the same conditions. The ‘Traksoy’ genotype had the maximum concentration of linoleic acid (34.02 %) under inoculation circumstances, whereas ‘Gapsoy’ had the lowest concentration (30.80 %) under the same conditions. The linolenic acid concentration varied between 7.45 % in ‘İlksoy’ under inoculated conditions and 7.12 % in ‘İlksoy’ under non-inoculated conditions. The arachidic acid level was greatest in the ‘Gapsoy’ genotype under inoculation circumstances, measuring 1.43 %. Conversely, the ‘Traksoy’ genotype under inoculated conditions had the lowest arachidic acid content, measuring 1.25 % (Table 4). IC = bacteria inoculation, NI = no bacteria inoculation, the means sharing different letters within a column are statistically similar (p > 0.05).
Fatty acids
Gapsoy
İlksoy
Traksoy
IC
NI
IC
NI
IC
NI
2018
Palmitic acid (%)
16.05 cd
16.01 d
16.93 a
16.56b
16.13c
16.13c
Stearic acid (%)
14.20 a
13.32b
12.68 d
12.84c
11.77f
12.39 e
Oleic acid (%)
30.320 a
30.05b
28.75 e
29.64 cd
29.60 d
29.71c
Linoleic acid (%)
30.80f
31.62 e
32.87c
32.48 d
34.02 a
33.30b
Linolenic acid (%)
7.21b
7.15 bc
7.45 a
7.12c
7.22b
7.19 bc
Arachidic acid (%)
1.43 a
1.40 ab
1.32 bcd
1.36 abc
1.25 d
1.28 cd
2019
Palmitic acid (%)
15.86b
16.15b
16.89 a
16.93 a
16.09b
15.91b
Stearic acid (%)
13.90 a
13.75 a
13.72 a
11.90b
11.89b
12.06b
Oleic acid (%)
30.67 a
30.25 ab
27.85 d
28.88c
29.75b
30.01 ab
Linoleic acid (%)
31.02c
31.21c
32.82b
33.53 a
33.82 a
33.55 a
Linolenic acid (%)
7.18b
7.13b
7.46 a
7.50 a
7.22b
7.19b
Arachidic acid (%)
1.44 a
1.38 a
1.27b
1.26b
1.23b
1.28b
The PCA divided the genotype by seed inoculation interactions into different groups. Overall genotype ‘Traksoy’ with or without seed inoculation proved better for essential fatty acids (Fig. 3). The PCA indicated that genotype has a substantial influence on the fatty acid profile. Furthermore, ‘Traksoy’ consistently demonstrated a beneficial composition of essential fatty acids, independent of its inoculation status. The data highlights that while inoculation may have a little influence on some fatty acids, the overall genotype remains a crucial determinant of fatty acid quality.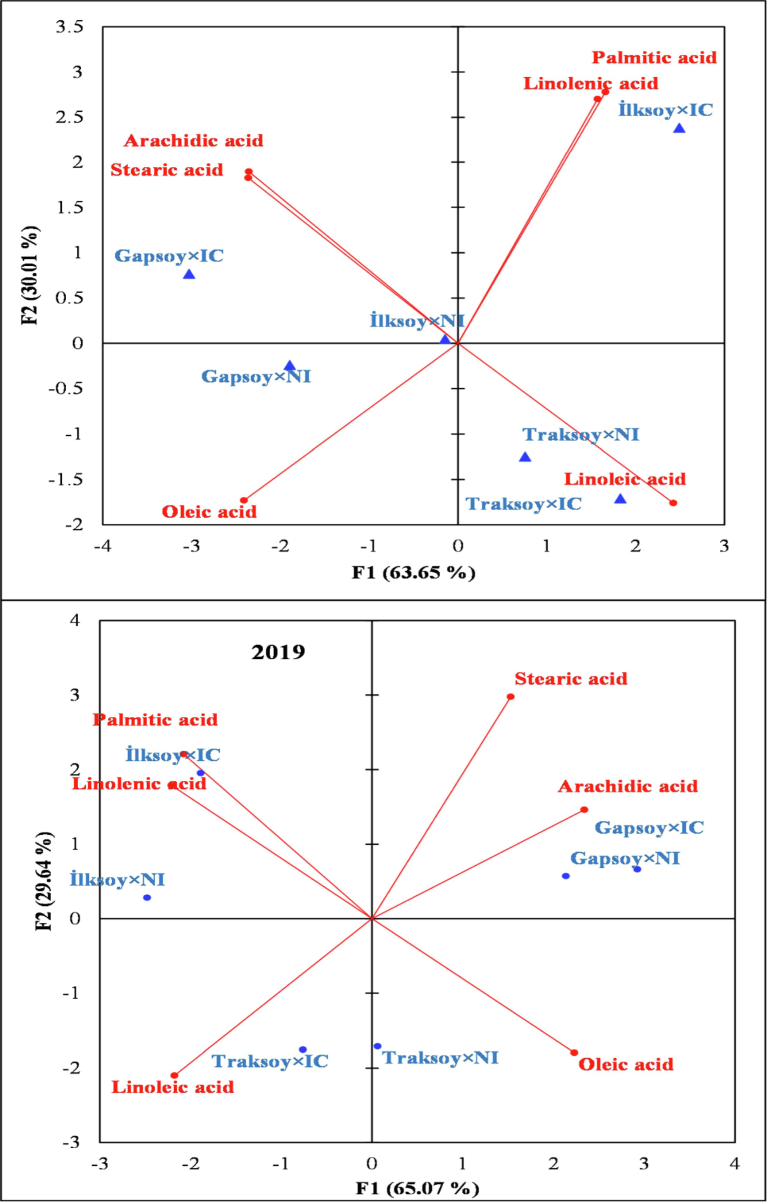
Biplot of the principal component analysis indicating the relationship between fatty acid profile of different soybean genotypes and bacterial inoculation treatments.
4 Discussion
Soybean is a highly cultivated legume crop that plays a crucial role in worldwide food and feed supply (Rose et al., 2016; Ziska, 2000). The production of soybeans is significantly impacted by the symbiotic association between nitrogen-fixing bacteria and soybean (Israilov et al., 2023; Szpunar-Krok et al., 2021b). Several research have examined the impact of Rhizobium inoculation on soybean development, productivity, and fatty acid composition (Israilov et al., 2023; Silva et al., 2017; Szpunar-Krok et al., 2021b; Wei et al., 2023). The combination of Rhizobium inoculation and appropriate fertilization may significantly modify the fatty acid content of soybean seeds by reducing harmful saturated fats and increasing beneficial unsaturated fatty acids (Han et al., 2020; Wei et al., 2023). The effects differ depending on the specific Rhizobium strains, soybean cultivars, and environmental conditions (Silva et al., 2017; Wei et al., 2023). This study has examined several Rhizobium strains, different cultivars of soybeans, and diverse climatic conditions.
The consumption of legumes with high content of proteins, fiber, minerals, and vitamins is advantageous for human well-being. Moreover, the cultivation of leguminous crops is also regarded beneficial for sustainable agriculture as these crops assimilate atmospheric nitrogen by interacting with soil bacteria known as Rhizobia. The induction of these bacteria to the plant lead to significant alterations in plant metabolism, resulting in N and protein contents. The use of bacteria has increased the yield of several legume crops. Nevertheless, legumes are regarded as functional foods and induction of bacteria could alter their seed composition, particularly fatty acid profiles (Silva et al., 2017).
Soybeans with elevated quantities of monounsaturated fatty acids (oleic acid) or polyunsaturated fatty acids (linoleic or linolenic acid) are preferable for human consumption compared to saturated fatty acids. Nevertheless, polyunsaturated fatty acids are responsible for oil’s susceptibility to oxidation, limited shelf life, and development of rancidity. Thus, soybean seeds with elevated amounts of oleic acids and reduced amounts of linoleic or linolenic acids are preferred to decrease the hydrogenation of the oil. The hydrogenation process has been shown to have negative health impacts, including an increased risk of coronary heart disease and elevated levels of LDL-cholesterol and reduced levels of HDL-cholesterol. Based on this information, it can be concluded that the compositions in our research suggest that the oil is suitable for human consumption owing to its high levels of Linoleic acid (C18:2) and Oleic acid.
Rhizobium is a Gram-negative bacterium that is found in soil. It has a wide distribution and the potential to cause the production of root nodules in legumes, which makes it easier for legumes to fix nitrogen via symbiotic relationships (Han et al., 2020). In addition to promoting the absorption of iron and phosphorus, Rhizobium also enhances the production of plant hormones that drive the growth of crops. This results in improved crop growth and enhanced nutrient absorption. In addition, they have the potential to enhance agricultural output, reduce the occurrence of diseases and parasites, and increase the abundance of good microorganisms.
An earlier study (Rahim et al., 2015) investigated the impact of seven distinct indigenous Bradyrhizobium inoculations and three different N fertilization methods on the qualitative attributes of soybean seeds Bradyrhizobium inoculation and N fertilization resulted in a considerable increase in oil content compared to the control group. The oil content of the seeds ranged from 16.2 % to 21.5 % and consisted mostly of linoleic acid (47 %) and oleic acid (24 %). The application of both inoculation and N fertilization resulted in a reduction in saturated fatty acids (namely palmitic and stearic acids) and an increase in unsaturated fatty acids (specifically linoleic acid and oleic acid). The linoleic acid levels in our research are greater than those reported in previous studies, whereas the oleic acid values are somewhat lower than our own findings.
5 Conclusions
The impact of seed inoculation on the fatty acid composition was mostly non-significant for certain fatty acids, indicating that inoculation alone may not significantly modify the amounts of palmitic, stearic, oleic, linolenic, and arachidic acids. Nevertheless, the interaction between genotype and inoculation was significant during both years, suggesting that genotype has a pivotal influence on the fatty acid composition when paired with inoculation. The ‘İlksoy’ genotype has the highest palmitic acid content during both years. The ‘Gapsoy’ genotype had the largest concentrations of stearic acid, which had a substantial impact on the composition of fatty acids. On the other hand, ‘Traksoy’ had higher quantities of oleic and linoleic acids. The ‘Traksoy’ exhibited better profiles for important fatty acids under both inoculated and non-inoculated circumstances, highlighting its strong ability to retain high levels of beneficial fatty acids independent of inoculation status. Inoculation had little impact on certain fatty acids. However, the results indicated that 'Traksoy’ variety is better for essential fatty acids. Seed inoculation with R. japonicum increased the essential fatty acid composition in ‘Traksoy’ genotype. However, ‘İlksoy’ genotype recorded a decrease in unsaturated fatty acids. Therefore, ‘Traksoy’ can be inoculated with R. japonicum to improve fatty acid profile.
CRediT authorship contribution statement
Aynur Bilmez Özçınar: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Natural variation in fatty acid composition of diverse world soybean germplasms grown in China. Agronomy. 2020;10
- [CrossRef] [Google Scholar]
- Effect of Rhizobium sp. BARIRGm901 inoculation on nodulation, nitrogen fixation and yield of soybean (Glycine max) genotypes in gray terrace soil. Biosci. Biotech. Bioch.. 2015;79:1660-1668.
- [CrossRef] [Google Scholar]
- Nonhypothesis analysis of a mutagenic soybean (glycine max [L.]) population for protein and fatty-acid composition. J. Am. Oil Chem. Soc.. 2018;95:461-471.
- [CrossRef] [Google Scholar]
- Variations of quality characteristics among oils of different soybean varieties. J. King Saud Univ. Sci.. 2016;28:332-338.
- [CrossRef] [Google Scholar]
- Assessing variation in US soybean seed composition (protein and oil) Front. Plant Sci.. 2019;10
- [CrossRef] [Google Scholar]
- Breeding for sustainable oilseed crop yield and quality in a changing climate. Theor. Appl. Genet. 2021
- [CrossRef] [Google Scholar]
- Profiling and associations of seed nutritional characteristics in Chinese and USA soybean cultivars. J. Food Compos. Anal.. 2021;98:103803
- [CrossRef] [Google Scholar]
- Soybean seed composition is influenced by within-field variability in soil nutrients. Crop Management. 2009;8:1-12.
- [CrossRef] [Google Scholar]
- Climate-based variability in the essential fatty acid composition of soybean oil. Am. J. Clin. Nutr.. 2024;119
- [CrossRef] [Google Scholar]
- Soybean production in the Americas. Principles of Plant-Microbe Interactions. Springer International Publishing, Cham. 2015:393-400.
- [CrossRef] [Google Scholar]
- A high-oleic-acid and low-palmitic-acid soybean: agronomic performance and evaluation as a feedstock for biodiesel. Plant Biotechnol. J.. 2009;7:411-421.
- [CrossRef] [Google Scholar]
- Variation in rhizosphere microbial communities and its association with the symbiotic efficiency of rhizobia in soybean. ISME J.. 2020;14:1915-1928.
- [CrossRef] [Google Scholar]
- IBM SPSS Inc., 2012. SPSS Statistics for Windows. IBM Corp. Released 2012 Version 20, 1–8.
- Interactive effects of N fertilization and bradirhizobia japanicum on agronomical traits of soybean in salt affected soils. Turkish J. Field Crops. 2023;28:15-25.
- [CrossRef] [Google Scholar]
- SoyNet: a database of co-functional networks for soybean Glycine max. Nucleic Acids Res.. 2017;45:D1082-D1089.
- [CrossRef] [Google Scholar]
- Prediction of crude protein and oil content of soybeans using Raman spectroscopy. Sens Actuators B Chem.. 2013;185:694-700.
- [CrossRef] [Google Scholar]
- Dietary fats, human nutrition and the environment: balance and sustainability. Front Nutr. 2022
- [CrossRef] [Google Scholar]
- Further insight into the latex metabolite profile of Ficus carica. J. Agric. Food Chem.. 2010;58:10855-10863.
- [Google Scholar]
- Soybean seed quality characteristics in response to indigenous bradyrhizobium inoculation and N fertilization in Kashmir-Pakistan. J. Am. Oil Chem. Soc.. 2015;92:1165-1174.
- [CrossRef] [Google Scholar]
- Impact of progressive global warming on the global-scale yield of maize and soybean. Clim. Change. 2016;134:417-428.
- [CrossRef] [Google Scholar]
- Physiological and phenological responses of historical soybean cultivar releases to earlier planting. Crop Sci.. 2014;54:804-816.
- [CrossRef] [Google Scholar]
- Legume bioactive compounds: influence of rhizobial inoculation. AIMS Microbiol.. 2017;3:267-278.
- [CrossRef] [Google Scholar]
- Inoculation with Bradyrhizobium japonicum enhances the organic and fatty acids content of soybean (glycine max (L.) merrill) seeds. Food Chem.. 2013;141
- [CrossRef] [Google Scholar]
- Principles and procedure of statistics. A biometrical approach (3rd Ed.). New York: McGraw HillBookCo. Inc.; 1997. p. :352-358.
- The pros and cons of soybean bioactive compounds: an overview. Food Rev. Intl. 2023
- [CrossRef] [Google Scholar]
- Effect of nitrogen fertilisation and inoculation with bradyrhizobium japonicum on the fatty acid profile of soybean (glycine max (L.) merrill) seeds. Agronomy 2021:11.
- [CrossRef] [Google Scholar]
- Effect of nitrogen fertilisation and inoculation with bradyrhizobium japonicum on the fatty acid profile of soybean (glycine max (L.) merrill) seeds. Agronomy. 2021;11:941.
- [CrossRef] [Google Scholar]
- Effects of rhizobium, nitrogen and phosphorus fertilizers on growth, nodulation, yield and yield attributes of soybean at pawe northwestern ethiopia. Int. J. Microbiology and Biotechnology 2017:2.
- [Google Scholar]
- Current agronomic practices, harvest & post-harvest processing of soybeans (glycine max)—a review †. Agronomy 2023
- [CrossRef] [Google Scholar]
- Long-term fertilization coupled with rhizobium inoculation promotes soybean yield and alters soil bacterial community composition. Front. Microbiol.. 2023;14
- [CrossRef] [Google Scholar]
- RNA-seq analysis of differential gene expression responding to different rhizobium strains in soybean (glycine max) roots. Front. Plant Sci.. 2016;7
- [CrossRef] [Google Scholar]
- The impact of elevated CO 2 on yield loss from a C 3 and C 4 weed in field-grown soybean. Glob. Chang. Biol.. 2000;6:899-905.
- [CrossRef] [Google Scholar]







