Translate this page into:
Scorpion crude venom induced apoptosis and structural changes of Echinococcus granulosus protoscolices
⁎Corresponding author. N.Alazaly@azhar.edu.eg (Naser Abdelsater),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Echinococcus granulosus parasitic infection in humans causes hydatidosis (also known as echinococcosis), which is a severe zoonotic illness that puts public health at risk.
This research was designed to assess the status of morphological, ultrastructure, and cell apoptosis of E. granulosus protoscolices. For this purpose, the protoscolices were isolated and collected from the hydatid cysts in the lungs and livers of camels, then incubated for 4 h in Androctonus crassicauda crude venom (ACCV) (at the concentrations of 50 and 100 µg/ml). The changes of E. granulosus protoscolices were monitored using light, transmission, and scanning electron microscopy, and the caspase-3 expression was detected by immunohistochemistry. The current study results have demonstrated the caspase-3 expression was substantially higher in the protoscolices incubated at 100 µg/ml than 50 µg/ml. Also, ACCV caused distinct changes in the morphology and ultrastructure of protoscolices detected by light, TEM, and SEM microscopy. In conclusion, Androctonus crassicauda crude venom (ACCV) may provide an alternative and nonsurgical treatment for hydatidosis. Hence, further studies are recommended to explore the impact of ACCV on inducing apoptosis and structural changes for this parasitic disease.
Keywords
Echinococcus granulosus
Apoptosis
Scorpion venom
Structural changes
Protoscolices
1 Introduction
Zoonotic parasitic illness is known as CE (“Cystic Echinococcosis”) produced by the larval stage of E. granulosus. It is endemic and has significant economic and health impacts in various regions of the world, such as Egypt and the Middle East (El-Shazly et al., 2007; Sadjjadi, 2006 Almalki et al., 2017; Abdel-Baki et al., 2018; El-Meleh et al., 2019; Abdelbaset et al., 2021). Definitive hosts are dogs and other canids, while herbivores act as intermediate hosts and infecting them by ingesting the parasite's eggs excreted in the feces of the definitive host. Humans get infected when they ingest E. granulosus eggs by accident (Sarkari and Rezaei, 2015). Hydatid cysts are primarily found in the liver (50% to 80%) and lungs (5% to 30%). The cysts also were identified in the central nervous system, bones, heart, kidney, spleen, and other organs, although with less frequency (Sayek et al., 1980). Protoscolices are microscopic larvae inside the cysts that may grow into adult worms in the final host intestine or secondary hydatid cysts in the intermediate host viscera (Sharafi et al., 2017).
There were four treatment choices for hydatid disease: percutaneous aspiration, medical treatment, surgery, and watch & wait (Brunetti et al., 2010). Surgery remains the most efficient and widely used treatment modality. However, recurrence is often caused by the spread of protoscolex-rich fluid during surgery. Secondary disseminated intraperitoneal hydatidosis may result from operative spillage (Rajabi, 2009). The most common method of preventing this severe consequence is to instill a scolicidal agent into a hepatic hydatid cyst (Tozar et al., 2005). Till now, various scolicidal agents, including plant extracts, Albendazole, hypertonic saline, hypertonic glucose, ethyl alcohol, silver, and selenium nanoparticles were tried with various successes (Rahimi et al., 2015; Mahmoudvand et al., 2014; Moazeni and Roozitalab, 2012; Moazeni and Nazer, 2010; Hosseini et al., 2006; Kayaalp et al., 2001; Besim et al., 1998; Pérez-Serrano et al., 1994). Unfortunately, most of these scolicidal agents may cause undesirable side effects. Thus, their usage is restricted (Moazeni and Alipour-Chaharmahali, 2011). Thus, finding new scolicidal agents with fewer side effects, low cost, and high efficacy urgently needed surgeons (Adas et al., 2009).
Venomous animals, including scorpions, are new candidates for future drugs and can play as new approaches with fewer side effects. Literature showed that the snake and scorpion venoms have significant effects on some life-threatening human parasites, including Plasmodium, Leishmania, and Trypanosoma (Jafari et al., 2019). Many pharmacological peptides are found in scorpion venoms, attracting the interest of scientists in the domain of drug development. (Perumal et al., 2017). The scorpion species A. crassicauda (Olivier, 1807) (the Arabian fat-tailed scorpion) is one of the most dangerous species of the family Buthidae with medical significance in Saudi Arabia and the Middle East. Although A. crassicauda causes the most human mortalities, its venom components are well known among many other scorpion species. The composition and function of A. crassicauda venom are still relatively limited; however, the first molecular and biochemical analysis of the venom components was reported by (Caliskan et al., 2006). The Antimicrobial Peptides extracted from A. crassicauda venom revealed an inhibitory effect against gram-negative as well as gram-positive bacteria (Du et al., 2014). Apoptosis, as a kind of cell death initiated with external aspects and eventually results in the cell's self-destruction offers an effective method to killing the parasitic E. granulosus. Protoscolices are important component of the parasite and share many identical or similar cell structures (Wang et al., 1994; Morsth, 1967).
The main objective of current research is to detect the possible effects of ACCV on the structure and ultrastructure of E. granulosus protoscolices. This study also was planned to reveal the possibility of ACCV impact in inducing apoptosis for the Echinococcus granulosus protoscoleces.
2 Materials & methods
2.1 Scorpion crude venom collection
One hundred A. crassicauda individuals (Olivier, 1807) scorpion species were collected from Wadi Feiran, southern Sinai, Egypt. The gathered scorpions were maintained individually in plastic containers at 25 °C in the Parasitology Lab, Zoology Department, Faculty of Science, “Al-Azhar University” Assuit branch. Electrical stimulation (20 V) in the telson articulation collected venom as reported by (Sarhan et al., 2012; Alajmi et al., 2020). Purified venom droplets were collected in an Eppendorf tube as well as centrifuged for 15 min at 14,000 rpm at 4 °C. The supernatant was collected, freeze-dried, and kept at –20 °C. The lyophilized specimens were dissolved in purified water and centrifuged at 4 °C for 15 min at 15,000 rpm.
2.2 Protoscolices selection and viability analysis
E. granulosus Hydatid cysts were obtained from livers and lungs of camels killed at a governmental slaughterhouse in Assiut. The obtained specimens were instantly sent to the Parasitology Lab, Zoology Department, Faculty of Science, “Al-Azhar University”, Assiut branch, Egypt. The hydatid fluid was extracted using a sterile syringe and set aside for 45 min before precipitating in a sterile falcon tube. The gathered protoscolices were cleaned thrice with normal saline, and the feasibility of protoscolices was evaluated by 0.1 percent eosin stain. The motility features and muscular motions were observed by light microscopy. In subsequent experiments, protoscolices with viability of more than 90 percent were chosen (Smyth and Barret, 1980) Fig. 1.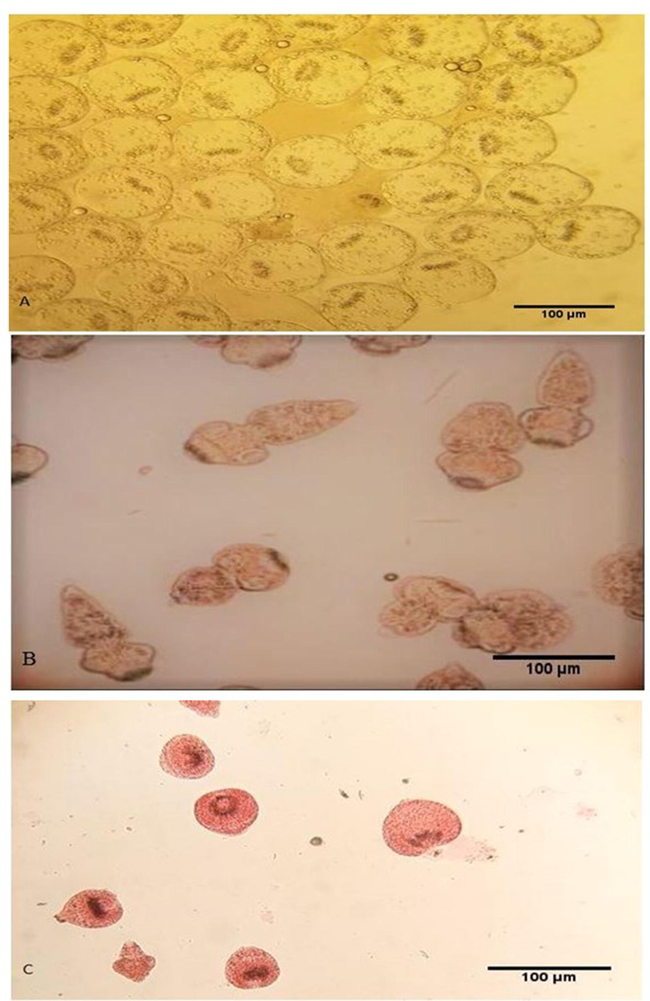
Live (control) unstained protoscolices (A), live (control) protoscolices after staining with 0.1 percent eosin (B), dead protoscolices after treatment with ACCV and staining with 0.1 percent eosin (C). Scale-bar = 100 µm.
2.3 Crude venom impact on protoscolices
The viable protoscolices specimens were washed by sterile saline, cultured in sterile RPMI 1640 medium, and split into three groups; one negative control group and two (50 and 100 µg/mL) scorpion venom treated groups (The sterile RPMI 1640 medium was prepared by adding 100 mg/mL streptomycin and100 IU penicillin). For each, 2.5 ml of the medium was added to a test tube, around 5x103 protoscolices was then placed to the tube and gently mixed and incubated at 37 °C in a 5% CO₂ incubator for 4hrs (Al-Malki and Abdelsater, 2020).
2.4 Light microscopy
Both control and ACCV incubated protoscolices were fixed in 10 percent formalin, treated to paraffin blocks, sectioned, dehydrated, stained by H&E (“Hematoxylin and Eosin”), and assessed by light microscope (Alam-Eldin and Badawy, 2015).
2.5 Transmission electron microscopy
TEM was utilized to analyze the ultrastructural changes in both control and ACCV-incubated protoscolices. Parasite specimens were kept in a 0.1 cacodylate solution containing 2.5 percent (v/v) glutaraldehyde for 1 h at pH 7.2. PBS was used to clean the fixed specimens two times before using 1 percent (v/v) osmium tetroxide in 0.1 M cacodylate buffer for 30mins at 7.2pH and room temperature post-fix the samples. They were dehydrated with rising ethanol levels. Propylene oxide was used to dehydrate the samples entirely before they were embedded in Epoxy Resin 812. An ultra-microtome was used to cut thin sections, then put on copper grids and stained using uranyl acetate and lead citrate. Sections were studied under the TEM JEOL JEM-1200 EX II. (Tawfik, 2018).
2.6 Scanning electron microscopy
Specimens were fixed for 24 hrs at 4 °C with 3 percent glutaraldehyde in sodium cacodylate buffer, washed several times in cacodylate buffer, and then dehydrated with rising concentrations of ethanol (50 to100%) before being immersed in hexamethyldisilazane for a 1 h and overnight. The samples were sputter-covered with gold (100 Å thick) and examined on a JEOL JSM-6460LV scanning electron microscopy at 15KV (Elissondo et al., 2006).
2.7 Immunohistochemistry
Caspase-3 antibodies were diluted 1:300, and Sections were exposed to a high temperature, high-pressure antigen extraction, and staining procedure according to the kit instructions (Dako). The slides were colored with DAB, counterstained with hematoxylin, and examined under a light microscope. Cytoplasm brownish staining (may comprise nuclei) was seen as caspase-3 positive (Hu et al., 2011).
3 Results
3.1 Morphological changes by light microscopy
The calcareous corpuscles were bright and clear with clear hooks and suction cups, and the control protoscolices were clear and undamaged. The specimens incubated in 50 µg/ml ACCV showed: cuticle discontinuation, loss of hooks & suction cups, free hooks and calcareous corpuscles, and vacuole formation with deformity. Whereas those incubated in 100 µg/ml ACCV showed: cuticle detachment and discontinuation, hooks loss, shrunken with deformity, and some were completely lost the integrity and shape (Fig. 2).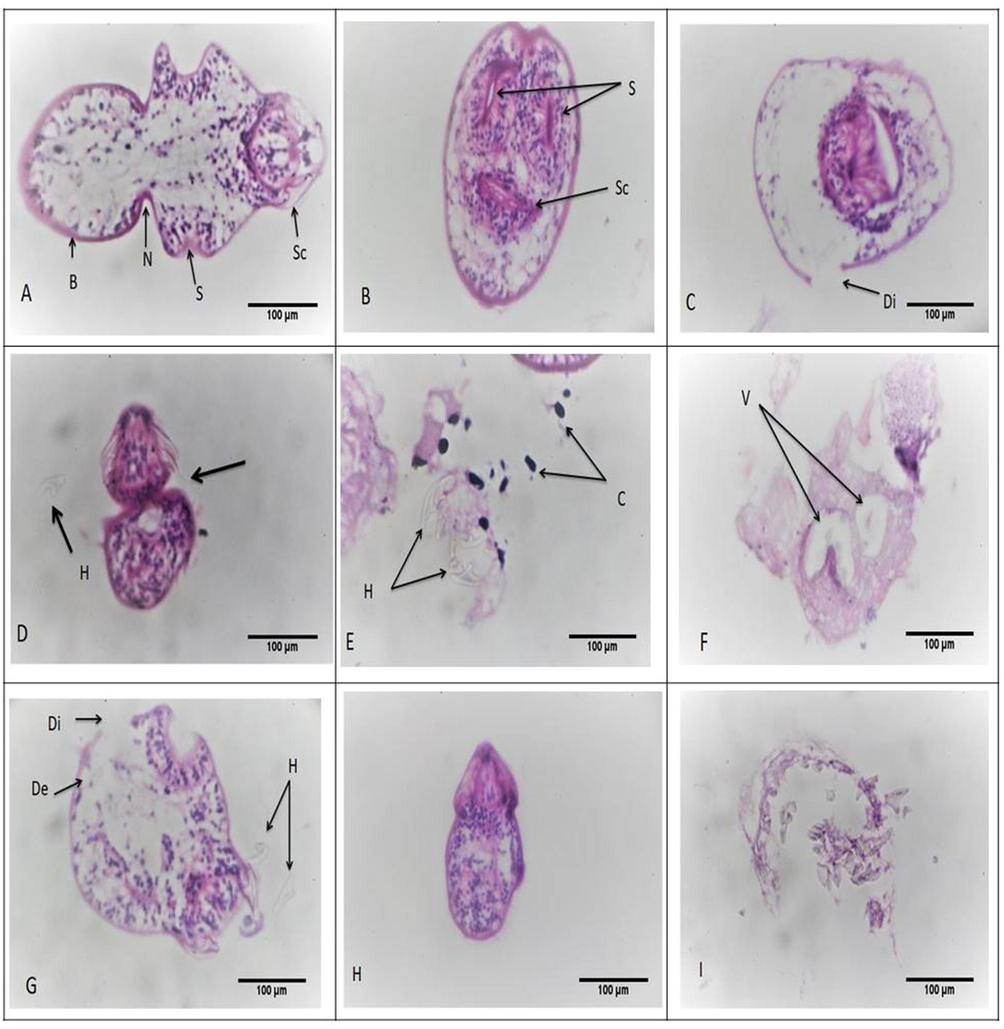
Morphological alterations of protoscolices stained with H&E. (a-b) showed normal intact protoscolices with hooks and suction cups. Sc, scolex region; B, body region; N, neck region; S, sucker region. (c-f) showed protoscolices incubated with 50 µg/ml ACCV; (c) showed cuticle discontinuation (di). (d) showed loss of suction cups (arrow) and loss of hooks (h). (e) showed free hooks (h) and calcareous corpuscles (c). (f) showed vacuoles (v) with deformity. (g-i) showed protoscolices incubated with 100 µg/ml ACCV; (g) revealed cuticle detachment (de), cuticle discontinuation (di) and hooks loss (h). (h) showed shrunken protoscolex with deformity. (i) showed complete loss of integrity and shape (x400).
3.2 Ultrastructural changes by TEM
Control protoscolices contain distinctive germinal layer (GL) with numerous cell types (e.g., round or oval internal parenchymal cells with clear along with large nuclei and nucleoli at the center). The nuclei chromatins were fine & smooth and contained a small quantity of heterochromatin. Also, the control protoscolices showed a tegument syncytium with aligned microtriches coated with a thick layer of PAS (“Periodic Acid-Schiff”) positive material. Protoscolices incubated with ACCV showed apoptotic changes; these changes were patchy in protoscolices incubated with 50 µg/ml ACCV and generalized in those incubated with 100 µg/ml ACCV. Moreover, protoscolices incubated with 50 µg/ml ACCV showed a start loss of the PAS-positive substance outside the tegument microtriches and vacuolation and the occurrence of lipid droplets in their cytoplasm. Whereas the protoscolices incubated with 100 µg/ml ACCV showed a start loss of the PAS-positive substance and shedding of microtriches, some showed complete loss of PAS, microtriches, and the presence of lipid droplets. There was also increased vacuolation of the distal cytoplasm, compressed cytoplasm in the inside parenchymal cells, apoptotic bodies, heterochromatin mass in the form of the crescent on the nuclear membrane of parenchyma cell, residual lamellar bodies, and the extensive damage of the internal tissue (Fig. 3).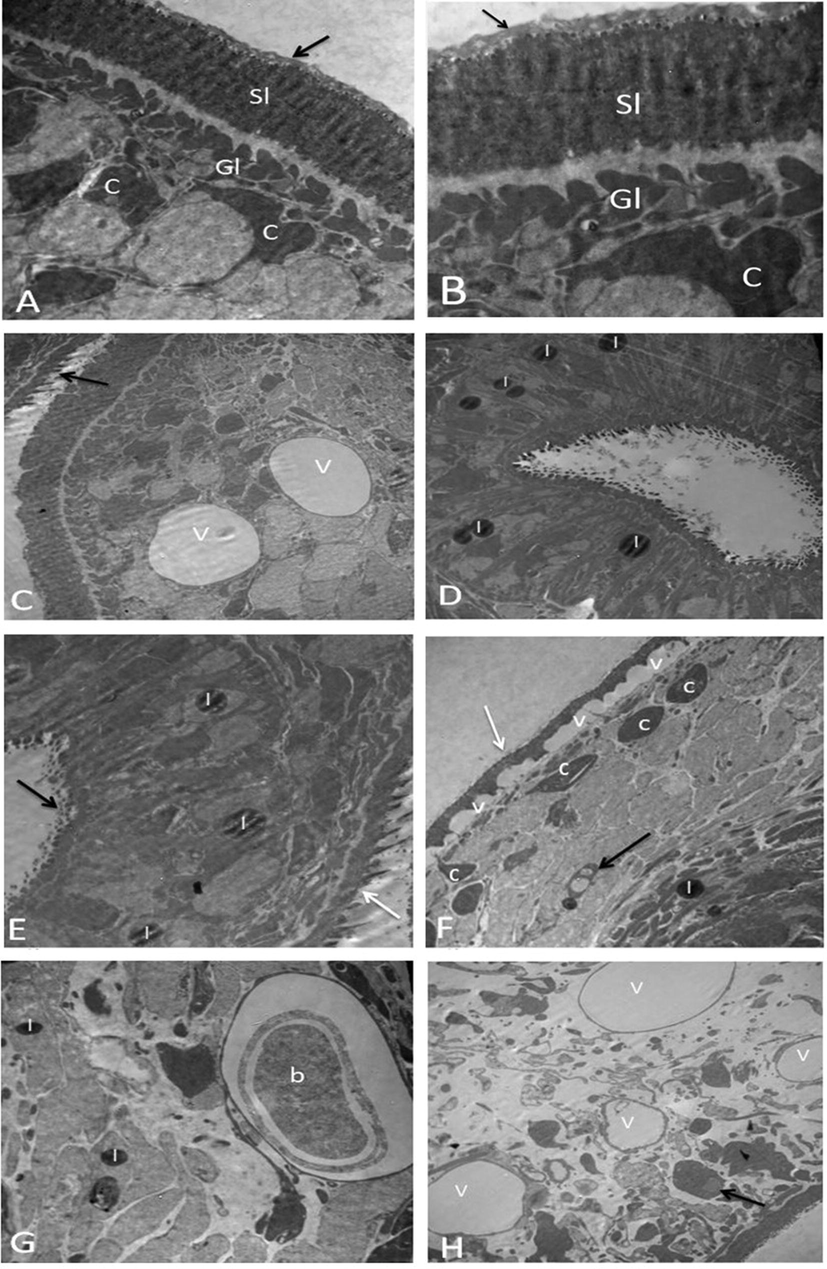
Protoscolices by TEM. (A-B) Normal protoscolices with distinctive features like; Gl (Germinal layer), which contains numerous cell forms, such as parenchymal cells (c), a syncytial layer of tegument (Sl), and PAS-positive material (arrow) (A) (X5810) and (B) (X10000). (C-D) Protoscolices incubated with 50 µg/ml ACCV (C) showed start loss of PAS-positive material (arrow) and vacuolation of cytoplasm (v) (X2900). (D) showed the presence of lipid droplets (l) (X3600). (E-H) Protoscolices incubated with 100 µg/ml ACCV (E) showed start loss of PAS-positive material (white arrow), shedding of microtriches (black arrow), and the presence of lipid droplets (l) (X4810). (F) showed vacuolation of distal cytoplasm (v), condensed cytoplasm of parenchymal cells (c), apoptotic bodies (black arrow), lipid droplets (l), and complete loss of PAS-positive material and microtriches (white arrow) (X3600). (G) showed residual lamellar bodies (b) and the presence of lipid droplets (l) (X4810). (H) showed cytoplasm vacuolation (v), The nuclear membrane of a parenchyma cell has a crescent-shaped heterochromatin mass (arrow), and extensive damage of the internal tissue (X2900).
3.3 Ultrastructural changes by SEM
Control protoscolices exhibited no ultrastructural changes during the whole period of incubation. In contrast, the treated protoscolices had ultrastructural damage, with the tegument of the parasite being the main source of damage.
After the 4th hour of treatment with ACCV, protoscolices incubated with 50 µg/ml ACCV showed the existence of tegumental changes and soma region contraction. The ultrastructural changes included rostellar disorganization, hooks loss, and shedding of scolex regions microtriches. Protoscolices incubated with 100 µg/ml ACCV showed the production of tegumental vesicles. Morphology loss was seen in certain protoscoleces, and the bursting of the soma area shows a loss of tegumental integrity, resulting in osmoregulatory damage (Fig. 4).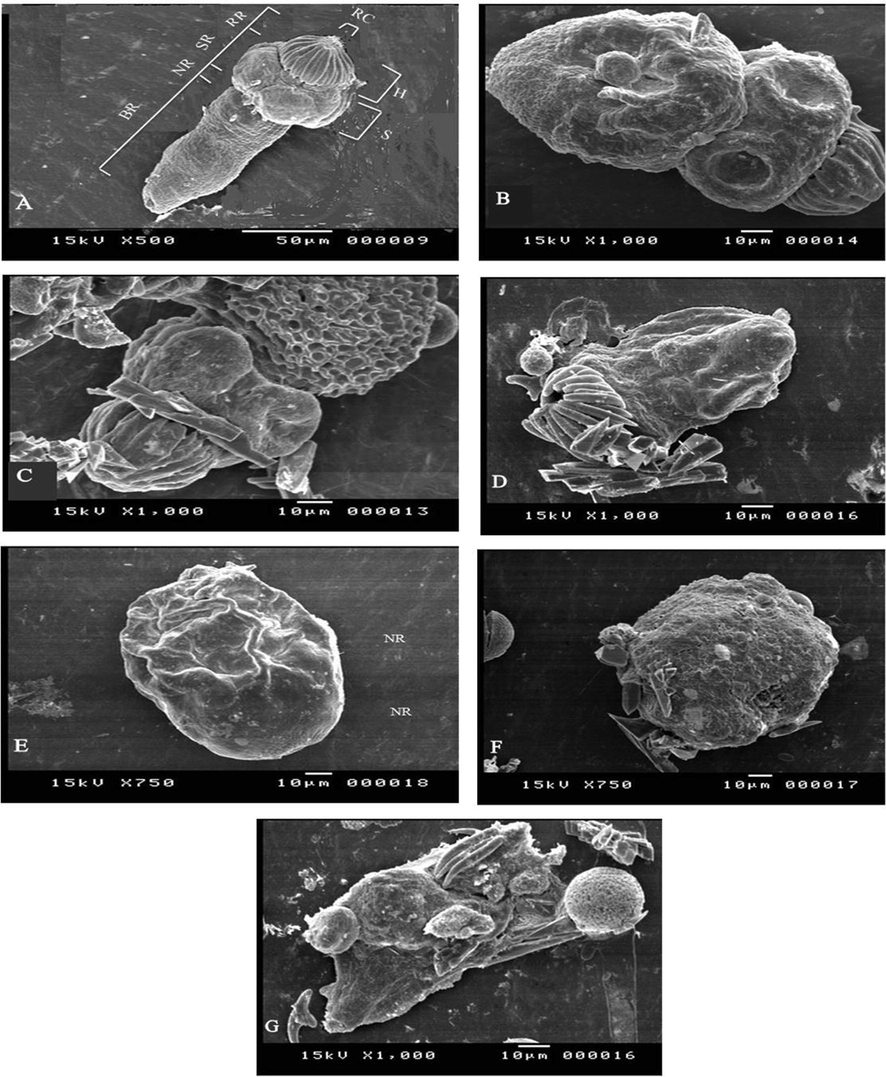
Protoscolices by SEM: (A) Evaginated control protoscolices; Body Region (BR); Neck Region (NR); Sucker Region (SR); Rostellar Region (RR); Suckers (S); Hooks (H), Rostellar Cone (RC). (B-D) Protoscolices incubated with ACCV (50 µg/mL) during 4 h of treatment; (B) Presence of tegumental changes, contraction of the soma region and shedding of microtriches (C) Presence of tegumental changes (D) Rostellar disorganization, hooks loss, and microtriches shedding of the scolex region. (E-G) Protoscolices incubated with100 µg/ml ACCV during 4 h of treatment; (E) Formation of tegumental vesicles, (F) Morphological deformation of protoscolices (G) The loss of tegumental integrity, which leads to osmoregulatory impairment, is shown by the bursting of the soma region.
3.4 Immunohistochemistry
Control protoscolices did not show any caspase-3 expression, whereas caspase-3 expression in the protoscolices incubated with ACCV was significantly high. Protoscolices incubated with 50 µg/ml ACCV revealed large areas of brownish staining, while those incubated in 100 µg/ml ACCV showed complete brownish staining (Fig. 5). However, when the PBS replaced the primary antibodies in the staining procedure, all slides did not show any brownish staining, excluding false-positive.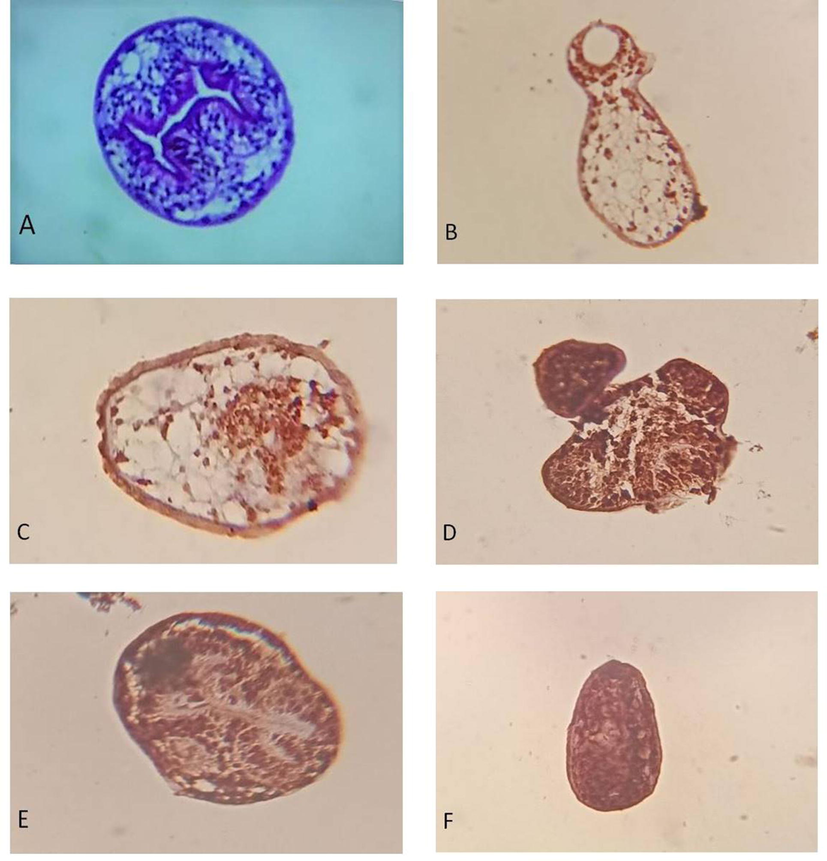
Caspase-3 expression in protoscolices using immunohistochemistry. (A) control protoscolices; (B-C) Protoscolices incubated with 50 µg/ml ACCV for 4 h, the results showed few spots stained brownish; (D-F) Protoscolices incubated with 100 µg/ml ACCV for 4 h, the results showed whole protoscolices stained brown (x400).
4 Discussion
Protoscolices are essential targets for therapeutic agents to stop the development of hydatid cysts since they can develop into adult worms in a definitive host or into new hydatid cysts in an intermediate host (Zou et al., 2009). Numerous scolicidal agents were used to inactivate hydatid cyst contents. However, the majority of these scolicidal agents have many side effects. The optimal scolicidal agent would have fast and comprehensive scolicidal actions, with minimum local or systemic side consequences (Altindis et al., 2004).
Natural venoms have a broad variety of antimicrobial actions against viruses, fungi, bacteria, and parasites, (Bahar and Ren, 2013). Biologically, the venoms of scorpions are diverse and have activity because of their defensive and predatory usage in nature (Rodríguez de la Vega and Possani, 2005; Martin-Eauclaire and Couraud, 1995). Borges et al. (2006) found that Tityus discrepans crude venom and its components inhibited the development of promastigote forms of Leishmania spp. and ultimately leads to parasite mortality. Xu et al. (2008) and El-Asmar et al. (1980) reported that scorpion venom has a cytotoxicity effect on “Ancylostoma caninum” as well as Schistosoma mansoni cercariae. Some in vitro analyses had demonstrated that some scorpion venoms have anti-Trypanosomal and anti-malarial activities (Perumal et al., 2017). The venom of scorpions contains multiple compounds such as a peptide, proteins, hyaluronidases, lipolysis activating peptides (LVPs), metalloproteases, serine proteases, and phospholipases A2 (Abdel-Rahman et al., 2016; Pessini et al., 2001). Phospholipase A2 degrades the integrity of the cell membrane via hydrolyzing the two-acyl bonds in membrane phospholipids, producing fatty acids and lysophospholipids that induce secondary membrane damage (Habermann, 1972). This enzyme displayed bactericidal activity towards gram-negative enterobacteria and was able to lyse Trypanosoma brucei brucei in vitro at a 1 mg/ml concentration (Boutrin et al., 2008). Flores-Solis et al. (2016) examined the impact of two peptides, Hoffmannihadrurus gertschi Hge36 and HgeD, on Entamoeba histolytica trophozoites and Taenia crassiceps cysticerci. They found that these parasites had considerably decreased while both peptides had little impact on lymphocytes. The ACCV was first revealed experimentally versus E. granulosus protoscolices by (Al-Malki and Abdelsater, 2020). They found that A. crassicauda crude venom could kill all protoscolices during 240 min.
The findings of the current study revealed that ACCV is not only affected the morphology and ultrastructure of protoscolices but also induces apoptosis. The visible changes of morphology and ultrastructure of protoscolices by light microscopy, SEM, and TEM were consistent with those reported in previous investigations using drugs or other compounds (Zahran et al., 2020; Tawfik, 2018; Pensel et al., 2017; Verma et al., 2013; Hu et al., 2011; Elissondo et al., 2006, 2008, 2009; Hosseini et al., 2006; Walker et al., 2004). The studies mentioned above confirmed the occurrence of apoptosis and indicated the presence of a CED-3 like apoptotic gene in protoscoleces.
Apoptosis was the most common form of eukaryotic cell death (Kerr et al., 1972). Caspase-3 is a cysteine aspartic acid protease that plays a central role in the execution-phase of apoptosis. It is the major terminal proteolytic enzyme in the process of apoptosis (Hu et al., 2011). The detection of caspase-3 was used to distinguish cell death pathways between apoptosis and necrosis, however the exact mechanism of apoptosis in E. granulosus stages was not quite clear (Van Cruchten and Van Den Broeck, 2002).
Nevertheless, understanding the exact mechanisms of apoptosis in E. granulosus stages will help the development of new treatments in the future. In the current study, the incubation of protoscolices with 50 µg/ml and 100 µg/ml of ACCV led to caspase activation at 37 °C after 4hrs. This finding was in line with the study of (Al-Asmari et al., 2018), who had proven the inhibitory effect of scorpion venoms on the proliferation of breast and colorectal cell lines through induction of apoptosis. Also, it was in accordance with the studies of Hu et al. (2011), who found that dexamethasone along with H2O2 can induces the cell apoptosis of protoscoleces and that of Tawfik (2018), who had illustrated that the cyst protoscoleces incubation with 50 ppm and 100 ppm of bee venom after 30 min at 37 °C leading to caspase activation. Naseri et al. (2016) showed the pro-apoptotic impact of “albendazole sulfoxide” as well as soluble albendazole nano polymeric particles on protoscoleces. The impact of bile acids on protoscoleces viability in vitro was investigated by Shi et al. (2016), they reported that using chenodeoxycholic acid in culture for six days at a concentration of 3 mM led to a 100 percent death rate of protoscoleces due to increased caspase-3 activity. Shahnazi et al. (2017) found incubating cyst protoscoleces with extracts of 50 to 100 mg/mL of Myrtus communis led to activated caspases for 4hrs at 37 °C. Zahran et al. (2020) exposed protoscolices and metacestode layers to different concentrations of fluralaner, which induced apoptosis in treated protoscolices due to Caspase-3 activity.
5 Conclusion
Protoscolices are the essential targets for therapeutic agents to stop the development of hydatid cysts since they can develop into adult worms in a definitive host or into new hydatid cysts in an intermediate host. Numerous scolicidal agents were used to inactivate hydatid cyst contents, however, the majority of these have many side effects. The findings of the current study revealed that Androctonus crassicauda crude venom (ACCV) has prominent and promising effects on the structure and / or ultrastructure of the protoscolices and apoptosis of their cells. However, further in vivo studies are recommended to find out the impacts of ACCV on both E. granulosus protoscolices and their hosting animals. Also, to conclude the possible use of it as therapeutic agent for this parasite and its similar ones.
6 Compliance with ethical standards
All methods used in the present study were approved and allowed by the ethical Committee of scientific research at the Faculty of Science, “Al-Azhar University” (Assiut Branch), Egypt, under the ethical norms of the relevant national guidelines on the care and usage of laboratory animals.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Prevalence and characterization of hydatidosis in Najdi sheep slaughtered in Riyadh city, Saudi Arabia. Saudi J. Biol. Sci.. 2018;25(7):1375-1379.
- [CrossRef] [Google Scholar]
- Abdelbaset A.E., Yagi, K., Nonaka, N., Nakao, R., 2021. Cystic echinococcosis in humans and animals in Egypt: An epidemiological overview. Curr. Res. Parasitol. Vector-Borne Dis. 1, 100061, ISSN 2667-114X, https://doi.org/10.1016/j.crpvbd.2021.100061.
- Abdel-Rahman, M.A., Quintero-Hernández, V., Possani, L.D., 2016. Scorpion venom gland transcriptomics and proteomics: An overview. In: Venom Genomics Proteomics; Springer Science+Business Media Dordrecht: Berlin/Heidelberg, Germany, pp. 105–124.
- Use of albendazole sulfoxide, albendazole sulfone, and combined solutions as scolicidal agents on hydatid cysts (in-vitro study) Wld. J. Gastroenterol. (WJG). 2009;15(1):112-116.
- [Google Scholar]
- Antimicrobial Activity of Two Novel Venoms from Saudi Arabian Scorpions (Leiurus quinquestriatus and Androctonus crassicauda) Int. J. Pept. Res. Ther.. 2020;26(1):67-74.
- [Google Scholar]
- Destructive effect of gamma irradiation on Echinococcus granulosus metacestodes. Parasitol. Res.. 2015;114(8):3145-3150.
- [Google Scholar]
- Scorpion Venom Causes Apoptosis by Increasing Reactive Oxygen Species and Cell Cycle Arrest in MDA-MB-231 and HCT-8 Cancer Cell Lines. J. Evid. Based Integr. Med.. 2018;23:1-8.
- [Google Scholar]
- In vitro Scolicidal effects of Androctonus crassicauda (Olivier, 1807) venom against the protoscolices of Echinococcus granulosus. Saudi J. Biol. Sci.. 2020;27(7):1760-1765.
- [Google Scholar]
- Assessment of prevalence of hydatidosis in slaughtered Sawakny sheep in Riyadh city, Saudi Arabia. Saudi J. Biol. Sci.. 2017;24(7):1534-1537.
- [CrossRef] [Google Scholar]
- In vitro leishmanicidal activity of Tityus discrepans scorpion venom. Parasitol. Res.. 2006;99(2):167-173.
- [Google Scholar]
- Boutrin, M.C., Foster, H.A., Pentreath, V.W., 2008. The effects of bee (Apis mellifera) venom phospholipase A2 on Trypanosoma brucei brucei and enterobacteria. Exp. Parasitol. 119(2), 246–251.
- Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop.. 2010;114(1):1-16.
- [Google Scholar]
- Characterization of venom components from the scorpion Androctonus crassicauda of Turkey: Peptides and genes. Toxicon. 2006;48(1):12-22.
- [Google Scholar]
- Cationicity-Enhanced Analogues of the Antimicrobial Peptides, AcrAP1 and AcrAP2, from the Venom of the Scorpion, Androctonus crassicauda, Display Potent Growth Modulation Effects on Human Cancer Cell Lines. Int. J. Biol. Sci.. 2014;10(10):1097-1107.
- [Google Scholar]
- Factor (s) in the venom of scorpions toxic to Schistosoma mansoni (intestinal belharzia) cercariae. Toxicon. 1980;18(5-6):711-715.
- [Google Scholar]
- Efficacy of thymol against Echinococcus granulosus protoscolices. Parasitol. Int.. 2008;57(2):185-190.
- [Google Scholar]
- Flubendazole and ivermectinin vitro combination therapy produces a marked effect on Echinococcus granulosus protoscoleces and metacestodes. Parasitol. Res.. 2009;105(3):835-842.
- [Google Scholar]
- In vitro effects of flubendazole on Echinococcus granulosus protoscoleces. Parasitol. Res.. 2006;98(4):317-323.
- [Google Scholar]
- El-Meleh, G., El-Meghanawy, R., Sabike, I., Hassan, M., 2019. Parasitic affections of edible offals of slaughtered animals at El-Shohada abattoir, Menofia Governorate. Egypt Benha Vet. Med. J., 36, 117–128, 10.21608/bvmj.2019.13933.1031.
- Echinococcosis granulosus/hydatidosis an endemic zoonotic disease in Egypt. J. Egypt. Soc. Parasitol.. 2007;37(2):609-622.
- [Google Scholar]
- Solution structure and antiparasitic activity of scorpine-like peptides from hoffmannihadrurus gertschi. FEBS Lett.. 2016;590(14):2286-2296.
- [Google Scholar]
- Bee and Wasp Venoms: The biochemistry and pharmacology of their peptides and enzymes are reviewed. Science. 1972;177(4046):314-322.
- [Google Scholar]
- In vitro protoscolicidal effects of hypertonic glucose on protoscolices of hydatid cyst. Korean J. Parasitol.. 2006;44(3):239-242.
- [Google Scholar]
- Drug induced apoptosis of Echinococcus granulosus protoscoleces. Parasitol. Res.. 2011;109(2):453-459.
- [Google Scholar]
- Jafari, H., Nemati, M., Haddad Molayan, P., Khaleghi Rostamkolaie, L., Hamidinejat, H., 2019. Scolicidal activity of mesobuthus eupeus venom against the protoscolices of echinococcus granulosus. Arch. Razi Inst. https://doi.org/10.22092/ari.2018.121416.1213.
- Apoptosis: A basic biological phenomenon with wideranging implications in tissue kinetics. Brit. J. Cancer. 1972;26(4):239.
- [Google Scholar]
- Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int. J. Surg.. 2014;12(5):399-403.
- [Google Scholar]
- Martin-Eauclaire, M.F., Couraud, F., 1995. Scorpion neurotoxins: Effects and mechanisms. In: Handbook of Neurotoxicity; Chang, L.W., Dyer, R.S., Eds.; Marcel Drekker: New York, NY, USA, pp. 683–716.
- Echinococcus granulosus: in vitro effectiveness of warm water on protoscolices. Exp. Parasitol.. 2011;127(1):14-17.
- [Google Scholar]
- In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. Wld. J. Surg.. 2010;34(11):2677-2681.
- [Google Scholar]
- High scolicidal effect of Zataria multiflora on protoccoleces of hydatid cyst: an in vitro study. Compar. Clin. Pathol.. 2012;21(1):99-104.
- [Google Scholar]
- Fine structure of the hydatid cyst and protoscolex of Echinococcus granulosus. Parasitol. 1967;53:312-325.
- [Google Scholar]
- Scolicidal and apoptotic activities of albendazole sulfoxide and albendazole sulfoxideloaded PLGA-PEG as a novel nanopolymeric particle against Echinococcus granulosus protoscoleces. Parasitol. Res.. 2016;115(12):4595-4603.
- [Google Scholar]
- Experimental cystic echinococcosis therapy: In vitro and in vivo combined 5-fluorouracil/albendazole treatment. Vet. Parasitol.. 2017;245:62-70.
- [Google Scholar]
- The effects of albendazole and albendazole sulphoxide combination-therapy on Echinococcus granulosus in vitro. Int. J. Parasitol.. 1994;24:219-224.
- [Google Scholar]
- A hyaluronidase from Tityus serrulatus scorpion venom: Isolation, characterization and inhibition by flavonoids. Toxicon. 2001;39:1495-1504.
- [Google Scholar]
- Scolicidal Activity of Biosynthesized Silver Nanoparticles against Echinococcus Granulosus Protoscolices. Int. J. Surg.. 2015;19:128-133.
- [CrossRef] [Google Scholar]
- Rajabi, M.A., 2009. Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst. Surg. Pract. 13(1), 2–7.
- Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure function relationships and evolution. Toxicon. 2005;46:831-844.
- [Google Scholar]
- Present situation of echinococcosis in the middle east and Arabic North Africa. Parasitol. Int.. 2006;55:S197-S202.
- [Google Scholar]
- Sarhan, M.M., Fouda, M.M., Elbitar, M.A., Aly, A.H., Sayed, B.A., 2012. Variation of protein profile among consecutive stings of the scorpion Parabuthus leiosoma (family: buthidae) from Egypt, supports the venom-metering hypothesis in scorpions. Al-Azhar Bull. Sci. 23, 61–71.
- Sarkari, B., Rezaei, Z., 2015. Immunodiagnosis of human hydatid disease: where do we stand? Wld. J. Metho. 5(4), 185.
- Sayek, I., Yalin, R., Sanaç, Y., 1980. Surgical treatment of hydatid disease of the liver. Arch. Surg. 115(7), 847–850.
- Evaluating the effect of Myrtus communis on programmed cell death in hydatid cyst protoscolices. Asian Pacific J. Trop. Med.. 2017;10(11):1072-1076.
- [Google Scholar]
- Sharafi, S.M., Sefiddashti, R.R., Sanei, B., Yousefi, M., Darani, H.Y., 2017. Scolicidal agents for protoscolicesof Echinococcus granulosus hydatid cyst: Review of literature. J. Res. Med. Sci.: Offic. J. Isfahan Univ. Med. Sci. 22, 92–99.
- Protoscolicidal effects of chenodeoxycholic acid on protoscoleces of Echinococcus granulosus. Exp. Parasitol.. 2016;167:76-82.
- [Google Scholar]
- Procedures for testing the viability of human hydatid cyst following surgical removal, especially after chemotherapy. Trans. R. Soc. Trop. Med. Hyg.. 1980;74:649-652.
- [Google Scholar]
- In vitro scolicidal effect of bee venom on Echinococcus granulosus protoscolices. J. Egypt. Soc. Parasitol.. 2018;48(3):689-697.
- [Google Scholar]
- The Effects of cetrimide-chlorhexidine combination on the hepatopancreaticobiliary system. Wld. J. Surg.. 2005;29:6:754-758.
- [Google Scholar]
- Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anatom. Histol. Embryol. 2002;31:214-223.
- [Google Scholar]
- Verma, V.C., Gangwar, M., Yashpal, M., Nath, G., 2013. Anticestodal activity of endophytic Pestalotiopsis sp. on protoscoleces of hydatid cyst Echinococcus granulosus. Biomed. Res. Int. (1), 308515.
- In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J. Antimicrob. Chemother.. 2004;54(3):609-616.
- [Google Scholar]
- Observations on the ultrastructure of protoscoleces from different hosts by transmission electron microscope in Qinghai plateau. Bull. Endemic Dis.. 1994;9:19-22.
- [Google Scholar]
- In vitro effect of medicinal scorpion on the larvae of Ancylostoma caninum. Chin. J. Parasitol. Parasitic Dis.. 2008;26(5):387-391.
- [Google Scholar]
- Study on the effect of an ion channel inhibitor “Fluralaner” on Echinococcus granulosus protoscolices and metacestode layers in vitro. J. Parasitic Dis.. 2020;44(2):411-419.
- [Google Scholar]
- Echinococcus granulosus: protoscolicidal effect of high intensity focused ultrasound. Exp. Parasitol.. 2009;121(4):312-316.
- [Google Scholar]







